Abstract
This study was carried out to determine the relationships between various morphological parameters, body condition data, and digestive and stress enzyme activities due to silvering in European eel (Anguilla anguilla L.) collected from four locations in Turkey. The yellow and silver eels were caught from Asi River, Köyceğiz, Güllük Bay, and Bafa Lake in November–December 2020. Total body length, eye diameter, and pectoral fin length were measured as morphological parameters. In addition, body fat percentage, gonado-somatic index, and Fulton’s condition factor were calculated, and also weight was assayed as body condition data. The variations of α-amylase, lipase, trypsin, and pepsin enzymes, and superoxide dismutase, catalase, and glutathione peroxidase enzyme activities were measured. The results supported the differences of yellow and silver stage samples in terms of both morphological and physiological parameters and also showed that the digestive and stress enzyme activities of the silver and yellow stage samples were significantly different (p ≤ 0.05). We think this study will make a physiological contribution in terms of digestive and stress enzyme levels to better understand and elaborate the silvering stage of eels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European eel (Anguilla anguilla L.) experiences two periods of metamorphosis during its life cycle. The first one is the change from the larval stage (Leptocephalus larvae) to the glass eel (Balm et al. 2007). The second (partial) metamorphosis occurs in inland waters during the growth and maturation phase. Eels transform from the yellow eel stage to the silver eel stage, and this process is called "silvering" (Balm et al. 2007).
The variations between the yellow and silver stages are physiologically significant (Durif et al. 2005). To discriminate these two stages, various criteria such as colors of skin and fins, eye size, and pectoral fin size are generally used. The color factor appears to be the most commonly used criterion to identify the stage of the eel. Research has shown that eye size is a common descriptor that also increases following sexual maturity (D’Ancona 1927, 1929; Bertin 1956; Carliske and Denton 1959; Böetius et al. 1962; Böetius and Böetius 1967, 1980; Stramke 1972; Pankhurst 1982). Pectoral fin darkening and elongation are also frequently used indicators of eels' transition to the silver stage. Similarly, significant differences in intestinal size were detected during the silvering phase in the study of Durif et al. (2005). It was observed that the cessation of nutrition started with the downstream movement of the individuals and the regression in the intestines increased as the migration process progressed.
Although some morphological changes have been revealed, the mechanisms that trigger these changes and the physiological stages that characterize this metamorphosis are still largely debated (Balm et al. 2007). Since silvering occurs in eels, individuals at this stage are more likely to need more energy sources. It is known that these individuals do not feed (Durif et al. 2005; Aarestrup et al. 2009) during migration, and the fat ratio in the muscle tissues increases during the silvering process (Svedäng and Wickstrom 1997). The cessation in migrant individuals suggests that they do not need digestive enzymes, and it is expected that digestive enzyme levels are low in silver individuals. Although there have experimental studies in which eels were starved (Larsson and Lewander 1973; Dave et al. 1975; Olivereau and Olivereau 1997; Caruso et al. 2010), there has been no detailed study on this subject in natural environment samples.
In addition to digestive enzymes, eels that are in the silvering process are expected to be under more stress. Therefore, it is highly likely that antioxidant responses are also high. Studies on stress enzyme levels in eels have also been associated with various environmental pollutants, and various antioxidant responses, stress enzyme levels, and oxidative stress parameters were investigated in these studies (Regoli et al. 2003; Santos et al. 2004; Ahmad et al. 2004, 2006). There is limited study on the stress enzyme levels during the silvering process (Mortelette et al. 2010). It has been reported that an experimental study on antioxidant activities of yellow and silver European eels may be useful to observe muscular effort during migration (Amérand et al. 2017). However, in natural conditions, a stage-dependent evaluation presented within the scope of this study has not been encountered among previous studies.
