Abstract
Lead, zinc, and associated minerals have been mined in the Enyigba, Nigeria area for over 92 years, leaving accumulated sulphide dumps and contaminated mine water. Stream sediment, mine tailings, mine water, stream water, and groundwater were sampled and analysed to determine the sources of potentially harmful elements in domestic water. The mine water contained mean concentrations of Cd, As, Co, Cr, Mn, Pb, and Ni that greatly exceeded those of the stream water, but samples from the streams and groundwater exceeded Nigerian drinking water and WHO thresholds for Al, Fe, and Pb, except for acceptable Pb concentrations in the borehole. Mn concentrations in one stream also exceeded Nigerian and WHO threshold for drinking water. In addition to the Pb–Zn mining, geological weathering also contributed to Pb, Al, Fe, and Mn contamination in the area.
Zusammenfassung
In der Enyigba-Region (Nigeria) werden Blei, Zink und weitere Minerale seit 92 Jahren bergbaulich gewonnen. Die Rückstände werden auf sulfidhaltigen Halden gelagert, was die Bildung von schadstoffhaltigen Grubenwasser begünstigt. In dieser Studie wurden Sedimentproben aus Vorflutern und Tailings sowie Wasserproben des Grubenabwassers und des Oberflächen- und Grundwassers untersucht, um mögliche Schadstoffquellen im genutzten Wasser zu identifizieren. Die mittleren Cd-, As-, Co-, Cr-, Mn-, Pb- und Ni- Konzentrationen des Grubenwassers liegen weit über den Werten des Oberflächenwassers. Die Proben aus dem Oberflächen- und Grundwasser liegen sowohl über den nigerianischen Trinkwasser- als auch über den WHO-Grenzwerten für Al, Fe und Pb. Ausnahmen existieren für Pb-Konzentrationen in Bohrlöchern. Die Mn-Gehalte in einem der Vorfluter übersteigen ebenfalls die gültigen Trinkwassergrenzwerte Nigerias und der WHO. Neben bergbaubedingten Freisetzungsprozessen aus dem Pb-Zn-Bergbau spielen auch geogene Verwitterungsprozesse eine Rolle, welche zusätzlich zu einer Erhöhung der Pb-, Al-, Fe- und Mn-Werte in der Region beitragen.
Resumen
El plomo, el zinc y los minerales asociados han sido extraídos en el área de Enyigba, Nigeria, durante más de 92 años, dejando relaves con sulfuro y agua de mina contaminada. Los sedimentos fluviales, los relaves de la mina, el agua de mina y las aguas superficiales y subterráneas fueron muestreados y analizados para determinar las fuentes de elementos potencialmente dañinos en el agua de consumo doméstico. El agua de la mina contenía concentraciones medias de Cd, As, Co, Cr, Mn, Pb y Ni que superaban en gran medida a las de las aguas superficiales y subterráneas pero éstas superaban los niveles aceptados para el agua potable por la legislación nigeriana y por la OMS para Al, Fe y Pb, excepto por las concentraciones aceptables de Pb en el pozo. Las concentraciones de Mn en el agua superficial también superaron los mínimos aceptados para el agua potable. Además de la minería Pb-Zn, la meteorización geológica también contribuyó a la contaminación por Pb, Al, Fe y Mn en el área.
抽象
尼日利亚Enyigba的铅、锌及其它矿物开采历史已经92年,遗留大量含硫化物的固体废物和污染的采矿废水。采集和测试了研究区河底污泥、尾矿、采矿废水、河水和地下水以识别生活用水的潜在有害元素污染源。采矿废水的Cd、As、Co、Cr、Mn、Pb和Ni平均浓度远大于河水;除了钻孔水Pb浓度处于可容许范围,河水和地下水的Al、Fe和Pb浓度已经超过尼日利亚和世界卫生组织饮用水标准。一条河水的Mn浓度也超过了尼日利亚和世卫组织饮用水标准。除了Pb-Zn开采,地质风化也使Pb、Al、Fe和Mn浓度提高。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining and ore processing produce huge quantities of tailings, and the mine wastewater provides a medium to transport the contaminants offsite (Adriano 1986). Studies of contaminant fluxes at abandoned mines in different parts of the world have often indicated high levels of contaminants in soils, streams, and plants which, through food chain transfer, pose a potential health risk to human and animals (Khan et al. 2008, 2010; Li et al. 2014; Martinez–Martinez et al. 2013; Moreno-Jimenez et al. 2009; Musah et al. 2013; Obiora 2012; Obiora et al. 2016a, b). The dangers associated with acid mine drainage (AMD) and discharge of water containing high concentrations of dissolved metals from mine wastes to nearby water bodies are environmental problems of global interest (Blowes et al. 2003). In developing countries, these problems can have a severe effect on the environment due to the inadequacy of mine waste management. Trace element toxicity in water is strongly affected by site-specific water quality factors, like pH, hardness, and dissolved constituents (Besser and Leib 1999; Diamond et al. 1992). Determination of trace element concentrations, their bioavailability, and transportation mechanisms by surface and groundwater is required to predict potential contamination of soil and water (Burt et al. 2011). Nieto et al. (2007) have shown that acidic waters can degrade groundwater, stream and river water, and even river basins.
