Abstract
Chichankanab is a small, shallow, ⁓8,000-year-old, tropical lake where the adaptive radiation of seven sympatric Cyprinodon species have been described. Since the variation in size, morphology, and diet play a key role in facilitating niche partitioning, this study aims to determine if the sympatric species can be independently identified by their variation in size, morphology, and diet and to understand the role of those variables in the ongoing diversification. To fulfill our aim we gathered, from fish collections, an updated sample of the seven sympatric species and the sister species Cyprinodon artifrons from whom we took their size and photographs to develop geometric morphometric analyses, and collected information on their diet. According to our results, size range allows the separation of Cyprinodon beltrani as the largest species, geometric morphometric allows the segregation C. beltrani, Cyprinodon simus, Cyprinodon suavium, and Cyprinodon labiosus, and a morphotype of three species, Cyprinodon esconditus, Cyprinodon verecundus, and Cyprinodon maya, which can be segregated by body measurements and the size of the species. The diet can help to segregate C. beltrani herbivore, C. maya piscivore/omnivore, C. simus zooplanktivore/detritivore, C. suavium and C. verecundus carnivore/molluscivore, and C. esconditus and C. labiosus carnivores. The ongoing hybridization and the changes in the lake are driving the diversification and decrease of these species. According to our results, there is not one character that differentiated all the species, differentiation was only possible through their specific variation in one character or by a specific combination of characters. It is important to monitor and keep updating information on these evolving species since all seven are listed in the IUCN as vulnerable and near threatened, and according to official Mexican categories as in danger of extinction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
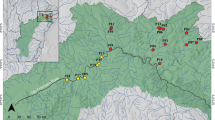
The paleogeographic development of the Yucatan Peninsula shaped its current diversity of ichthyofauna. The eastern coast was especially affected by eustatic changes, which favor the formation of coastal lakes that separate from the sea over time and become inland lakes, as is the case of the Chichankanab (Wilkens 1982). This small (~20 km long), shallow, tropical lake is the largest inland body of water in the Yucatan Peninsula (Covich and Stuiver 1974; Hodell et al. 1995; Ramsar 2004). It is a body of freshwater saturated with calcium sulfate (average salinity of 2.4 g/l; Strecker 2006b), where the invertebrate and fish fauna is low in diversity because the water saturated with calcium sulfate it is intolerable to most species (Humphries and Miller 1981; Strecker et al. 1996; Strecker 2006a). However, paleontological studies indicate that when it was first filled about 8,200 years ago it had high concentrations of sulfates and salinity ⁓13–40 g/l (Humphries and Miller 1981; Strecker 2006b; Perry et al. 2002; Brenner et al. 2003). The climate in the region is warm sub-humid with summer rains, total annual precipitation of 1,100–1,200 mm, and an average annual temperature of 26–28 °C (Ramsar 2004). It is located at the eastern end of the Sierra de Ticul and is presumably the result of the same fault system that produced those mountain ranges (Humphries and Miller 1981; Fig. 1).
Left: Chichankanab lagoon and its position (red dot) in the Yucatan Peninsula, Mexico. Right: methods used to quantify the morphological variation of the Cyprinodon species from Chichankanab lagoon and C. artifrons; top right: geometric morphometric protocol of landmarks (red dots) and semilandmarks (blue dots); bottom right: five body measurements (red lines) and the standard length (yellow line)
Cyprinodon species are mainly known for their isolation in desert hot springs throughout the southwestern United States and Mexico (Miller 1948; Miller et al. 2005). However, in Chichankanab the adaptive radiation of seven monophyletic sympatric Cyprinodon species has been described (Humphries and Miller 1981; Humphries 1984a; Strecker 2002, 2005). In adaptive radiations, two main factors seem to play an important role, selective mating, which leads to reproductive isolation (Strecker and Kodric-Brown 1999; Cruz et al. 2004; Boughman et al. 2005), and morphological differentiation which allows the differential use the resources and habitat choice (Feulner et al. 2007).
It has been indicated that Cyprinodon species from Chichankanab evolve isolated from predators and competitors (Fuselier 2001) and show differences mainly in the head morphology, suggesting trophic divergence and the exploitation of different feeding niches (Stevenson 1992). Some of the morphological characters that help to distinguish the species are, Cyprinodon beltrani is a deep-bodied species, morphologically similar to Cyprinodon artifrons the sister species of the group; Cyprinodon maya is the largest species with the larger mouth opening; Cyprinodon labiosus present protuberant fleshy lips and an elongate body; Cyprinodon simus has a vertical lower jaw, larger eye size, and is the smallest species; Cyprinodon verecundus presents a broad mouth opening, large fins, and large eyes; Cyprinodon esconditus has broad mouth opening and smaller eye; and Cyprinodon suavium presents terminal mouth with thickened lips (Humphries and Miller 1981; Humphries 1984a; Strecker 2002, 2005).
Despite this morphological variation, different studies have indicated an incipient species partitioning. Trophic studies have shown 40% to over 95% niche overlap among species (Horstkotte and Strecker 2005); laboratory crossings reveal that all species are interfertile (Strecker and Kodric-Brown 1999, 2000; Kodric-Brown and Strecker 2001); and genetic analyses have indicated significant genetic differentiation only for C. maya (Strecker 2006a). It has been suggested that this incipient partitioning may result from speciation proceeded by ongoing hybridization (Humphries 1984b; Strecker et al. 1996; Strecker 2006a).
The holotypes and paratypes of the Cyprinodon species from Chichankanab were collected and/or described between the years 1949 and 2000. Changes have occurred in the ecosystem that may affect the species. For example, in 1988 the introduction of an Oreochromis spp. was registered (Stevenson 1992; Strecker 2006b), in 1996 the invasion of Astyanax fasciatus (Strecker 2002), and in 2000, a study registered a nematode infestation with strong prevalence and intensity, which cause an enormous decrease in the relative abundances of the Cyprinodon species from Chichankanab (Strecker 2002).