The determination of the silvering process and the ratio of silver stage eel to escape in the population are very important for the continuity of the species generations, population dynamics, and stock estimation. There is no partitioning of landings between the yellow and silver stage European eel in many exploited local stocks in nearly all distributional areas (Moriarty and Dekker 1997), and the silver eel estimation is recorded as biased and overestimated in some areas (Dekker 2000). In general, yellow and silver eel mixed data is inappropriate for stock assessment (Han et al. 2001). The gap particularly in the time series of silver stage eel throughout the distribution area has been discussed in ICES (2021). Durif et al. (2005) contributed to filling this gap in the literature by making a more robust estimation of silver stages by using morphological and physiological data. However, European eel has a wide distributional area and high plasticity with spatial heterogeneity in the silvering process in terms of silver at length and silver at age (Frost 1945; Hansen and Eversole 1984; Helfman et al. 1984; Vøllestad 1992; Svedäng et al. 1996; Holmgren et al. 1997; Oliveira 1999; Durif et al. 2005). Moreover, the environmental conditions affect the silvering process and this process may stop and revert to the yellow stage in unfavorable conditions (Svedäng and Wickstrom 1997; Durif et al. 2003; van den Thillart et al. 2004). The flexibility of eels on the physiological and behavioral patterns makes this species more open to detailed studies on the silvering process, particularly in exploited populations exposed to a wide range of local environmental gradients. For this reason, it is crucial to determine a decision tree to a robust description of silver at an example of the extreme geography of the distribution area of eel, such as Turkey, in terms of filling the gap in the literature. The digestive and stress enzyme levels with prominent morphometric characteristics of eels mentioned above may be a good tool used in the discrimination of silver stage eels. The silvering stage classification defined by Durif et al. (2005) was validated by some physiological and morphometric characteristics of eels. However, the geography of eels also affects these characteristics and the determined stages may differ according to the regions. We emphasize the possibility that the morphological distinction will not be sufficient to give the true silver percentage and therefore more accurate predictions can be made by using digestive enzymes and stress enzymes.
The European eel is declining in trend and thus was listed as Critically Endangered in IUCN in 2008 (Jacoby and Gollock 2014), and the data on local population structures have a critical role in the overall population trend evaluation of this panmictic species (ICES 2020). However, there are a few studies on the eel biology to evaluate local population structures in Turkey freshwater habitats (Küçük et al. 2005; Yalçın Özdilek and Solak 2007; Rad et al. 2013) and the gap in the biological and fisheries data of eel limits estimation of local stocks which has been already disregarded in a whole continental stock.
In this study, it was aimed to determine the yellow/silver ratio, which is frequently needed in stock estimation and management of European eels found in the waters of our own country, by observation-based, morphological parameters and especially digestive enzymes-based methods. With this in mind, we separated the samples captured from different parts of the country as yellow and silver by observation and supported them with morphological parameters. Thus, by evaluating the possible physiological differences between yellow and silver eels from an enzymatic perspective, facilitating the staging of eels will also be beneficial for population dynamics and stock estimation studies considering that individuals who complete the silvering period in eels will leave the population.
Materials and methods
Sampling sites and animal collection. In this study, female eel samples (n = 60) were evaluated from four locations (1. Asi River, 2. Köyceğiz, 3. Güllük, and 4. Bafa Lake) at Turkey's southern and western coasts (Table 1). The eels are exploited commercially and recreationally from Asi River (since 2020), Güllük and Bafa, but in Köyceğiz the eels have been caught commercially since 1974. Eel samples were caught by fishermen via fyke net from November to December 2020. The samples were brought to the Aquatic Ecology Laboratory of the Biology Department of Çanakkale Onsekiz Mart University under suitable cold chain conditions.
Morphological measurements and dissection. The stages of specimens were defined taking into consideration the morphological characters, such as pectoral fin color and size, eye size, and the thickness of skin, and marked as yellow or silver. Eel samples were grouped according to study regions, and morphological measurements such as total length (TL), horizontal (HED) and vertical eye diameter (VED), and pectoral fin length (PFL) in mm were made with the help of calipers. The eye index (EI) was calculated according to the formula given in Pankhurst (1982). The pectoral fin index (PFI) (PFI = 100PFL × TL−1) was calculated.
After the morphological analyses, like body condition data, the body masses of the samples (W) were weighed in g (Table 2). Fat percentage (FP) in the samples was calculated by the method used in Folch et al. (1957), as revised by Post and Parkinson (2001) and Arrington et al. (2006). Similarly, the Fulton condition factor was calculated using the equation K = 100 × W/TL−5 (Table 2).
