Abstract
The environmental benefits of waste desulfurization were evaluated in the Santa Catarina coal field, Brazil. Coal waste from a beneficiation plant was separated into three density fractions, using a two stage process. Characterization of these fractions indicated that the low (D < 2.2 g/cm3) and high (D > 2.7 g/cm3) density fractions were potentially suitable for energy and sulfuric acid production, respectively. The waste fraction of intermediate density (2.2 < D < 2.7 g/cm3) represented 69% of the total mass studied and had a relatively low sulfide content, and it was postulated that it may be suitable for land disposal with minimum risk to the surrounding environment. This hypothesis was tested using laboratory-scale static and kinetic tests, which indicated that although the fraction remained net acid generating, the rate and net amount of metals, salts, and acidity that leached was considerably less than that of the discards before separation. It was concluded that this approach could reduce the amount of waste generated, as well as the associated pollution risk.
抽象

Zusammenfassung
Die Vorteile einer Abfallentschwefelung wurden für das Santa Caterina Kohlevorkommen in Brasilien bewertet. Kohleabfälle einer Aufbereitungsanlage wurden mittels eines zweistufigen Prozesses in drei Dichtefraktionen getrennt. Die Charakterisierung dieser Dichtefraktionen zeigte, das die Fraktionen niedriger (D<2.2 g/cm3) und hoher (D>2.7 g/cm3) Dichte potentiell für die Energie- bzw. Schwefelsäuregewinnung geeignet sind. Die Abfallfraktion mittlerer Dichte (2.2<D<2.7 g/cm3) entsprach 69% der untersuchten Gesamtmasse und besaß einen relativ geringen Sulfidgehalt. Es wurde daher angenommen, dass diese Fraktion mit geringem Risiko für die umgebende Umwelt deponiert werden könnten. Diese Hypothese wurde mit statischen und kinetischen Labortests geprüft. Die Tests ergaben, dass die Fraktion zwar weiterhin eine Netto-Aziditätsproduktion aufwies, aber eine erheblich kleinere als der unfraktionierte Gesamtabfall. Daraus wurde geschlossen, dass mit der Trennung in die Dichtefraktionen sowohl die Abfallmenge als auch das mit dem Abfall verbundene Risiko von Umweltverunreinigungen verringert werden kann.
Resumen
Se evaluaron los beneficios ambientales de la desulfuración de residuos en el campo de carbón Santa Catarina, Brasil. Los residuos de carbón provenientes de una planta de beneficio fueron separados en tres fracciones de distinta densidad, usando un proceso de dos etapas. La caracterización de estas fracciones indicó que las fracciones de baja (D<2,2 g/cm3) y alta (D>2,7 g/cm3) densidad, eran potencialmente generadoras de energía y ácido sulfúrico, respectivamente. La fracción de densidad intermedia (2,2<D<2,7 g/cm3) representaba 69% del total de la masa estudidada y tenía un contenido relativamente bajo de sulfuros por lo que se postuló que era adecuada para su disposición con mínimo riesgo ambiental. Esta hipótesis fue estudiada usando ensayos cinéticos y estáticos a escala de laboratorio los que indicaron que aunque la fracción remanente aún era generadora neta de acidez, la velocidad y la cantidad de metales, sales y acidez que se lixiviaban era considerablemente menor que la que había antes de la separación. Se concluyó que esta aproximación podría reducir la cantidad de residuo generado y del riesgo de polución asociado.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal mining and beneficiation operations generate a considerable amount of industrial solid waste in terms of production, accumulation volume, occupied area, and acid rock drainage (ARD) generation (Bell et al. 2001; Bian et al. 2010; Komnitsas et al. 2001; Simate and Ndlovu 2014). The ARD can contaminate regional surface and groundwater or the land, with toxicity levels depending on discharge volume, pH, total acidity, concentration of dissolved metals, and buffering capacity of the receiving streams (Akcil and Koldas 2006; Kontopoulos 1998).