Because no study had included all seven recognized species, changes have occurred in the lake since species description (Stevenson 1992; Strecker 2002, 2006b), species hybridization (Strecker 2006a), and their morphological variation have been described using traditional linear morphometry and anatomical observations (Humphries and Miller 1981; Humphries 1984a, b; Strecker 2002, 2005). In the present study, using a more updated sample of Cyprinodon species, collected between 2005 and 2015, we analyze the ecomorphological variation and its potential role in the ongoing diversification of the Cyprinodon species from Chichankanab, Quintana Roo, Mexico.
Materials and methods
Specimens. Eight species were considered in this study, the seven endemic Cyprinodon species, C. beltrani, C. esconditus, C. labiosus, C. maya, C. simus, C. suavium, C. verecundus, and the sister species of these group C. artifrons (Strecker 2006a) [Electronic Supplementary Material (ESM) Fig. S1]. Photographs of the specimens used in this study were obtained from three ichthyological collections, Colegio de la Frontera Sur (ECOSUR), Chetumal, Quintana Roo, Centro de Investigaciones y Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV), Mérida, Yucatán, and Universidad Autónoma de Yucatán (UADY), Mérida, Yucatán, and were supplemented with images of the holotypes and paratypes originally published.
From ECOSUR, we use photographs already classified into species by morphological and molecular tools, from CINVESTAV, we use photographs already classified into species by morphological and molecular tools, and specimens classified into species by morphological tools on which we take the photographs, and from UADY, we use specimens already classified into species by morphological tools on which we take the photographs (ESM Table S1). In this study, we seek to only use adult specimens, to ensure this and have a range of adult sizes, we use the sizes indicated in the original descriptions of the species and scientific literature, using only specimens close to those sizes or larger, and since Strecker (2006b) indicated that the size of the species may be changing in some species (getting smaller C. maya) due to changes in the system, to help us ensure the specimens are adults, we review the eye size which changes from juveniles to adults (juveniles present larger eyes in relation to head size). No live specimens were used in this study.
Supporting ecological data. The standard length of the specimens (from the tip of the snout to the posterior end of the caudal peduncle) used in this study was reviewed by a jitter plot including the size of the holotypes and paratypes, followed by an Analysis of variance (ANOVA) to determine differences between species. By a bibliographic revision of quantitative gut content analyses of the seven species of the complex and C. artifrons (Stevenson 1992; Horstkotte and Strecker 2005; Miller et al. 2005), a binary matrix was constructed using nine broad diet categories: detritus, plants, bivalvia, worms, crustaceans, larvae of aquatic insects, gastropods, zooplankton, and teleost. Indicating presence (1), if it has been indicated in the literature that the species feeds on that item, or absence (0) if that item has not been reported as part of its diet. This matrix was analyzed by a non-metric multidimensional scaling (NMDS) using the Jaccard similarity index which did not treat absences as evidence of similarity between groups (Clarke 1993; Kosman and Leonard 2005).
Morphological data. From the eight species, 138 adult specimens were photographed, between six (C. verecundus) and 52 (C. beltrani) specimens per species, according to availability (Table 1; ESM Table S1). These 138 specimens include 16 holotypes and paratypes, two per species, 80 specimens classified by both morphological and molecular tools (mitochondrial DNA), and 40 specimens classified only by morphological tools. Four of the eight species considered include a percentage of their specimens classified only by morphology, C. beltrani 34 specimens classified only by morphological and 16 specimens by morphology and molecular tools; C. maya five specimens classified only by morphological and five by morphology and molecular tools; C. suavium 17 specimens classified only by morphological; and C. verecundus two specimens classified only by morphological and two by morphology and molecular tools.
To quantify shape variation, a configuration of 16 landmarks and 24 semi-landmarks (Fig. 1) was analyzed by geometric morphometric methods (Rohlf 1999; Zelditch et al. 2004). A landmark is a point fixed on an anatomical structure, while semi-landmarks are points between landmarks, which help us include curves, surfaces, or contours of the organism that do not have an anatomical structure on which to fix a landmark. Landmark and semi-landmark configurations were superimposed using a generalized Procrustes analysis (Bookstein 1991; Rohlf 1999) to obtain a matrix of shape coordinates. With the shape matrix of the whole sample, cluster analysis using Euclidean distance was calculated to determine the level of overlap between species, the morphological variation was tested by Principal Component Analysis (PCA) to detect possible groups of species, and lastly, the species were analyzed for statistical differences using permutational multivariate analysis of variance (PERMANOVA), a non-parametric test of differences between two or more groups (Anderson 2021). To get a better appreciation of the relationship of the species, the first four PCs were included in a jitter plot marking the holotypes and paratypes, a simplified PCA using the consensus configuration of the species, and two simplified clusters, one using the holotypes and paratypes morphology, and a second using the consensus configuration of the species were developed. The cophenetic correlation coefficient (CCC) was used as a measure of the goodness of fit of the dendrograms to the original data (Sokal and Rohlf 1962).
Finally, using jitter plots, five body measurements were analyzed, including the holotypes and paratypes of the species: length of the cephalic region, eye, jaw, caudal peduncle height, and maximum body height (Fig. 1). Lengths were standardized using the standard length, by dividing the standard length by each length and expressed as times in the standard length (TSL), i.e., the times the structure fit in the standard length.