Gonad, liver, muscle, and total digestive system were removed from the samples dissected following the ethical rules. The gonads were examined macroscopically and microscopically to determine the sex and also the gonado-somatic index was calculated. A piece of a tissue sample taken from the liver was stored at −45°C until analysis was performed to determine antioxidant enzyme activities. The small intestine part of the digestive tract was separated and kept at −45°C until digestive enzyme analysis was performed. The muscle tissues taken to determine the fat percentage were dried in the oven at 60°C for 48 h.
Digestive enzyme analysis. Stomach and small intestine tissue homogenates of eel specimens were prepared, and α-amylase, lipase, pepsin, and trypsin enzyme activities were determined. Bradford’s (1976) method was used to determine the amount of protein in each homogenate in the measurement of digestive enzyme activities, just as in the measurement of antioxidant enzyme activities.
α-Amylase enzyme activity: To monitor the α-amylase enzyme activity, the analysis method used in the study by Métais and Bieth (1968) was taken as a basis, and measurements were completed at 540 nm using soluble starch as substrate.
Lipase enzyme activity: The analysis method suggested by Versaw et al. (1989) was used to measure the lipase enzyme activity. During the measurements at 490 nm, α-naphthyl caprylate was preferred as the substrate.
Pepsin enzyme activity: For the determination of pepsin enzyme activity, the analysis method reported by Worthington (1982) as revised by Zambonino Infante and Cahu (1994) was used and measurements were taken at 280 nm. Hemoglobin was used as the substrate.
Trypsin enzyme activity: Determination of trypsin enzyme activity was done according to the method of Tseng et al. (1982) and measured at 253 nm using Na-benzoyl-DL-arginine-p-nitroanilide (BAPNA) as substrate.
Stress enzyme analysis. The activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) enzymes were measured spectrophotometrically in the liver tissue homogenates of eels caught within the scope of this study. The amount of protein in each tissue homogenate was decided by the method of Bradford (1976).
SOD enzyme activity: SOD enzyme activity was measured according to the method of Flohé and Ötting (1984), based on the principle that superoxide radical produced by the xanthine oxidase/hypoxanthine system causes color formation by reducing nitro blue tetrazolium (NBT).
CAT enzyme activity: CAT activity was measured according to the Clairborne and Greenwald’s (1985) method, which was set up on the decomposition of hydrogen peroxide. Samples were measured every 15 s at 240 nm for a total of 90 s.
GPx enzyme activity: GPx activity was determined according to the Wendel’s (1980) method, which adopts the principle that GPx enzyme activity causes glutathione reductase degradation and absorbance decreases.
Data analysis. Descriptive statistics of morphological measurements for TL, EI, and PFL, body condition parameters for W, FP, and K, stress enzyme levels (SOD, CAT, GPx), and digestive enzyme levels (α-amylase, lipase, pepsin, and trypsin) of eels were made according to stations and stages.
To conduct our research, we employed the multiple logistic regression equation for each of the three subsets as the baseline model to discriminate the observed silver stage (y = silver) from the observed yellow stages (y = yellow).
The multiple logistic regression (MLR) model is a type of generalized linear model (GLM) that describes the mathematical relationship between an outcome and one or more predictors as both binary classification algorithm and a prediction model (Shanubhogue and Gore 1987; Pearce and Ferrier 2000).
The multiple logistic regression model can be expressed mathematically as in Equation (1):
where \(p\) is the probability of silver or yellow of stage: \(P\left(Y=silver\right)=p\), \({\beta }_{i}\) are model parameters, and k is the number of predictor variables \({x}_{i}\).
The multiple logistic regression model Eq. (1) enables us to determine the probabilities for each class of the outcome variable (Equation 2), and also the odds ratio (Equation 3), which is the ratio of the probability of silver to the probability of yellow, once the parameters have been estimated. Equation (1) is used to express the probability as a function of the predictors:
The odds ratio is:
Statistical significance was defined at p < 0.05.
Multiple logistic regression models generated by subsets are as follows:
Stepwise regression was used to select the variables to be included in the final model of the enzymes.