Approximately 6.5 million tonnes (t) a year of coal waste were generated in Brazil during the years 2008–2014, almost 80% of which was in the state of Santa Catarina (SIECESC 2014). The grade of coal deposits in Brazil is relatively low, and approximately 65% of the run-of-mine (ROM) coal extracted from underground mines in the carboniferous region of Santa Catarina is discarded in waste dump deposits. In addition, inadequate waste management in the past has left a devastating legacy in this region, with pollution plumes extending more than 6000 ha over several catchment areas. Local studies have indicated considerable contamination of the Araranguá, Tubarão and Urussanga river basins, with reduced pH and high concentrations of metals and sulfate (Gomes et al. 2011; SIECESC 2014).
Currently, Brazilian coal mining operations emphasize an end-of-pipe treatment approach to coal waste and ARD management (Silva and Rubio 2009; Silveira et al. 2009). Chemical ARD treatment techniques such as lime neutralization typically consume large amounts of expensive reagents, generate significant quantities of sludge, and are only effective in reducing ARD risks in the short term. As pointed out by Kontopoulos (1998), many of these shortcomings can be overcome by implementing preventative techniques that minimize the generation and the subsequent dispersion of ARD from waste dump deposits. One such approach entails the pre-disposal removal of ARD-generating sulfide minerals by means of physical separation techniques such as flotation and density separation. Apart from reducing ARD risk, integration of a sulfide removal step into the beneficiation circuit also offers opportunity for additional value recovery (Amaral Filho et al. 2013; Benzaazoua et al. 2008; Hesketh et al. 2010; Hilson 2000; Kazadi Mbamba et al. 2012).
The ability to accurately predict the ARD-generating potential of wastes plays an important and essential role in the development of effective approaches and technologies for mitigating associated impacts and liabilities. Methods for quantifying the ARD potential of sulfide wastes can be classified as either static or kinetic tests. Static testing methods are short term (hours to days) tests that ignore the relative rates of acid-forming and neutralizing reactions, while kinetic testing methods are long term (months to years) tests that allow for the study of the dynamic factors influencing ARD generation (Barbosa et al. 2009; Lapakko and Antonson 2006; Lengke et al. 2010; Sapsford et al. 2009, US EPA 1994). Although a number of kinetic test protocols have been developed and some adaptations and protocol improvements suggested—for example, to simulate conditions of waste rock piles in an arid environment (Lapakko and Trujillo 2015) or to avoid excessive drying of the sample (Bouzahzah et al. 2015)—humidity cell tests follow a standard procedure and are recommended for ARD prediction (ASTM 2007a).
The objective of this study was to evaluate the environmental implications of desulfurizing coal waste by dense medium separation. The separated fractions were characterized and the rate and extent of release of acid and metals from the bulk discards (separation feed) and separated sulphide-lean tailings fraction were determined using laboratory-scale static and humidity cell tests.
Methods
The coal waste was collected in the state of Santa Catarina from the Verdinho Mine preparation plant, which extracts the Barro Branco seam. Specifically, the sample was collected from the discards of the coarse (average diameter between 2 and 50 mm) particle processing (jigging) circuit, which is responsible for 85% of the total waste rock production. About 90% of the total sulfur is removed as part of this fraction during the coal beneficiation process. The material (bulk discards) was subjected to laboratory-scale dense medium (Fe–Si) separation tests to attain three density fractions (D): a low density (D < 2.2 g/cm3) coal-rich fraction; an intermediate density (2.2 < D < 2.7 g/cm3) sulphide-lean fraction; and a high density (D > 2.7 g/cm3) sulphide-rich fraction. Atomized ferrosilicon was mixed with water to obtain suspensions of 2.2 and 2.7 g/cm3. Suspension densities were measured by a densimeter. All three density fractions (products) were weighed and subjected to standard proximate (ASTM 2007b), ultimate (ASTM 2009), and chemical speciation (ASTM 2002) analysis.
Static and kinetic ARD prediction tests were carried out on the bulk (pre-intervention) discards and low-sulfide intermediate density fraction (2.2 < D < 2.7 g/cm3) samples to evaluate and compare their acid-generating potentials before and after coal and pyrite recovery.
Static ABA methods are widely used as a screening procedure. In this study, the static tests were performed by both acid-base accounting (ABA) and modified acid-base accounting (MABA) (Sobek et al. 1978, US EPA 1994) to determine the balance between acid production and consumption (neutralization) by the mineral components of the samples. The particle size of the samples was reduced to less than 0.25 mm. Acidity potential (AP) was determined by total sulfur analysis for ABA and sulphide sulfur for MABA. Total sulfur was measured using a Leco Analyzer and sulphide sulfur by ASTM D 2492 (ASTM 2002).