Since it has been indicated that no statistical differences were found between some species using mitochondrial DNA, but significant genetic structuring among species was evident (Strecker 2006a), we considered the genetic classification of the species as a hypothesis to prove, running different sets of PCA, all including the holotypes and paratypes. First, using all the data, indicating which specimens were identified by morphology and which by both morphology and molecular tools, a second only including the specimens identified by both morphology and molecular tools, a third including the four species including specimens classified by only morphology and morphology and molecular tools, and a fourth PCA using the consensus morphology of each classification, i.e. only morphology vs. morphology and molecular tools. Finally, to determine if there are statistical differences between classifications, ANOVA was run using the consensus morphology of each classification of the four species including both specimen classifications. Geometric morphometric analyses were performed in the tps software series (Rohlf 2015), and statics analyses were completed in PAST V 4.05 (Hammer et al. 2001).
Results
Supporting ecological data. The jitter plot using the size (Fig. 2a) shows that the largest specimens belong to Cyprinodon maya (max. 6.25 cm), however, this species shows the second-largest mean value (3.51 cm), and the largest mean belongs to C. beltrani (mean 3.70 cm). Two species present the smaller mean sizes C. esconditus and C. verecundus (2.75 cm; 2.83 cm). While C. simus, C. labiosus, and C. suavium present average mean values (3.03 cm, 3.16 cm, and 3.27 cm). Finally, C. artifrons presents the smallest mean size (2.02 cm). Cyprinodon artifrons and C. maya are the only two species where the holotypes and paratypes are not in the range of the species analyzed. ANOVA only shows differences between C. beltrani as the largest species and the rest of the species C. artifrons, C. esconditus, C. labiosus, C. simus, and C. verecundus (Table 2).
a Jitter plot of the variation in size (SL: standard length, cm) of the Cyprinodon species from the Chichankanab lagoon and C. artifrons. Holotypes and paratype, red x female, blue x male; black dot specimens used in this study. b Non-metric multidimensional scaling (NMDS; stress: 0.1486) on the feeding item on the diet of the species. Items in the diet, D detritus, P plants, B bivalvia, C crustaceans, Li larvae of aquatic insects, G gastropods, Z zooplankton, T teleost
On the other hand, the NMDS first axis of the diet (Fig. 2b) segregates in the positive edge the species that consume four or more items including bivalvia and gastropoda as C. maya, C. suavium, C. verecundus, and C. labiosus, from the species that consume maximum three items not including bivalvia and gastropoda, C. beltrani, C. simus, C. artifrons, and C. esconditus. From the first group with the wider diet, C. maya segregates in the positive edge of the second axis due to the presence of teleost and plants in its diet, while teleost is an exclusive item for this species, plants are an item shared with C. beltrani, which segregates from the group with the restrictive diet. As well, from the first group with the wider diet, C. labiosus is located in the negative extreme of the second axis due to the presence of larvae of aquatic insects which share with C. verecundus and C. esconditus. Finally, crustaceans are an item shared by five of the seven species, C. esconditus, C. labiosus, C. maya, C. suavium, and C. verecundus, except C. beltrani and C. simus. All feed on detritus.
Morphologic variation. The cluster analysis of the whole sample shows an intricate pattern with overlap between all the species. The cluster is divided into three main groups, one with C. artifrons and C. beltrani with the holotypes and paratypes of C. artifrons, and one of C. beltrani. A second includes specimens of C. beltrani, C. suavium, C. maya, C. esconditus, and C. simus. The third group includes specimens of all species, and holotypes and paratypes of all species but C. artifrons. In this third group, C. beltrani is the basal species of two subgroups, one integrated by specimens of C. esconditus, C. suavium, C. simus, C. labiosus, C. maya, C. verecundus, and C. artifrons, with the holotypes and paratypes of C. esconditus, C. suavium, and C. simus. The second was integrated by specimens of C. verecundus, C. labiosus, C. maya, and C. artifrons, with the holotypes and paratypes of C. verecundus, C. labiosus, and C. maya (Fig. 3a).
Cluster analyses of the Cyprinodon species from the Chichankanab lagoon and C. artifrons. Cophenetic correlation coefficient (CCC). a Cluster analysis using the complete sample, and the holotypes and paratypes which are indicated with an asterisk. b Cluster using only the holotypes and paratypes. c Cluster using the consensus shapes per species
When using only the holotypes and paratypes (Fig. 3b), C. artifrons first segregate as basal species from the Cyprinodon of Chichankanab. From the seven Cyprinodon, C. beltrani first segregate as basal species of the group, then three groups are formed, C. maya and C. verecundus form a group; C. labiosus and C. simus form another group, however, the holotype and paratype of C. labiosus separate. The third group is integrated by C. esconditus and C. suavium.
Finally, using the consensus shapes (Fig. 3c) C. artifrons separates first. Then two groups are formed, one including C. beltrani, C. simus, and C. suavium, and a second including C. labiosus, C. esconditus, C. verecundus, and C. maya. The patterns conserved in the three clusters were, C. artifrons as the sister species of the group, C. beltrani is basal to the group, and the morphological closeness of C. maya and C. verecundus. PERMANOA (F = 9.49; p = 0.0001) shows differences between most species but C. esconditus, C. verecundus, and C. maya (Table 3).