The classification success of the models was compared using tenfold cross-validation to evaluate the classification success according to the stage of the three logistic models. When deciding which model was better, the Akaike information criteria (AIC) (Akaike 1974) were utilized (Burnham et al. 2011). According to the others, the model with the small AIC is the better model. Accuracy, sensitivity, specificity, and kappa from the metrics goodness of prediction were used to evaluate the prediction success in the comparison. Accuracy is the rate of correct classification, and a rate of 70% or more is a suitable value for classification success (Berrar 2019). Sensitivity, or positive observations that were predicted correctly, reflects a model’s ability to detect eels’ silver stage status. Specificity or proportion of negative observations correctly predicted reflects the ability of the model to predict the yellow stage status of eels. The kappa metrics show the accuracy of classified stages (Freeman and Moisen 2008). Kappa > 0.40 is a good prediction.
The difference between the ratios obtained from the models was analyzed using the proportion test. Decision tree analysis, one of the most popular machine learning techniques, was carried out using the variables in the models established for morphologic parameters in terms of being a more practical method during field studies. All analyses were performed in the statistical program R version 4.0.4 (R core team 2021).
Results
Morphological and body condition measurements. Morphological measurements of eels caught from four different stations and their descriptive statistics are presented in Table 2. When morphological measurements were evaluated, it was found that all measurements were generally higher in samples at the silver stage. It was determined that the samples with the highest total lengths were in the silver stage (585.7–757.6 mm). The highest values in terms of eye index were found in silver samples (7.3–11.1), and the highest pectoral fin index values were determined in silver samples (4.3–5.3).
W, FP, and K values and GSI were measured in body condition data. In terms of weight, the highest values were found in silver samples (395.9–1154.2 g) and the highest fat percentage was again in silver samples (37.4–44.2). In terms of Fulton’s condition factor, the highest values were observed in silver samples (0.19–0.3). GSI values were found to be higher in silver samples, just as in other measured parameters.
Digestive enzyme analysis. While demonstrating whether there was any difference in digestive enzyme values at different stages of eels, we assumed that there was no difference between different stations (Fig. 1). Trypsin enzyme levels varied between 1.68 and 32.96 U mg−1 in silver stage samples, while it was found between 3.36 and 41.45 U mg−1 in yellow stage samples. This difference seen in different stage samples in terms of trypsin activity was statistically notable (p < 0.05). Similarly, pepsin activity values measured in silver stage samples varied between 3.97 and 38.4 U mg−1, and 7.37 and 107.63 U mg−1 in yellow ones. The difference between the stages was significant (p < 0.01). Amylase activity was between 0.06 and 1.6 U mg−1 in silver stage samples, and 0.14 and 6.52 U mg−1 in yellow stage samples. This difference seen in different stage samples in terms of amylase activity was statistically significant (p < 0.01). The lipase enzyme activity varied between 0.01 and 0.05 U mg−1 in the silver stage samples, and between 0.01 and 0.06 U mg−1 in the yellow stage samples. The distinction between the stages was not significant (p > 0.05).
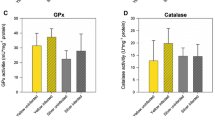
Stress enzyme analysis. The mean values of stress enzyme levels of the eel samples depend on the stage and sex presented in Fig. 2. The SOD values varied between 11.55 and 123.55 U mg−1 in silver eels, and 1.99 and 50.83 U mg−1 in yellow eels. The changes seen depending on the stage were statistically significant (p < 0.01). CAT enzyme values were between 1.43 and 72.84 U mg−1 in silver eels, and between 1 and 43.42 U mg−1 in yellow eels. These values were significant in terms of the stage (p < 0.05). GPx enzyme activity varied between 5.57 and 33.91 U mg−1 in the silver samples, and between 0.7 and 25.9 U mg−1 in the yellow samples. This difference in terms of the stage was statistically significant (p < 0.01). Overall, measured stress enzyme levels were found to be higher in silver eels than in yellow ones.