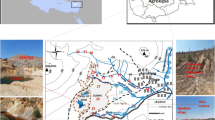
Mining of lead–zinc in Enyigba and adjoining areas in southeastern Nigeria has lasted for over 92 years, and small-scale mining is still going on. This has led to an abundance of mine wastes and abandoned mine sites in the area. Some of these tills have been converted to farmlands; while old mine pits are used as ponds for domestic water supply. Some of the mines were located next to seasonal stream channels and tailings were deposited less than 4 m from the stream (Fig. 1). These streams, which are a major domestic water supply source, have been a major accumulator of potentially harmful elements (PHEs).
The Enyigba community is located about 15 km south of Abakaliki, in southeastern Nigeria. The mining area is drained by three major waterways: the Nwangele-Akpara River, the Ina stream, and the Ngele-Odicha stream, which flows in a dendritic pattern to the River Akpara. Most of the mines are located in sporadic small hills that drain into these streams, which are located in the lowlands (Fig. 1). The mines occur in three areas: the Ishiagu-Enyigba mines, Alibaruhu Enyigba mines, and Ndinwanu Ishiagu-Enyigba–Ikwo mines (Ikwo-Ameri). The region experiences dry and rainy seasons and the streams are seasonal. The dry months are from January to March, while the other months are characterized by precipitation and heavy rainfall. During the dry periods, vegetables are cultivated near the streams and stream beds, and irrigated using the contaminated streams and mine water. The study area is predominantly underlain by fractured shales and intermittent siltstones and limestones of Albian age.
Studies in neighbouring African countries have indicated elevated concentrations of Cu, Co, Ni, and Zn in domestic water ponds and tap water (e.g. Hartwig et al. 2005; Mwesigye et al. 2016; Owor et al. 2007). Abraham and Susan (2017) reported an increase in Cu, Co, Ni, Pb, and As concentrations in River Nyamwamba, near the Kilembe copper mine in western Uganda. The work of Obiora et al. (2016a, b), in the study area (Enyigba community) has shown that the elevated concentrations of Mn, Pb, and Zn in soils, food crops, and vegetables may pose a health risk in the area. Since only a few boreholes (wells) have been drilled in the area, most of the Enyigba inhabitants depend on stream water and ponds for their domestic water supply. The main subject of this work was determining the point sources of PHEs in the Enyigba Pb–Zn mining district and the proportion of the total element budget in public and domestic water supplies in the area.
Materials and Methods
Water Sample Collection and Analytical Procedures
The water samples were collected in three batches: water samples from the three mine locations, n = 7; samples from public water sources including stream waters (n = 13), collected upstream and downstream of the mines; and a sample from an available borehole, which is located up-gradient of the mines. The water samples from the stream channels were collected 50–100 m intervals from at least 2 sample points at a depth greater than 5 cm below the surface. All of the samples were homogenized in a rinsed 100–200 mL beaker and transferred into a prepared 100 mL bottle. The borehole samples, on the other hand, were collected directly from the pump into 100 mL beakers that had been rinsed three times with the borehole water, without homogenization. The water samples were filtered in the field using disposable filters of 0.45 µm diameter to remove suspended solids and were then stored in prepared bottles. Samples for PHE analysis were acidified with 1.0 mL of pure concentrated HNO3 using a syringe to prevent sorption on the walls of the container and stored in an ice pack container to minimize temperature changes. Beakers and sample bottles were thoroughly washed and then filled with distilled water acidified with 1.0 mL of HNO3 for at least 3 days before the sampling. The beakers and sample bottles were cleaned with diluted HNO3 acid at every sample location and also rinsed at least 3 times with water from the sampling points. The acidified waters were analyzed by inductively coupled plasma mass spectrometry (ICP-MS) at ACME Laboratories, Vancouver, Canada for 36 elements. However, only 11 PHEs (Pb, Zn, Cd, Co, Ni, As, Cr, Cu, Mn, Fe, and Al) are discussed in this paper. Water temperature and pH were measured in the field.