The PC1 of the analysis using the seven species and C. artifrons (Fig. 4a) sum 32% of the variance and mainly segregates in the positive axis C. artifrons and C. labiosus from C. beltrani in the negative axis. In the PC2, which adds 16% to the variation, C. artifrons in the negative axis, is segregated from C. labiosus in the positive axis. The rest of the species are located in the center of both axes. In the PC1-, C. beltrani segregates due to an angular short head, and the lower jaw insertion is posterior to the superior edge of the mouth, producing an ~120° angle of insertion, a deep and long trunk, and an elongated and thin caudal peduncle. In the PC1+/PC2+ C. labiosus presents the contrary characteristics, a downward deep head profile, the lower jaw is anterior than the superior edge of the mouth, producing an ~70° angle of insertion, the trunk is short and slender, and the caudal peduncle is short and deep, while in the PC1+/PC2-, C. artifrons shows a short and deep body.
a Principal components analyses (PCA) on the variation of the body-shape of Cyprinodon species from the Chichankanab lagoon and C. artifrons using the complete sample. The transformation grids represent the edge of each axis. Color code: red, C. artifrons; blue, C. beltrani; aqua, C. esconditus; yellow, C. labiosus; orange, C. maya; green, C. simus; pink, C. suavium; black, C. verecundus. b PCA on body-shape variation using the consensus shapes by species. The transformation grids represent the consensus morphology of each species against the mean.
The PCA using only the consensus shapes shows a clearer pattern (Fig. 4b). PC1 sums 44% of the variance and PC2 adds 30%. In the PC1-, closer to the extreme of the axis, there is a cluster of three species C. suavium, C. beltrani, and C. simus, still in the PC1- but closer to the axis intersection is C. esconditus. The abdomen of C. beltrani is extended and protruded, producing a more angular ventral profile towards the head and a deeper and longer trunk, the position of the dorsal fin is over the anal fin but little anterior than in C. simus, C. suavium, and C. esconditus. On the other extreme of this variation, C. esconditus presents a less protruded abdomen and more elongated cephalic profile, elongated body, and the dorsal fin is located posterior than in C. beltrani, C. simus and C. suavium. In C. beltrani, the position of the pectoral fin is further down than the mouth and the inferior edge of the interopercle, while in C. esconditus the superior edge of the pectoral fin is a little bit higher than the inferior edge of the mouth and the inferior edge of the pectoral fin is align with the inferior edge of the interopercle, as well, the superior and inferior edges of the mouth in C. esconditus are aligned (~90° angle), while in C. beltrani they are arranged in a ~120° angle. In C. beltrani the posterior edge of the dorsal fin is located anterior to half the anal fin, while in C. esconditus the posterior edge of the dorsal fin is posterior to half the anal fin. In this way, C. simus and C. suavium present intermedium morphological variation between C. beltrani and C. esconditus (Fig. 4b).
In the PC1+, closer to the axis intersection are C. maya and C. verecundus, and then closer to the edge of the axis are C. labiosus and C. artifrons. Cyprinodon maya and C. verecundus are morphologically similar, C. maya presents a slenderer body, the superior edge of the pectoral fin is aligned to the inferior edge of the mouth, and the inferior edge of the pectoral fin aligns with the inferior edge of the interopercle, while C. verecundus presents a little deeper trunk and the position of the pectoral fin is a little lower than the inferior edge of the mouth and the inferior edge of the interopercle. In both species, the superior and inferior edges of the mouth are aligned (~90° angle), and the posterior edge of the dorsal fin is posterior to half the anal fin.
On the extreme of the PC1+ and PC2+ is C. labiosus, which presents opposite characteristics to C. beltrani, both species represent extremes C. beltrani of PC1 and C. labiosus of PC2. Cyprinodon labiosus presents a slender body, the head is elongated, the mouth is larger, the trunk is short, and the caudal peduncle is deep. The superior edge of the pectoral fin is a little up than the inferior edge of the mouth and the inferior edge of the pectoral fin aligns with the inferior edge of the interopercle; the inferior and superior edges of the mouth from a ~70° angle and the position of the dorsal fin is almost completely over the anal fin. Lastly, C. artifrons shows a deep and short trunk and caudal peduncle, the superior and inferior edges of the mouth are aligned, the position of the pectoral fin is further down than the mouth and the inferior edge of the interopercle, and the posterior edge of the dorsal fin is posterior to half the anal fin (Fig. 4b).
The jitter plots of the first four principal components of the geometric morphometric data showed the holotypes and paratypes in the range of the specimens used (Fig. 5). In the plot of the first and second PCs, the holotype and paratype of C. maya looks a little out of phase, however, in PC3 and PC4 it looks among the range. As can be observed in these plots C. artifrons, C. beltrani, and C. labiosus are the species that segregate more from the group.
Jitter plots of the Cyprinodon species from the Chichankanab lagoon and C. artifrons. x Holotypes and paratypes; red x female, blue x male; black dot specimens used in this study. Above: scores of the first four principal components (PC) of the geometric morphometric analyses. Bottom: standardized body measurements as times in the standard length (TSL)
The jitter plots of the body measurements showed the holotypes and paratypes generally in the range of the specimens used (Fig. 5). Only C. maya looks a little out of phase in the jaw measurement. As in the geometric morphometric analyses, C. artifrons, and C. beltrani are the ones that segregate more from the group. The cephalic measurements show C. artifrons and C. labiosus as the species with the largest cephalic region in TSL, C. beltrani shows a wide range, and the rest of the species show similar mean rage. The species with the largest eyes are C. artifrons, C. simus, and C. verecundus, again C. beltrani presents a wide variation, C. maya and C. labiosus show the smallest eyes, and C. esconditus and C. suavium mean sizes. The larger jaws belong to C. labiosus, C. maya, and C. simus, the smallest to C. beltrani, C. esconditus, and C. suavium, while C. verecundus and C. artifrons presented mean sizes. The species with the highest caudal peduncle is C. artifrons, followed by C. verecundus and C. labiosus, the rest of the species present mean highs. Finally, C. artifrons presents some of the higher body specimens, however, it presents a wide variation including covering most of the variation of the seven species, among the Chichankanab species, C. beltrani and C. verecundus presents the highest bodies, the rest of the species present mean values (Fig. 5).