The bulk discard and intermediate density fraction samples were also subjected to long-term humidity cell tests, in accordance with the ASTM D 5744 procedure, “Standard test method for accelerated weathering of solid materials using a modified humidity cell” (ASTM 2007a). This procedure was carried out for 92 weeks (21 months) and the leachates were analyzed for the following parameters: pH, redox potential (Eh), acidity, and concentrations of sulfate and metals (Al, Mn, Zn, and Fe). Analyses were conducted weekly, following the procedures of the Standard Methods for the Examination of Water and Wastewater (APHA 2005). The procedure was carried out without bacteria inoculation; however, the presence of Acidithiobacillus ferrooxidans in the leachates was monitored, and reached 105 MPN:100 mL after the 15th week. Based on the kinetic test results, the pyrite oxidation rates were calculated in terms of kg of pyrite per ton of coal waste per day (kg t−1 day−1). Weekly average temperatures were obtained from a local weather station to assess the potential influence of temperature on the experimental results.
Results and Discussion
Density Separation
Results of the density separation tests indicate that 17% of the coal discards had a density less than 2.2 g/cm3, 69% had a density between 2.2 and 2.7 g/cm3, and 14% had a density greater than 2.7 g/cm3. The characterization results for the bulk discards and discard fractions after density separation are shown in Table 1.
While all samples had a relatively high total ash content, the low density fraction (<2.2 g/cm3) was enriched in carbonaceous matter (16.4% carbon) and depleted in sulfur (1.8% total sulfur). Previous studies (Li et al. 2006, 2011; Muthuraman et al. 2010) have demonstrated the feasibility of co-combusting high ash coal with carbonaceous wastes to produce energy. Most (≈80%) of the pyritic sulfur in the feed is reported to the high density fraction. This fraction had a pyritic sulfur content of 32.5%, equivalent to 61% pyrite. Pyrite roasting has been used worldwide to produce sulfuric acid (Runkel and Sturm 2009). Although comprising the bulk of the discard material (69% by mass), the pyritic sulfur content in the fraction of intermediate density (2.2 < D < 2.7 g/cm3) was relatively low (0.7%), amounting to less than 10% of the pyritic sulfur in the feed discards.
Static ARD Tests
The results of the subsequent static ARD tests, conducted on the bulk discards (before density separation) and the intermediate density fraction (2.2 < D < 2.7 g/cm3), are summarized in Table 2. Both samples were classified as acid forming by both the traditional (ABA) and modified (MABA) test results. This can be attributed to the negligible neutralizing capacity (NP = 0) of both samples. Nevertheless, a comparison of the static test results indicates that the fraction of intermediate density has a significantly lower acid production (AP) potential and higher net neutralizing potential (NNP) than the bulk discards, due to the reduced pyritic sulfur content.
Humidity Cell Tests
The time-related profiles for the humidity cell tests conducted on the bulk discard and intermediate density fraction samples are summarized in Fig. 1.
Both samples generated slightly acidic leachates from the beginning of the tests, with pH values in the region of 4.5. These pH values continued to decline steadily, stabilizing at approximately 2.0–2.5 after 30 weeks. The bulk discard sample presented slightly lower pH values than the intermediate density fraction sample throughout the experiment. It is also possible to observe that the pH of the leachate of the intermediate fraction increased slowly after a period of 80 weeks. Redox potentials increased from initially low values of around 300 mV to peak values between 550 and 600 mV after 13 and 22 weeks for the bulk discard and intermediate density fraction samples, respectively. Redox potentials >550–600 mV at pH values <3 are generally indicative of rapid oxidation of ferrous iron and sulfide minerals, and are normally associated with microbial activity (Acharya et al. 2001; Hesketh et al. 2010; Kazadi Mbamba et al. 2012). The significant increases in the soluble iron, sulfate, and acidity after 13 and 22 weeks for the bulk discard and intermediate fractions, respectively, are consistent with the onset of rapid and extensive pyrite oxidation at these time intervals. Similar leach profiles were obtained for other metals, with the onset of rapid pyrite oxidation being accompanied by a significant increase in the soluble concentrations of Al, Zn, and Mn. A comparison of the humidity cell leach profiles for the bulk discards and intermediate density fractions indicates that the pyrite oxidation and release of acidity occurs at a faster rate and to a significantly greater extent in the case of the bulk discard sample (Fig. 2).