Considering all parameters, three different generalized linear models were developed to determine the eel stage status, using morphological parameters, body condition values, and some digestive (pepsin and lipase) and some stress (SOD and CAT) enzyme activities separately. (Table 3) It was determined how much the eel samples, which were separated as yellow or silver by observation when caught, were supported by morphological parameters, body condition values and enzyme activities by these models.
p < 0.05 was statistically significant in all three models, and when AIC values in Table 3 for model selection were compared, the body condition model had the minimum AIC value. Even though the body condition model has a lower AIC value, the three models have different practical importance. It would be better for researchers to develop modeling strategies by taking this situation into account. Three models were sufficient in terms of classification success in terms of stage status.
Utilizing the model created using morphological parameters, the number of silver individuals determined as 42 by observation alone was announced as 39, and the number of yellow individuals determined as 14 by observation alone was determined as 18. Similarly, in the model established with body condition values, the number of gray individuals was 39 and the number of yellow individuals was 16. Forty-one of the silver individuals identified as 42 during the study with the enzyme model were declared as silver. Similarly, the number of yellow individuals was determined as 18 and 14 of them were identified as yellow with this model. When the silver/yellow ratios explained by these three models were compared, there was no difference between the ratios obtained in the three models (p > 0.05).
From the data obtained as a result of the study, we created two decision trees, which will contribute to determining the yellow or silver eel in our country’s waters. One of these was organized using morphological parameters and the other using body condition data. Morphological and body condition data, which can be determinative in the silvering stage of the eel, were based on the variation according to the habitat, as stated by Durif et al. (2005) (Fig. 3).
EI was found to be the most effective parameter in distinguishing the silver–yellow phase in the decision tree created with the morphological data. The eye index as a particular parameter separated the population by 55%. An EI value greater than 8.6 confirmed that the sample was in the silver phase. In cases where the EI was less than 8.6, the TL parameter came to the fore. If the samples are more than 525 mm, it can be concluded that they are in the silver phase, and if they are smaller, they are in the yellow phase (Fig. 3a).
When the weight data was used in the decision tree secured with body condition data, it was determined that the sample was in the yellow phase if it was lighter than 226 g. Another parameter checked for heavier samples was FP. This diagram explained the probability of an individual being silver with an FP value greater than 22 and yellow with a small FP value (Fig. 3b).
Discussion
Although there is extensive research on eels in addition to Schmidt's (1923, 1925, 1935) work, information on many aspects of the life cycle of the species is very limited. The silvering mechanism seen in eels was examined by dividing it into two stages (yellow and silver), ignoring that it could be a possible preparation stage for a long time (Durif et al. 2005). The first different classification was recommended by Fontaine (1994), and then Feunteun et al. (2000) grouped eels as yellow, yellow-silver, and silver. However, this classification was based solely on visual variables (skin color, lateral line, and eye surface visibility). The silvering stages of eels were detailed by Durif et al. (2005) as the growth phase, the preparation phase, and the migration phase. Using such detailed classifications to describe this important population instead of classifying eels into yellow and silver stages will allow the dynamics to be evaluated from a more realistic and complete perspective. However, when estimating stock indicators, it is practical to know the yellow/silver ratio to evaluate the escape rate in the total landing data. Therefore, the basic discrimination of two stages taking into consideration the morphology, body condition, and physiology in this study will be beneficial for the assessment of local stocks.
Based on this purpose, in this study, we aimed to reveal the enzymatic levels of the silver and yellow stage eels and the relationship with morphological parameters used in the literature. In this context, it was determined that the morphological characters (TL, EI, and PFI) and body condition data measured in eels caught in our country's territorial waters and separated into yellow and silver based on observation were higher in silver samples, just like in the literature (D’Ancona 1927, 1929; Bertin 1956; Carliske and Denton 1959; Böetius et al. 1962; Böetius and Böetius 1967, 1980; Stramke 1972; Pankhurst 1982; Feunteun et al. 2000; Durif 2003; Durif et al. 2005; Balm et al. 2007). However, the silver/yellow ratio of 60 samples used in this study was determined as 42/18. It was observed that this ratio was 19/41 when I, II and III stages were accepted as yellow, and IV and V stages as silver as evaluated by Durif's silvering stage method. This obvious difference can be explained by the distance from the area where the samples are collected in the preparation area.