Stream Sediments and Mine Tailings
Stream sediments (n = 10) were collected from the three Enyigba stream channels at the same points as the water samples by scraping the top 5 cm of the stream bed with a plastic scoop. At least 3 sediment sub-samples were collected from each sampling point and homogenized. The samples were collected upstream and downstream of the mines. The samples were stored in plastic bags and transported to Ebonyi State University, Abakaliki, and air dried in the laboratory for 2 weeks, then pulverized and sieved to less than 2 mm and stored in plastic bags for analysis. Samples of the mine tailings (n = 7) were collected from various old tailings sites and prepared for analysis using the procedure defined in the UNESCO-SIDA sponsored Abandoned Mines Project (Kribek 2013). Trace elements concentrations were also determined in the ACME laboratories (Vancouver, Canada). One gram of stream sediment and tailing samples were mixed with 15 mL of a mixture of HNO3, H2SO4, and HClO4 in the ratio 5:1:1 at 100 °C, until complete digestion. Whatman No. 42 filter paper was used to filter the digested samples, and filtrates were diluted in 50 mL of distilled water and stored for further analysis. Sampling was conducted between April and May 2014.
Quality Control Analysis
The tailings and stream sediment samples were prepared and tested in duplicate standard optimization forms with a confidence level of 95%, as a quality control measure. Standard solutions of the analyzed elements were set up by adding 1000 mg L− 1 of corresponding certified standards. Blank reagents and standard reference materials were used to test analytical correctness and precision (see http://www.acmelab.com for further inquiries).
Enrichment Factor in Stream Sediments
Metals from natural sources in sediments can be differentiated from anthropogenic sources by using Fe to normalize trace metal contents (Baptista et al. 2000; Martinez-Martinez et al. 2013; Obiora et al. 2016b). The enrichment factor (EF) is computed using Eq. 1 (Buat-Menard and Chesselet 1979).
where C is the elemental concentration in a sediment sample, Cn is the Fe concentration in the sediment sample, m is the elemental concentration in background sediments and Fe is the respective concentration of Fe in the background sediments. The Fe background concentration is 35,900 mg/kg (Martin and Meybeck 1979). EF values between 0.5 and 1.5 indicate that the metal is entirely from natural sources, while EF values greater than 1.5 suggest a possible anthropogenic source (Zhang and Liu 2002). Considering the degrees of contamination, Lacatuso (1998) showed that EF values of 1.1–2 indicate slight contamination; EF: 2.1–4 moderate contamination; EF: 4.1–8 severe contamination; EF: 8.1–16 very severe contamination; and EF > 16 excessive contamination.
Social Interactions and Survey
A survey was conducted by interviewing men and housewives randomly selected from 51 households across the localities using a questionnaire, to identify the domestic water sources in the Enyigba mining district. The survey data were statistically processed using SPSS, version 20.0, and Microsoft Excel (2010). The results were presented as mean values and standard deviations. The stream sediment, tailings, and water analytical data were subjected to statistical tests, including Person’s correlation, to determine a possible linear association between the analyzed trace elements. Significant differences were determined by ANOVA analysis.
Results and Discussion
Mine Tailings
The concentrations of PHEs in the Enyigba mine tailings are presented in Table 1. The topography of the area, characterized by a number of small hills, is the loci of the tailings deposits. These deposits are frequently exposed to erosion by rainfall and subsequently downhill transport into the surrounding soils and streams. In the process of reclaiming land near some of the abandoned mines, especially in the Enyigba-Ishiagu mines (Fig. 1), the inhabitants use old tailings to fill the abandoned mine pits, thereby exposing the surface area to more PHEs, which interact with the soil and stream waters through surface erosion. The erosion of the tailings is evident through the development of sheets and gullies. The tailings near the Ikwo (Amari) mines are very close to Ina stream and consequently erode into it. The concentrations of Pb, Zn, Cu, As, Mn, Fe, and Cd in the tailings are up to 450 times their average values of the upper crust (Table 1; McLennan 2001).