Finally, using the geometric morphometric data, four PCA comparing classification tools, morphological vs. morphological and molecular tools were carried out, showing similar patterns and no statistical differences between classifications. ANOVA results between C. beltrani F = 0.2866; p = 0.5932; C. maya F = 0.9032; p = 0.3434; C. suavium F = 0.9808; p = 0.3766; C. verecundus F = 0.9258; p = 0.3375.
The pattern between the whole sample (Fig. 6a) and just those classified by both morphological and molecular tools (Fig. 6b) is similar since most specimens were classified by both tools. The PCA of the species that include both classifications (Fig. 6c) shows an overlap between classifications for C. maya and C. beltrani. In the PCA using the consensus shapes (Fig. 6d), we can observe that the consensus of C. maya and C. beltrani classified by morphology are closer to those classified by both morphological and molecular tools than to the holotypes and paratypes, while in C. verecundus the consensus classified only by morphological data are closer to the holotype and paratype than to the specimens classified by both morphological and molecular data, finally, the sample of C. suavium includes holotype and paratype, and specimens classified only by morphological tools, however, ANOVA did not show differences between them.
Principal components analyses (PCA) on the variation of the body-shape of Cyprinodon species from the Chichankanab lagoon and C. artifrons, using a the complete sample, b only the specimens identified by both morphological and molecular tools, c only the species that include specimens identified by only morphology and by both morphological and molecular tools, and d mean shape of the specimens identified by only morphology and by both morphological and molecular tools. Cross Holotypes and paratypes, squares specimens identified by morphological tools, dots specimens identified by both morphological and molecular tools. Color code: red, C. artifrons; blue, C. beltrani; aqua, C. esconditus; yellow, C. labiosus; orange, C. maya; green, C. simus; pink, C. suavium; black, C. verecundus
Discussion
An important topic to first discuss is the possibility of wrong determination due to the difficulty in distinguishing the nominal species, especially knowing there is hybridization ongoing (Strecker 2006a) and changes occurring in the ecosystem (Fuselier 2001; Strecker 2006b) since the species were first described. To ensure we used the correct specimens, we included the holotypes and paratypes of the species in the morphological comparison, more than half of our sample was diagnosed by both morphological and molecular tools, and, statistical analyses were done to ensure that the sample only diagnosed by morphological characters was not significantly different than that diagnosed by both morphological and molecular variables.
The cluster plot using the whole sample (Fig. 3) does not show a completely cohesive pattern among species. It is divided into three main groups; the basal cluster segregates Cyprinodon artifrons and C. beltrani with their holotypes and paratypes. Corroborating the differential morphology of these species, since C. artifrons is the sister species of the group and C. beltrani is the basal species of the Cyprinodon from the Chichankanab (Humphries and Miller 1981; Horstkotte and Strecker 2005). Most holotypes and paratypes are in one cluster, although, they are not grouped by species, this group may represent the morphology of the species when collected for the original descriptions (1949–2000) and some specimens with strong similarity to those morphologies. A second cluster, which does not include holotypes and paratypes, shows more consistency among species, which may indicate the state of the morphology in the range of time of the sample (2005–2015).
Based on our analysis of variance, using the geometric morphometric results, most species are distinguishable from each other, but three species, C. esconditus, C. verecundus, and C. maya. A supporting variable that can help in the segregation of C. maya is the size; C. maya showed a mean SL (standard length) of 3.51 cm, while C. esconditus 2.75 cm and C. verecundus 2.83 cm, and the body measurements can help us segregate C. esconditus which presents a shorter cephalic region, eye, and jaw in TSL, than C. verecundus. Thus, with these specimens and these variables, we can achieve species segregation using different variables.
Our sample of C. maya did not reach the large SL originally described for this species (male SL = 6.25 cm; female SL = 5.63 cm; Humphries and Miller 1981). In this regard, Fuselier (2001) indicated that the presence of Oreochromis spp. caused a shift in the use of the habitat of C. maya and C. labiosus pushing them to habitats occupied by C. beltrani. Later, Strecker (2006b) indicates that the large sizes of C. maya documented before the cichlid invasion were not detected in all the subsequent sampling, where no C. maya specimens were observed with a standard length surpassing 5 cm. The mean standard length of the C. maya specimens used in our analyses was 3.51 cm, and they were collected between the years 2006, 2008, 2009, and 2011. Size depends on the intrinsic characteristics of the species and environmental variables. The environmental stressors may cause slower growth and alter the age and size of maturity, forcing the maximization of fitness by changing the life history tactics to mature earlier at smaller sizes (Berrigan and Koella 1994). Thus, the smaller sizes may be a response to the changes and pressures imposed by the environment.
In Fig. 4, we can notice the holotype and paratype of C. maya show larger jaws than the rest of the C. maya sample. Yet, in the other body measurements, the difference between the holotype, paratype, and the rest of the sample is not as strong as in the jaw. To determine if the characteristic large jaw of C. maya is changing or if our findings are the result of the use of hybrid (Strecker 2006a) or younger specimens, actualized sampling and morphological analyses supported by molecular analyses are needed, thus we recommend discretion when analyzing our results of C. maya.
The morphological variation found in this study indicates some lines of morphological diversification between the Cyprinodon species of Chichankanab in the cephalic profile, trunk, caudal peduncle, fins, mouth, and eyes. Trunk morphology is related to stability and thrust (Drucker and Lauder 2001, 2005; Fulton 2007; Standen 2008). In this way, deep trunks favor stability at slow swimming speeds and are likely advantageous in structurally complex habitats (Aguilar-Medrano et al. 2016). While long, slender trunks favor fast swimming, which is advantageous for exploiting midwater and near-surface habitats (Webb and Weihs 1986; Lauder 2000). Thus, we can hypothesize that the use of the ecosystem was an important character that allows the diversification of this group, favoring stability in C. beltrani, C. simus, and C. suavium, and speed in C. labiosus, C. esconditus, C. verecundus, and C. maya.