A comparison of the accumulated release values over the 92 week leach period (Table 3) confirms that, with the exception of Mn, density separation decreased soluble metal concentrations by up to 60%, and decreased the release of sulfate ions by 51% and acidity by 55%.
In the case of the bulk discards, at 92 weeks, 8595 mg/kg of cumulative Fe was leached whilst cumulative releases of Mn, Zn, and Al were 27, 92, and 424 mg/kg, respectively. In contrast, the cumulative amount of Fe leached from the intermediate density fraction, obtained from processing the bulk discard, amounted to 3434 mg/kg after the same time, with cumulative releases of Mn, Zn, and Al of 27, 48, and 196 mg/kg, respectively.
Based on sulfate release, calculated pyrite oxidation rates averaged 64 and 32 g FeS2 t−1 day−1 for the bulk discards and desulfurized tailings fraction, respectively. However, the pyrite oxidation rate of the bulk discards remained approximately constant while it started to decrease for the intermediate density fraction over the second half of the experiment. Sulfur balances furthermore indicated that 98% of the S present in the desulfurized coal waste fraction was consumed in the 92 week period compared to only 31% of the S in the bulk discards. This is indicative of incomplete oxidation of the pyrite and release of acidity, salts, and metals from both samples, but more so from the bulk discards. Longer leach periods are thus likely to produce significantly higher total cumulative release values for the bulk discard sample relative to the desulfurized fraction.
The molar Fe:S ratio in the leach liquors was consistently less than the stoichiometric ratio of 0.5 for pyrite (Fig. 3). This is attributed to the high solubility of sulfate relative to that of ferric iron (Fe3+), which is partially adsorbed onto coal waste particles or precipitated as hydroxides/oxyhydroxides. The precipitation/adsorption of iron is pH dependent and favored at pH values greater than 3.0, which occurred during the first weeks in both humidity cells and, in the case of the desulfurized fraction, after week 80. Similar trends were observed by Sapsford et al. (2009) during humidity cell leaching of a metalliferous (Cu–Zn–Pb) mine waste.
Figure 4 compares the release rate of acidity for the bulk discards sample with temperature fluctuations. With the exception of an initial lag period for the first 20 weeks, the graph shows a trend of increasing acidity release from the bulk discards in the hottest period (summer). This trend can probably be attributed to increased activity of A. ferrooxidans bacteria, whose optimum growth temperature is 30 °C (Johnson and Hallberg 2005; Lundgren et al. 1972), in the warmer months. The lag period may be due to the time required for establishment of the microbial population and acclimatization.
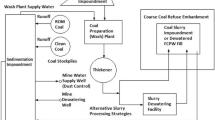
Conceptual Approach
Traditionally, ARD generated by coal waste piles is treated using conventional techniques such as lime neutralization. This represent a long-term cost to the coal producers, who are coming under increased pressure (by public and government spheres) to adopt more preventive approaches in line with cleaner production and sustainable development principles. In the approach proposed here, bulk discards are separated into three density fractions, a coal-rich low density fraction, a sulfide-rich high density fraction, and an intermediate density fraction with a reduced sulfide content. It is further proposed that the coal-rich and sulfide-rich fractions have the potential to be used and integrated back into the local economy through their use as feedstock for energy production, by combustion or gasification, and sulfuric acid production, by roasting, respectively. Apart from improving resource efficiency and providing opportunities for coal mines to enhance their profitability through product diversification, the proposed approach also has a number of environmental benefits. These include a reduction in the net volume of waste requiring disposal and a leachate containing less salts and toxic components. Although leachate treatment is still likely to be required to meet acceptable water quality standards, the reduced acidity and salt loads will lower capital and operating costs, and reduce the amount and hazardous properties of the sludge generated by conventional ARD treatment. Despite the obvious economic and environmental benefits, the successful implementation of such an approach will require effective engagement and collaboration between all potential stakeholders (Fan et al. 2014; Haibin and Zhenling 2010; Hilson 2003; Reddick et al. 2008; McLellan et al. 2009).