Umeji (2000) and Obiora et al. (2016b) have studied the principal ore minerals exploited in the area: galena (PbS), sphalerite (ZnS), chalcopyrite (CuFeS2) and bornite (Cu5FeS4). Smithsonite (ZnCO3), cerussite (PbCO3), and azurite [Cu3(CO3)2(OH)2] occur as supergenetic enrichment products. Siderite (FeCO3), calcite (CaCO3), pyrite (FeS2), marcasite (FeS2), and quartz (SiO2) occur as gangue minerals. Hence, the elevated concentrations of Pb, Zn, Cu, and Fe in the tailings are associated with the ore minerals in the area, while Cd is a by-product from the smelting of the Pb–Zn–Cu ores, and As is a by-product of the AMD. Stream water and stream sediments collected 2 m from the tailing sites, especially from the Ina stream, have a mean pH value of 5.5 and contain high Pb, Al, and Fe levels in the water and high Pb, Zn, As, and Cr levels in the sediment, compared with its concentrations in waters and sediment collected from ≈ 10 m from the tailings. This variability indicated that tailings erosion had increased the concentration of PHEs and acidity in the stream waters by dissolution. Abraham and Susan (2017) similarly showed that rivers near the western Uganda copper mine tailings were more contaminated by Cu, Co, and Ni than river water further from the tailing sites.
Surface Mine Waters
Water discharged from the Enyigba Pb–Zn mines and processing areas are characterized by extremely high concentrations of PHEs compared with water collected from about 2 km upstream of the mined areas (Fig. 2). The average concentrations of Pb, Zn, Cd, Co, Ni, As, Cr, Cu, Mn, Fe, and Al were 4000, 2612, 750, 652, 332, 350, 300, 80, 31,503, 13,951, and 6286 µg/L, respectively, in surface mine waters, while PHE concentrations in upstream water (control) were 23, 72.5, 0.165, 1.59, 3.7, 0.6, 2.85, 4.75, 96.62, 1077, and 1565.5 µg/L, respectively. The mean electrical conductivity of the surface mine water was over 150 times higher than the conductivity of the upstream waters, while the average pH (4.1) was strongly acidic compared to the pH of the upstream waters, which had an average pH of 7.6. The effect of the AMD decreases downstream of the tailings may be due to dilution, proportional to the rate of volume influx from adjacent tributaries.
Stream Sediments
The PHE concentrations in the stream sediments are presented in Table 2. The stream sediments in the mined zones had high concentration of Cu, Pb, Zn, Mn, As, and Cd compared with average concentrations in stream sediments collected downstream. The concentrations of PHEs, except for Cr, in both sets of samples (mined zones and downstream) exceeded the mean background values of samples collected upstream and background values of the upper crust (Table 2; McLennan 2001). Burt et al. (2011) reported similar soil and water contamination (Cd, Pb, and Zn) due to geologic and anthropogenic activities in the Willow Creek mining area, in Colorado, and Martinez-Martinez et al. (2013) observed elevated levels of Pb and Zn in soils and sediments in the Pb–Zn mining district of southeastern Spain. The high concentrations of Cu, Pb, Zn, As, and Cd along the stream channels near the mines compared with lower values downstream suggest that the effects of the tailings and mine waters are being diluted by water and sediment from the tributaries.
The EF of the stream sediments in the mined zone, and downstream and upstream of the mined area is presented in Table 3. The EF of the stream sediments were: Pb > Cd > Mn > Cu > Zn > Co > As > Ni > Cr > Al in the mined zone and Cd > Co > Mn > Zn > Pb > Cr > Cu > As > Ni > Al downstream. The EF values for Pb, Cd, Mn, Cu, and Zn in the mined zone all exceeded 1.5; downstream, the EF values for Cd and Mn were still > 1.5. Upstream of the mined area, only the EF for Cd was > 1.5. Enrichment factors above 1.5 suggest an anthropogenic origin, according to Zhang and Liu (2002). Therefore, Pb, with an EF > 40, much higher than the other contaminants, must have resulted from its release from tailings. At locations downstream, density settling and elemental precipitation must have played a role in bringing about the drastic decrease in Pb concentrations, unlike Cd, which had similar concentrations in all three zones (Table 3). In addition, the decrease in Pb downstream indicates its low solubility in water, compared with Zn, which is more soluble (Burgos et al. 2006; Conesa et al. 2008). The comparison of the concentrations of PHEs show relevant Person’s correlation coefficients for stream sediments; for example Mn and Fe (p < 0.001, r = 0.9664), Fe and Cd (p < 0.001, r = 0.9617), Cu and Pb (p < 0.01, r = 0.8185), Zn and Cu (p < 0.001, r = 0.9368), Pb and Zn (p < 0.01, r = 0.7202), Al and Cu (p < 0.01, r = − 0.7317), Zn and Mn (p < 0.01, r = 0.7149), Ni and As (p < 0.001, r = 0.9313), Al and Cr (p < 0.001, r = 0.9574), Co and Cr (p < 0.001, r = 0.8902), Zn and As (p < 0.01, r = 0.7467). The good correlations between the variables reflect the combined effects of adsorption, precipitation, and co-of metals, especially by aluminum, iron, and manganese oxy-hydroxides and organic matter (Fӧrstner and Wittmann 1981; Banks et al. 1997; Rubio et al. 2000; Holmstrom and Ohlande 2001; Xiangdong et al. 2001). However, adsorbed elements can be released back into the environment if the water chemistry changes (Blasco et al. 2000).