Cyprinodon beltrani has the deepest trunk, a mouth with an insertion angle of ~120°, the largest size (µ SL 3.70 cm), as well as the largest intestine, about six to eight times its SL, is more abundant on soft substrates (sand and gravel) around the lake margin, in loose aggregations, feeding on detritus and plant matter from the bottom (Humphries and Miller 1981; Horstkotte and Strecker 2005; Horstkotte and Plath 2008). Because algae and detritus are poor in nutrients (Wilson et al. 2003), herbivores and detritivores have long digestive tracts to extract adequate nutrition from these resources (Elliott and Bellwood 2003). Therefore, the deep trunk may be related to a long intestine in C. beltrani.
Cyprinodon suavium also has a deep trunk, which is not only related to a protruding abdomen but also a small hump, it is one of the largest species of the group (µ SL 3.27 cm), it has a relatively long caudal peduncle, it feeds of benthic mollusks, such as snails, ostracods, and bivalves (Strecker 2005). A hump increases drag (Portz and Tyus 2004), in other species it has been related to sexual selection (Bonduriansky and Rowe 2005), it has been suggested to be an advantage to defend against predation by increasing body depth (Portz and Tyus 2004), and it can also help to support stronger muscles to exert powerful compressive forces to feed on hard-shelled animals.
Cyprinodon simus has a short intestine (Horstkotte and Strecker 2005) and according to Humphries and Miller (1981), it feeds on zooplankton, however, it has a deep trunk, and according to a more updated analysis (Horstkotte and Strecker 2005), detritus represents 99% relative importance in its diet. It also has a vertical lower jaw and therefore the opening of the mouth is superior. According to Elias-Gutierrez et al. (2001) and Horstkotte and Strecker (2005), the lake now has almost no zooplankton, which is indicated as the reason for the decline in the C. simus population. In this way, the availability of food is a strong force in the divergence of this group, where changes in the availability of a resource produce disadvantageous scenarios for the species specialized in its consumption, producing a decrease in the population, a new adaptation process to other resources, or the extinction of the species.
Although C. beltrani, C. simus, and C. suavium have similar morphologies, they differ in some characteristics that allow them to access a different diet. Furthermore, these three species differ in size, C. beltrani, and C. suavium are two of the three largest species of the group (with C. maya), while C. simus is the third smallest species. Size influences the competitive ability, resource utilization, and ecological niche of a species (Malerba et al. 2017), also many life-history traits covary with body size (Peters 1983; Reznick et al. 1990), for example, smaller species tend to grow faster (Savage et al. 2004) and have a shorter lifespan (Marba et al. 2007; Malerba et al. 2017). In this way, although the morphology is similar, the size allows niche differentiation in these species.
On the other hand, C. labiosus, C. esconditus, C. verecundus, and C. maya are carnivorous species (Horstkotte and Strecker 2005), and have elongated bodies and vary in the depth of the caudal peduncle. An elongated trunk reduces drag when swimming favoring speed, but both the trunk and the caudal peduncle work together to increase and maintain speed during locomotion (Fulton 2007; Aguilar-Medrano et al. 2016). Cyprinodon esconditus has a slenderer and more elongated peduncle than the rest species of the clade, which favors swimming speed and efficiency (Videler 1993; Fulton 2007; Aguilar-Medrano et al. 2013), while C. labiosus and C. verecundus have a deeper caudal peduncle, which facilitates strong initial swimming bursts (Fulton 2007). In this way, C. esconditus could be a more efficient long-distance swimmer, while C. labiosus and C. verecundus can use this strong burst to hunt, push, or escape from predators.
Following this idea, C. labiosus is a carnivorous species that presents fleshy lips, with a larger lower lip. The long snout of C. labiosus has been indicated to be suitable for searching for invertebrates among pebbles (Horstkotte and Plath 2008), this being the case, a fleshy, strong, and long lower lip would be useful, as well as a deep caudal peduncle that facilitates a strong swimming burst, which can also be useful for pushing pebbles.
Cyprinodon maya shows a mean morphological variation between C. esconditus, C. labiosus, and C. verecundus, that is, an elongated body, with a deep caudal peduncle; it also differs from these species by presenting one of the largest sizes of the group (µ SL 3.51 cm). In addition, C. maya has the broadest diet and is the only species in the group that feeds on fish. In this way, we can consider C. maya as a predator, with highly energetic consumption, which is related to both diet and size (Tang et al. 2017).
Different studies have found that the trunk, the dorsal, pelvic, and anal fins make a module associated with stability and thrust, and specifically the pectoral fin with maneuverability (Drucker and Lauder 2001, 2005; Fulton 2007; Standen 2008). The dorsal and anal fins increase stability by increasing the lateral surface of the body during swimming, but at the expense of increasing drag (Grillner 2011), therefore a small misalignment between them can decrease resistance, especially useful for those species with an already deep trunk. These patterns were corroborated by this study, indicating a relationship between the dorsal, anal, and pectoral fins in this group. In C. beltrani, C. simus, and C. suavium the dorsal and anal fins are less aligned, that is, the dorsal fin covers less of the anal fin, and the pectoral fin is in a lower position to the mouth, the opposite is observed in C. labiosus, C. esconditus, C. verecundus, and C. maya, the dorsal and anal fins are more aligned, and the pectoral fin is more aligned with the mouth.
According to the characters analyzed here, C. beltrani is the most divergent species of the group, being specifically segregated by size, morphology, and diet, followed by C. simus segregated by morphology and diet, C. suavium by size and morphology, C. maya by size and diet, and C. labiosus by morphology. Cyprinodon esconditus and C. verecundus are similar in size and morphology, both are carnivores, but C. verecundus shows a tendency to molluscivory, a diet shared with C. suavium, which presents different size and morphology, on the other hand, the diet of C. esconditus is similar to that of C. labiosus, which has a different morphology.