Conclusion
The results of this test work show that pre-separation of coal waste discards into different density fractions reduces the amount and hazard of the disposable waste. The waste fraction of intermediate density, which contained less sulfur, represented 69% of the total mass studied. Static and humidity cell tests were used to assess the effect of coal waste desulfurization on ARD risk. They indicate that this separated waste fraction had significantly less ARD-generating potential than the bulk discard sample, resulting in reduced release of metals, salts, and acidity into solution over the long-term. Reprocessing coal waste thus has the potential to both reduce the economic and environmental burdens associated with coal waste, and improve the net material efficiency of the coal sector.
References
Acharya C, Kar RN, Sukla LB (2001) Bacterial removal of sulphur from three different coals. Fuel 80:2207–2216
Akcil A, Koldas S (2006) Acid mine drainage (AMD): causes, treatment and case studies. J Clean Prod 14:1139–1145
Amaral Filho JR, Schneider IAH, Brum IAS, Sampaio CH, Miltzarek G, Schneider C (2013) Caracterização de um depósito de rejeitos para o gerenciamento integrado dos resíduos de mineração na região carbonífera de Santa Catarina, Brasil. Rev Esc Minas 66:347–353
APHA (American Public Health Assoc) (2005) Standard methods for the examination of water and wastewater, 21st edit, American Public Health Assoc, Washington DC
ASTM (2002) ASTM D 2492: standard test method for forms of sulfur in coal. ASTM International, West Conshohocken, PA
ASTM (2007a) ASTM D 5744: standard test method for accelerated weathering of solid materials using a modified humidity cell. ASTM International, West Conshohocken, PA
ASTM (2007b) ASTM D 3172: standard test method for proximate analysis of coal and coke. ASTM International, West Conshohocken, PA
ASTM (American Society for Testing and Material) (2009) ASTM D 3176: standard test method for ultimate analysis of coal and coke. ASTM International, West Conshohocken, PA
Barbosa R, Lapa N, Boavida D, Lopes H, Gulyurtlu I, Mendes B (2009) Co-combustion of coal and sewage sludge: chemical and ecotoxicological properties of ashes. J Hazard Mater 170:902–909
Bell FG, Bullock SET, Hälbich TFJ, Lindsay P (2001) Environmental impacts associated with an abandoned mine in the Witbank Coalfield, South Africa. Int J Coal Geol 45:195–216
Benzaazoua M, Bussière B, Demers I, Aubertin M, Fried É, Blier A (2008) Integrated mine tailings management by combining environmental desulphurization and cemented paste backfill: application to mine Doyon, Quebec, Canada. Miner Eng 21:330–340
Bian Z, Inyang HI, Daniels JL, Otto F, Struthers S (2010) Environmental issues from coal mining and their solutions. Min Sci Technol 20:215–223
Bouzahzah H, Benzaazoua M, Bussière B, Plante B (2015) ASTM normalized humidity cell kinetic test: protocol improvements for optimal sulfide tailings reactivity. Mine Water Environ 34:242–257
Fan G, Zhang D, Wang X (2014) Reduction and utilization of coal mine waste rock in China: a case study in Tiefa coalfield. Resour Conserv Recycl 83:24–33
Gomes CJB, Mendes CAB, Costa JFCL (2011) The environmental impact of coal mining: a case study in Brazil’s Sangão watershed. Mine Water Environ 30:159–168
Haibin L, Zhenling L (2010) Recycling utilization patterns of coal mining waste in China. Resour Conserv Recycl 54:1331–1340
Hesketh AH, Broadhurst JL, Bryan CG, Van Hille RP, Harrison STL (2010) Biokinetic test for the characterisation of AMD generation potential of sulfide mineral wastes. Hydrometallurgy 104:459–464
Hilson G (2000) Barriers to implementing cleaner technologies and cleaner production (CP) practices in the mining industry: a case study of the Americas. Miner Eng 13:699–717
Hilson G (2003) Defining “cleaner production” and “pollution prevention” in the mining context. Miner Eng 16:305–321