The concentrations of PHEs in the different water sources in the Enyigba area are presented in Table 4. Though the upper part of the Akpara stream is not known to be mined, water samples from the area showed that Al, Pb, and Fe concentrations clearly exceeded the Nigerian and UK thresholds for drinking water. The average concentration of Al, Pb and Fe exceeded the thresholds of 200, 10, and 300 µg/L, respectively for drinking water (SON 2007; UK 2010). The high PHE concentrations in the non-mining areas indicate that the underlying geology and Pb–Zn mineralization of the area are sources of these elements. In addition, the appreciable concentrations of Al and Fe in the borehole water, which is outside of the mines’ area of influence, also suggests dissolution of elements via geological weathering and mineralization. Obiora et al. (2016b) also showed that anthropogenic activities are not the only source of soil contamination in the area; the geochemical composition of the rocks in the area is also a contributing factor.
Quality of Domestic and Public Water in the Enyigba Area
The data obtained from the studied area indicates that 69% of the dwellers depend on running streams for their domestic water supply, 22% depend on well water, while 9% depend on old mine pond water. The concentrations of PHEs in streams and other public water supplies are presented in Table 4. The PHEs, except for the Al from the streams used for domestic water supply (Ina, Ngele-Odicha, and Akpara streams) decrease significantly downstream and upstream of the mine influenced area (control). This shows a significant contribution from the Pb–Zn mining activities. The Al concentration, which was significantly higher, further downstream than the mined zones, suggests a significant introduction of PHEs from weathering of the shaly bedrock rather than from the lead–zinc mines. Abraham and Susan (2017) reported a similar decline in Al near a Cu mine in western Uganda that could have been due to precipitation of metals in the acidic mined zone. The Al and Fe concentrations exceeded the Nigerian threshold for drinking water (SON 2007; UK 2010) of 200 and 300 µg/L, respectively in all the domestic water supply samples, including the borehole (Table 4). Only the borehole water, with a Pb concentration of 2.9 µg/L, was under the permissible limit for drinking water of 10 µg/L (SON 2007; WHO 2008). Mn concentrations in Ina stream waters exceeded the 200 and 400 µg/L threshold values for drinking water defined respectively by SON (2007) and WHO (2008) and was below the permissible drinking water threshold for other domestic water sources (Table 4). High Pb concentrations in the water sources could be attributed to elemental inputs from metal-rich acid mine waters and release of metals from the PHE-rich tailings in the Enyigba mining community. Gunsinger et al. (2006) showed that surface disposal of tailings leads to the oxidation of residual sulphide minerals and release of H+, SO4, and various metals to the pore waters in the tailings. The control samples upstream (located outside the mining influenced area) also contained concentrations of Pb (23 µg/L) that exceeded the drinking water limits (SON 2007; WHO 2008), thus signifying contamination from the area’s Pb–Zn mineralization.
The high concentrations of Al, which increase with pH, increased in domestic water downstream of the mine zones (pH < 5), which is concordant with the findings of Lee et al. (2002) and Abraham and Susan (2017), which they attributed to the precipitation of Al(OH)3. The lower Al concentrations in the mine water could also be associated with precipitation of Al-hydroxysulphate by sorption of SO42− on the surface of Al(OH)3 at low pH levels (Munk et al. 2002). Conversely, unlike the Al concentration, Fe concentration decreases downstream away from the mines, suggesting introduction of Fe from bornite (Cu5FeS4), siderite (FeCO3) and pyrite (FeS2) from the tailings and precipitation of Fe into colloids. The high Mn concentration in Ina stream can also be attributed to the trace element inputs from the tailings due to its proximity to the Ikwo-Ameri mine (Fig. 1). In addition, Mn requires high pH (pH > 8) for oxidation of soluble Mn (II) to insoluble form Mn (IV) (Hallberg and Johnson 2005; Stumm and Morgan 1981); hence it could not precipitate at pH 6.0-6.9 in the streams of Enyigba area.