In 2004, the Chichankanab Lake was designated a RAMSAR site (No. 1364) justifying its designation by the presence of the endemic Cyprinodon group, the presence of early Holocene sediments with remains of Ammonia beccarii, and the presence of Rhizophora mangle (https://rsis.ramsar.org/es/ris/1364). Chichankanab Lake is located on communal land, so to undertake conservation actions in the area it is of utmost importance to collaborate with local representatives of the communal system so that the conservation of the area is made by users and connoisseurs of the resource, who have developed an economic and social identity related to the lake.
References
Aguilar-Medrano R, Frederich B, Balart EF, de Luna E (2013) Diversification of the pectoral fin shape in damselfishes (Perciformes, Pomacentridae) of the Eastern Pacific. Zoomorphology 132:197–213
Aguilar-Medrano R, Frederich B, Barber PH (2016) Modular diversification of the locomotor system in damselfishes (Pomacentridae). J Morphol 277:603–614
Anderson, M. J. (2021). A new method for non-parametric multivariate analysis of variance. Austral Ecol, 26:32–46.
Berrigan D, Koella JC (1994) The evolution of reaction norms: simple-models for age and size at maturity. J Evol Biol 7:549–566
Bonduriansky R, Rowe L (2005) Sexual selection, genetic architecture, and the condition dependence of body shape in the sexually dimorphic fly Prochyliza xanthostoma (Piophilidae). Evolution 59:138–151
Bookstein FL (1991) Morphometric tools for landmark data geometry and biology. Cambridge University Press, Cambridge
Boughman JW, Rundle HD, Schluter D (2005) Parallel evolution of sexual isolation in sticklebacks. Evolution 59:361–373
Brenner M, Hodell DA, Curtis JH, Rosenmeier MF, Anselmetti FS, Arizteguiet D (2003) Paleolimnological approaches for inferring past climate change in the Maya Region: recent advances and methodological limitations. In: Gómez-Pompa A, Allen MF, Fedick SL, Jiménez-Osorio JJ (eds) The Lowland Maya area. Food Products Press, Binghamton, pp 45–75
Clarke KR (1993) Non-parametric multivariate analysis of changes in assemblage structure. Austral Ecol 18:117–143
Covich A, Stuiver M (1974) Changes in oxygen 18 as a measure of long-term fluctuations in tropical lake levels and molluscan populations. Limnol Oceanogr 19:682–691
Cruz R, Carballo P, Conde-Padín P, Rolán-Alvarez E (2004) Testing alternative models for sexual isolation in natural populations of Littorina saxatilis: indirect support for by-product ecological speciation? J Evol Biol 17:288–293
Drucker EG, Lauder GV (2001) Locomotor function of the dorsal fin in teleost fishes: experimental analysis of wake forces in sunfish. J Exp Biol 204:2943–2958
Drucker EG, Lauder GV (2005) Locomotor function of the dorsal fin in rainbow trout: kinematic patterns and hydrodynamic forces. J Exp Biol 208:4479–4494
Elias-Gutierrez M, Smirnow NN, Suarez-Morales E, Dimas-Flores N (2001) New and little known cladocerans (Crustacea: Anomopoda) from southeastern Mexico. Hydrobiologia 442:41–54
Elliott JP, Bellwood DR (2003) Alimentary tract morphology and diet in three coral reef fish families. J Fish Biol 63:1598–1609
Feulner PGD, Kirschbaum F, Mamonekene V, Ketmaier V, Tiedemann R (2007) Adaptive radiation in African weakly electric fish (Teleostei: Mormyridae: Campylomormyrus): a combined molecular and morphological approach. J Evol Biol 20:403–414
Fulton CJ (2007) Swimming speed performance in coral reef fishes: field validations reveal distinct functional groups. Coral Reefs 26:217–228
Fuselier L (2001) Impacts of Oreochromis mossambicus (Perciformes: Cichlidae) upon habitat segregation among cyprinodontids (Cyprinodontiformes) of a species flock in Mexico. Rev Biol Trop 49:647–656
Grillner S (2011) Brain and nervous system, motor control systems of fish. In: Farrell AP (ed) Encyclopedia of fish physiology. Academic Press, Cambridge, pp 56–65
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Palentological Statistics software package for education and data analysis. Palaeontol Electronica 4:1–9
Hodell DA, Curtis JH, Brenner M (1995) Possible role of climate in the collapse of classic maya civilization. Nature 375:391–394
Horstkotte J, Plath M (2008) Divergent evolution of feeding substrate preferences in a phylogenetically young species flock of pupfish (Cyprinodon spp.). Sci Nat 95:1175–1180
Horstkotte J, Strecker U (2005) Trophic differentiation in the phylogenetically young Cyprinodon species flock (Cyprinodontidae, Teleostei) from Laguna Chichancanab (Mexico). Biol J Linn Soc 85:125–134
Humphries JM (1984a) Cyprinodon verecundus, n. sp., a fifth species of pupfish from Laguna Chichancanab. Copeia 1984:58–68
Humphries JM (1984b) Genetics of speciation in pupfishes from Laguna Chichancanab, Mexico. In: Echelle AA, Kornfield I (eds) Evolution of fish species flocks. University of Maine Press, Orono, pp 129–139
Humphries JM, Miller RR (1981) A remarkable species flock of pupfishes, genus Cyprinodon, from Yucatán, México. Copeia 1981:52–64
Kodric-Brown A, Strecker U (2001) Responses of Cyprinodon maya and C. labiosus females to visual and olfactory cues of conspecific and heterospecific males. Biol J Linn Soc 74:541–548