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Kazadi Mbamba C, Harrison STL, Franzidis J, Broadhurst JL (2012) Mitigating acid rock drainage risks while recovering low-sulfur coal from ultrafine colliery wastes using froth flotation. Miner Eng 29:13–21
Komnitsas K, Paspaliaris I, Zilberchmidt M, Groudev SN (2001) Environmental impacts at coal waste disposal sites-efficiency of desulfurization technologies. Glob Nest Int J 3:109–116
Kontopoulos A (1998) Acid mine drainage control, In: Castro SH, Vergara F, Sanchez MA (eds) Effluent treatment in the mining industry. University of Concepciòn, Concepciòn, pp 57–118
Lapakko KA, Antonson DA (2006) Pyrite oxidation rates from humidity cell testing of greenstone rock. In: Barnhisel RI (ed), Proc, 7th International Conf on Acid Rock Drainage (ICARD), American Soc of Mining and Reclamation (ASMR), Lexington, USA, pp 1007–1025
Lapakko KA, Trujillo E (2015) Pyrite oxidation rates from laboratory tests on waste rock. Proc, 10th ICARD and IMWA Annual Conf, Santiago, Chile
Lengke MF, Davis A, Bucknam C (2010) Improving management of potentially acid generating waste rock. Mine Water Environ 29:29–44
Li XG, Ma BG, Xu L, Hu ZW, Wang XG (2006) Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim Acta 441:79–83
Li XG, Lv Y, Ma BG, Jian SW, Tan HB (2011) Thermogravimetric investigation on co-combustion characteristics of tobacco residue and high-ash anthracite coal. Bioresour Technol 102:9783–9787
Lundgren DG, Vestal JR, Tabita FR (1972) The microbiology of mine drainage pollution. In: Mitchell R (ed) Water pollution microbiology. Wiley Interscience, New York City, pp 69–88
McLellan BC, Corder GD, Giurco D, Green S (2009) Incorporating sustainable development in the design of mineral processing operations—review and analysis of current approaches. J Clean Prod 17:1414–1425
Muthuraman M, Namioka T, Yoshikawa K (2010) A comparative study on co-combustion performance of municipal solid waste and Indonesian coal with high ash Indian coal: a thermogravimetric analysis. Fuel Process Technol 91:550–558
Reddick JF, Blottnitz H Von, Kothuis B (2008) Cleaner production in the South African coal mining and processing industry: a case study investigation. Int J Coal Prep Util 28:224–236
Runkel M, Sturm P (2009) Pyrite roasting, an alternative to sulphur burning. J South African Inst Min Metall 109:491–496
Sapsford DJ, Bowell RJ, Dey M, Williams KP (2009) Humidity cell tests for the prediction of acid rock drainage. Miner Eng 22:25–36
SIECESC—Sindicato das Indústrias Extratoras de Carvão do Estado de Santa Catarina (2014) http://www.carvaomineral.com.br/conteudo/gm_estatisticas/estatisticas_2014.pdf. Accessed 28 Dec 2015
Silva R, Rubio J (2009) Treatment of acid mine drainage (AMD) from coal mines in south Brazil. Int J Coal Prep Util 29:192–202
Silveira AN, Silva R, Rubio J (2009) Treatment of acid mine drainage (AMD) in south Brazil. Int J Miner Process 93:103–1095
Simate GS, Ndlovu S (2014) Acid mine drainage: challenges and opportunities. J Environ Chem Eng 2:1785–1803
Sobek AA, Schuller WA, Freeman JR (1978) Field and laboratory methods applicable to overburdens and minesoils. EPA-600/2-78-054, Cincinnati
US EPA (Environmental Protection Agency) (1994) Acid mine drainage prediction. EPA 530-R-94-036, Washington DC
Acknowledgements
The authors thank the Brazilian Coal Network, CNPq, FINEP, CCSA, SATC, CTCL, and SIESESC/ABCM for the support provided for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
do Amaral Filho, J.R., Weiler, J., Broadhurst, J.L. et al. The Use of Static and Humidity Cell Tests to Assess the Effectiveness of Coal Waste Desulfurization on Acid Rock Drainage Risk. Mine Water Environ 36, 429–435 (2017). https://doi.org/10.1007/s10230-017-0435-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-017-0435-7