Although the Cd, Co, Zn, and Ni concentrations in the domestic waters were within the threshold limits for drinking water, their concentration in Ina stream was considerably greater than in the other domestic water sources in the area. This is attributed to the discharge of AMD from the mines and erosion of the tailings from Ikwo-Ameri mine to Ina stream, which almost transverses the mine (Fig. 1). Pearson’s correlation coefficients for PHE-concentrations in water also gave high correlations, as with the sediments, suggesting trace element retention in both, probably due to adsorption on organic matter and Fe, Al, and Mn complexes. Examples of the Pearson’s correlation in water are Pb and Zn (p < 0.001, r = 0.9808), Cu and Fe (p < 0.01, r = 0.9296), Al and Cr (p < 0.001, r = 0.9689), Fe and Al (p < 0.01, r = 0.8871), Cd and Mn (p < 0.001, r = 0.9575), Co and Cu (p < 0.01, r = 0.8167), Ni and Fe (p < 0.01, r = 0.9106), As and Cd (p < 0.001, r = 0.8113), Cr and Cu (p < 0.001, r = 0.9656), and Co and Mn (p < 0.001, r = 0.9962). Many of these correlations suggest possible oxidation of residual galena, sphalerite, and probably chalcopyrite in the tailings (Munk et al. 2002).
The concentrations of most PHEs in the sample from the borehole near the Alibaruhu locality of Enyigba met the drinking water standards (SON 2007; WHO 2008). The only exceptions were Al and Fe, with respective average concentrations of 3608 and 1474 µg/L, exceeding the respective Nigerian thresholds for drinking water (200 and 300 µg/L; SON 2007). The borehole, although cased for surface contamination, reflects the groundwater, which is susceptible to contamination by dissolution of mineralized zones and geological weathering.
Conclusions
The erosion of tailings, mine water discharges, and stream sediments are the major sources of PHE contamination in domestic and public water supplies in the Enyigba mining community. Natural sources, such as the mineralization of the area and geologic weathering, also contribute significantly to high levels of trace elements in the waters. The Pb, Al, Mn, Cd, Co, Zn, and Ni concentrations in the catchment waters downstream mostly result from the tailings, mine water, and stream sediments. The domestic waters in the area had high Pb concentrations, exceeding the 10 µg/L threshold value for drinking water (SON 2007; WHO 2008). The only water source with Pb concentrations within the permissible limits was the borehole water, with an average Pb concentration of 2.9 µg/L. Aluminium and iron concentrations exceed the 200 and 300 µg/L threshold values for drinking water (SON 2007) in all of the water sources, including upstream where there was no evidence of mining activities. Furthermore, Mn concentration from the Ina stream exceeded the 200 µg/L (SON 2007) and 400 µg/L threshold (WHO 2008) for drinking water. Hence, apart from the anthropogenic (mostly mining) activities, geologic processes also expose the inhabitants of Enyigba to water contaminated by PHEs.
This study revealed that most of the Enyigba dwellers, who depend on the streams for their domestic water supply, are highly exposed to toxic levels of Pb, Al, and Fe through their intake of this contaminated water. The Ina stream water contains the highest concentration of these contaminants, including Mn. The health risk could be higher during the dry season due to an increase in PHEs concentrations brought about by evaporation. Among the water sources, the borehole water had the lowest PHEs contents. Treatment of mine water before it discharges into the streams, containment of tailings, adoption of erosion reduction methods, and mapping of contaminated watercourses, followed by water treatment over long periods of time could help improve the quality of domestic water supplies. Borehole water should be harnessed and residents should be encouraged to use this water to meet their domestic requirements. Reclamation of the abandoned pits with materials of tested and known elemental compositions should be adopted to avoid re-introduction of contaminated soils in the already contaminated area. Finally, further research on domestic household water contamination is recommended. Public sensitization programs should be proposed by Government and non-governmental organizations to sensitize the inhabitants on water source contamination.