Kosman E, Leonard KJ (2005) Similarity coefficients for molecular markers in studies of genetic relationships between individuals for haploid, diploid, and polyploid species. Mol Ecol 14:415‒424
Lauder GV (2000) Function of the caudal fin during locomotion in fishes: kinematics, flow visualization, and evolutionary patterns. Am Zool 40:101–122
Malerba ME, White CR, Marshall DJ (2017) Eco-energetic consequences of evolutionary shifts in body size. Ecol Lett 21:54–62
Marba N, Duarte CM, Agusti S (2007) Allometric scaling of plant life history. Proc Natl Acad Sci U S A 104:15777–15780
Miller RR (1948) The Cyprinodont fishes of the Death Valley system of Eastern California and Southwestern Nevada. Miscellaneous Publications. Museum of Zoology, University of Michigan, no. 68. University of Michigan Press, Ann Arbor
Miller RR, Minckley WL, Norris SM (2005) Freshwater fishes of Mexico. University of Chicago Press, Chicago
Perry E, Velazquez-Oliman G, Marin L (2002) The hydrogeochemistry of the Karst Aquifer System of the Northern Yucatan Peninsula, Mexico. Int Geol Rev 44:191–221
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Portz DE, Tyus HM (2004) Fish humps in two Colorado River fishes: a morphological response to cyprinid predation? Environ Biol Fishes 71:233–245
Ramsar (2004) Ficha Informativa de los Humedales de Ramsar. Laguna de Chichankanab. https://rsis.ramsar.org/RISapp/files/RISrep/MX1364RIS.pdf . Accessed 07 05 2024
Reznick DA, Bryga H, Endler JA (1990) Experimentally induced life-history evolution in a natural population. Nature 346:357–359
Rohlf FJ (1999) Shape statistics: Procrustes superimpositions and tangent spaces. Methods Ecol Evol 16:197–223
Rohlf FJ (2015) The tps series of software. Hystrix 26:9–12
Savage VM, Gilloly JF, Brown JH, Charnov EL (2004) Effects of body size and temperature on population growth. Am Nat 163:429–441
Sokal RR, Rohlf FJ (1962) The comparison of dendrograms by objective methods. Taxon 11:33–40
Standen EM (2008) Pelvic fin locomotor function in fishes: three-dimensional kinematics in rainbow trout (Oncorhynchus mykiss). J Exp Biol 211:2931–2942
Stevenson MM (1992) Food habits within the Laguna Chichancanab Cyprinodon (Pisces: Cyprinodontidae) species flock. Southwest Nat 37:337–343
Strecker U (2002) Cyprinodon esconditus, a new pupfish from Laguna Chichancanab, Yucatan, Mexico (Cyprinodontidae). Cybium 26:301–307
Strecker U (2005) Description of a new species from Laguna Chichancanab, Yucatan, Mexico: Cyprinodon suavium (Pisces: Cyprinodontidae). Hydrobiologia 541:107–115
Strecker U (2006a) Genetic differentiation and reproductive isolation in a Cyprinodon fish species flock from Laguna Chichancanab, Mexico. Mol Phylogenet Evol 39:865–872
Strecker U (2006b) The impact of invasive fish on an endemic Cyprinodon species flock (Teleostei) from Laguna Chichancanab, Yucatan, Mexico. Ecol Freshw Fish 15:408–418
Strecker U, Kodric-Brown A (1999) Mate recognition systems in a species flock of Mexican pupfish. J Evol Biol 12:927–935
Strecker U, Kodric-Brown A (2000) Mating preferences in a species flock of Mexican pupfishes (Cyprinodon, Teleostei). Biol J Linn Soc 71:677–687
Strecker U, Meyer CG, Sturmbauer C, Wilkens H (1996) Genetic divergence and speciation in an extremely young species flock in Mexico formed by the genus Cyprinodon (Cyprinodontidae, Teleostei). Mol Phylogenet Evol 6:143–149
Tang L, Jacquin L, Lek S, Liu H, Li Z, Liu J, Zhang T (2017) Differences in anti-predator behavior and survival rate between hatchery-reared and wild grass carp (Ctenopharyngodon idellus). Int J Limnol 53:361–367
Videler JJ (1993) Fish swimming. Chapman and Hall/Springer, London
Webb PW, Weihs D (1986) Functional locomotor morphology of early life history stages of fishes. Am Fish Soc Symp 115:115–127
Wilkens H (1982) Regressive evolution and phylogenetic age: the history of colonization of freshwaters of Yucatan by fish and crustacea. Bull Texas Mem Mus 28:237–243
Wilson SK, Bellwood DR, Choat JH, Furnas ML (2003) Detritus in the epilithic algalmatrix and its use by coral reef fishes. Oceanogr Mar Biol 41:279–309
Zelditch ML, Swiderski DL, Sheets HD, Fink WL (2004) Geometric morphometrics for biologists: a primer. Elsevier Academic Press, London
Acknowledgments
We thank Mirella Hernández de Santillana and Mirna Erendira Estrella Martinez for their help in the museum collections. To Rodolfo Pérez Rodríguez for his very helpful comments. Finally, we would like to thank the anonymous reviewers and editor for their comments and suggestions that highly improved the document. Both authors are fellow recipients of the National System of Researchers (CONHACYT-SNII).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The handling of the specimens was carried out according to the guidelines of each fish collection and the University or Research Center that contains them. All specimens used were preserved specimens as no live specimens were used in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Aguilar-Medrano, R., Vega-Cendejas, M.E. Ecomorphological diversification of the Cyprinodon species complex from Lake Chichankanab, Yucatan, Mexico. Ichthyol Res (2024). https://doi.org/10.1007/s10228-024-00980-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10228-024-00980-2