References
Abraham MR, Susan TB (2017) Water contamination with heavy metals and trace elements from Kilembe copper mine and tailing sites in Western Uganda; implications for domestic water quality. Chemosphere 169:281–287
Adriano DC (1986) Trace elements in the terrestrial environment. Springer-Verlag, New York City
Banks D, Younger PL, Arnesen RT, Iversen ER, Banks S (1997) Mine water geochemistry: the good, the bad, the ugly. Environ Geol 32:157–174
Baptista NJA, Smith BJ, McAllister JJ (2000) Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, southeast Brazil. Environ Pollut 109:1–9
Besser JM, Leib KJ (1999) Modeling frequency of occurrence of toxic concentrations of zinc and copper in the upper Animas River. In: Morganwalp DW, Buxton HT (eds) Proceedings of Technical Meeting, U.S. Geological Survey (USGS) Toxic Substances Hydrology Program, USGS WRI Report 99-4018A, vol 1, pp 75–81
Blasco J, Sancheenz V, Gomez-Parra A (2000) Heavy metal fluxes at the sediment-water interface of three coastal ecosystems from southwest of the Iberian Peninsula. Sci Total Environ 247:189–199
Blowes DW, Ptacek CJ, Jambor JL, Weisener CG (2003) the geochemistry of acid mine drainage. In: Lollar BS (ed) Treatise on geochemistry, vol 9, Elsevier Ltd, New York, pp 149–204
Buat-Menard P, Chesselet R (1979) Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42:398–411
Burgos P, Madejon E, Perez-de-Mora A, Cabrera F (2006) Spatial variability of the chemical characteristics of a trace-element-contaminated soil before and after remediation. Geoderma 130:157–175
Burt R, Weber T, Park S, Yochum S, Ferguson R (2011) Trace element concentration and speciation in selected mining-contaminated soils and water in Willow Creek flood plain, Colorado. Appl Environ Soil Sci. https://doi.org/10.1155/2011/237071
Conesa HM, Robinson BH, Schulin R, Nowack B (2008) Metal extractability in acidic and neutral mine tailings from the Cartagena-La Union Mining District (SE Spain). Appl Geochem 23:1232–1240
Diamond JM, Winchester EL, Mackler DG, Rasnake WJ, Fanelli JK, Gruber D (1992) Toxicity of cobalt to freshwater indicator species as a function of water hardness. Aquat Toxicol 22(3):163–180
FÓ§rstner U, Wittmann GTW (1981) Metal pollution in the aquatic environment. Springer-Verlag, Berlin
Gunsinger MR, Ptacek CJ, Blowes DW, Jambor JL (2006) Evaluation of the long term sulfide oxidation processes within pyrrhotite-rich tailings, Lynn Lake, Manitoba. J Contam Hydrol 83:149–170
Hallberg KB, Johnson DB (2005) Biological manganese removal from acid mine drainage in constructed wetlands and prototype bioreactors. Sci Total Environ 338:115–124
Hartwig T, Owor MA, Zachmann D, Pohl W (2005) Lake George as a sink for contaminants derived from Kilembe copper mining area, western Uganda. Mine Water Environ 24:114–124
Holmstrom H, Ohlande B (2001) Layers rich in Fe- and Mn-oxyhydroxides formed at the tailings-pond water interface, a possible trap for trace elements in flooded mine tailings. J Geochem Explor 74:189–203
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut 152:686–692
Khan S, Rehman S, Khan AZ, Khan MA, Shah MT (2010) Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol Environ Saf 73:1820–1827
Kribek B (2013) Recommendations for the collection and processing of samples when assessing the degree and extent of contamination of surface and ground waters, stream sediments, soils, and vegetation in areas affected by mining and mineral processing in countries of Sub-Saharan Africa. Unpublished Report. SIDA Project Planing Meeting, 2013. Prague, Czech Republic, pp 1–6
Lacatuso R (1998) Appraising levels of soil contamination and pollution with heavy metals. European Soil Bureau Research Report No. 4, pp 393–399
Lee G, Bingham JM, Faure G (2002) Removal of trace metals by co-precipitation with Fe, Al and Mn from natural waters contaminated with acid mine drainage in the Ducktown mining district, Tennessee. Appl Geochem 17:569–581
Li Z, Ma Z, Jan Van Der Kuijp T, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Enviro 468–469:843–853
Martin J, Meybeck M (1979) Elemental mass-balance of material carried by major world rivers. Mar Chem 7:173–206
Martinez-Martinez S, Acosta JA, Fazcano A, Carmona DM, Zornoza R, Cerda C (2013) Assessment of the lead and zinc contents in natural soils and tailing ponds from the Cartagena- La Union mining district, SE Spain. J Geochem Explor 124:166–175
McLennan MS (2001) Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem Geophys Geosyt. https://doi.org/10.1029/2000GC000109
Moreno-Jiménez E, Penalosa JM, Manzano R, Carpena-Ruiz RO, Gamarra R, Esteban E (2009) Heavy metals distribution in soils surrounding an abandoned mine in NW Madrid (Spain) and their transference to wildflora. J Hazard Mater 162:854–859
Munk LA, Gunter F, Douglas E, Jerry P, Bigham M (2002) Sorption of trace metals to an aluminium precipitate in a stream receiving acid rock-drainage; Snake River, Summit County, Colorado. Appl Geochem 1:421–430
Musah SN, Maxiwel A, Boateng A (2013) Health risks of heavy metals in selected crops cultivated in small-scale gold-mining areas in Wassa-Amenfi-west district of Ghana. J Nat Sci Res 3(5):2224–3186
Mwesigye AR, Young SD, Bailey EH, Tumwebaze SB (2016) Population exposure to trace elements in the Kilembe copper mine area, western Uganda: a pilot study. Sci Total Environ 573:366–375
Nieto JM, Sarmiento AM, Olías M, Canovas CR, Riba I, Kalman J, Delvalls TA (2007) Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian pyrite belt, SW Spain) and bioavailability of the transported metals to the Huelva estuary. Environ Int 33:445–456
Obiora SC (2012) Mapping of abandoned mines in Nigeria. In: Presentation, 2nd Workshop, IGCP/SIDA/UNESCO Project held at the NODA HOTEL, Kumasi, Ghana, 27–30 June 2012
Obiora SC, Chukwu A, Davies TC (2016a) Heavy metals and health risk assessment of arable soils and food crops around Pb–Zn mining localities in Enyigba, southeastern Nigeria. J Afr Earth Sci 116:182–189
Obiora SC, Chukwu A, Toteu SF, Davies TC (2016b) Assessment of heavy metal contamination in soils around Lead (Pb)–Zinc (Zn) mining areas in Enyigba, southeastern Nigeria. J Geol Soc India 87:453–462
Owor M, Hartwig T, Muwanga A, Zachmann D, Pohl W (2007) Impact of tailings from the Kilembe copper mining district on Lake George, Uganda. Environ Geol 51:1065–1075
Rubio B, Nombela MA, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull 40:968–980
SON (Standard Organization of Nigeria) (2007) Nigerian standard for drinking water quality. https://www.unicef.org. Accessed 5 Jan 2017
Stumm W, Morgan J (1981) Aquatic chemistry, second edn. Willey, New York, p 780
UK Drinking Water Inspectorate (2010) Drinking water standards. http://www.dwi.gov.uk. Accessed 14 Jan 2016
Umeji AC (2000) Evolution of the Abakaliki and the Anambra Basins, Southeastern Nigeria. A report submitted to the Shell Petroleum Development Company Nigeria Limited, pp 155
US EPA (2016) Table of regulated drinking water contaminants. https://www.epa.gov/your-drinkingwater/table-regulated-drinking-water-contaminants. Accessed 14 Jan 2016
WHO (World Health Organization) (2008) Guidelines for drinking water quality. World Health Organization, Geneva
Wisconsin Department of Natural Resources (2011) Drinking water and groundwater quality standards/advisory levels
Xiangdong L, Zhengou S, Onyx WH, Yok-Sheung (2001) Chemical forms of Pb, Zn, and Cu in the sediment profile of the Pearl River estuary. Mar Pollut Bull 42:215–223
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China-weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Mar Sci 54:1051–1070
Acknowledgements
The authors thank Dr S. Felix Toteu, leader of the UNESCO-SIDA-sponsored project, for his approval of this project. This work is part of the UNESCO-SIDA sponsored Project 503RAF2000 on ‘Abandoned Mines in Sub-Saharan African Countries’, which itself is an offshoot of the IGCP-SIDA Projects 594 and 606. The authors appreciate the Chief editor B. Kleinmann and co-reviewers for their constructive reviews.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obiora, S.C., Chukwu, A. & Davies, T.C. Contamination of the Potable Water Supply in the Lead–Zinc Mining Communities of Enyigba, Southeastern Nigeria. Mine Water Environ 38, 148–157 (2019). https://doi.org/10.1007/s10230-018-0550-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-018-0550-0