In this field study, it was concluded that the digestive enzyme levels obtained were statistically different in the silver and yellow samples. Variations in digestive enzyme levels (and also other parameters such as GSI) may be due to individual starvation conditions of the samples or may be related to the evaluation of the data following the observational separation of yellow and silver at the beginning of the study. Although these enzyme activities show how many hours before the samples were fed, variations may be due to individual differences. There are studies in which the effects on some physiological parameters as a result of experimental starvation in eels were investigated. These studies evaluated the effects of fasting on certain metabolite levels in liver and muscle of eels (Larsson and Lewander 1973; Dave et al. 1975) and the effects of short-term fasting on hematological, biochemical, and some immune responses (Caruso et al. 2010). Olivereau and Olivereau (1997) found significant reductions in body weight in experimentally starved eels and reported hypertrophy of GH cells in the pituitary gland. Okamura et al. (2012) revealed how morphological criteria are affected by hunger in the leptocephali stage of eels. Although studies are evaluating how different parameters are affected by the starvation of eels under in vitro conditions, there are very few studies examining the results of nutritional cessation in silver individuals caught from the natural environment. Facey and Helfman (1985) suggested in their study that although silver individuals of Anguilla rostrata species found nutrients in their gut, eels stopped feeding during migration. In addition, intestinal regression has been reported in migrating eels due to the cessation of feeding (Durif et al. 2005). In this context, digestive enzyme analyses were found to be higher in yellow stage eels compared to silver stage samples. This difference between the stages in terms of four digestive enzymes whose levels were measured was also statistically significant. This situation supports the viewpoint that silver stage eels cease feeding, which is suggested in the literature.
Reactive oxygen species (ROS) are continuously produced by contaminant-stimulated phagocytes, and excessive ROS can result in local tissue damage. However, research is still ongoing on defense mechanisms and oxidative responses that neutralize the effect of ROS on fish (Ahmad et al. 1998, 2003; Fatima et al. 2000). For example, although it is known that environmental pollutants activate these defense mechanisms, stress enzymes have increased as a physiological response when extreme situations occur that go beyond their normal life, such as being locked in a cage (Ahmad et al. 2006). Within the scope of this study, it would not be correct to attribute high- or low-stress enzyme levels to the stage of the samples; of course, it is obvious that many factors affect the stress enzymes. However, the difference in stress enzyme levels in silver and yellow eel samples from the same station, which were revealed in the same environmental gradients, necessitated more detailed studies. When we compared the yellow stage and silver stage samples in terms of SOD, CAT, and GPx enzymes, we found that the enzyme levels changed depending on the stage. The higher enzyme levels in the silver stage samples can be explained as the adaptation of individuals to keep their defense systems high despite the possible adverse events during migration. This suggested the possibility that stress enzyme levels in eels preparing for or starting migration may differ from those in samples of the yellow stage. We did not come across a study based on this hypothesis in our literature research. In some studies, hormone levels were estimated as another physiological parameter that may be effective in the silvering phase of eel (Durif et al. 2005; Balm et al. 2007). In these studies, internal maturation parameters such as growth hormone, gonadotropin, gonado-somatic index, and vitellogenin were evaluated. In this study, we evaluated the levels of different enzyme groups, leaving out the hormone parameter. However, our results and literature data suggest that it would be appropriate to evaluate both hormone levels and enzyme activities together in new studies to determine the silvering process in eels.
In some studies, stress enzymes have been measured in eels in response to this pollution in habitats under the influence of various pollutants. These studies include exposure to internal water pollution (van der Oost et al. 1996), some environmental pollutants (BaP, DHAA, and BKMPE) (Pacheco and Santos 2002), industrial wastes (Santos et al. 2004), and high organochlorine, PAH and heavy metal pollution. Ribeiro et al. (2005) evaluated the stress enzymes levels of eels. In addition, oxidative stress responses in different tissues were examined by caging only eel samples (Ahmad et al. 2006), and also the oxidative stress sensitivity of the species was evaluated in an in vitro study (Regoli et al. 2003). As mentioned previously, the stress enzyme levels of eels are affected by environmental conditions; however, in the present study yellow and silver stage eels were collected in the same environment. The environmental constituents that possibly trigger the stress enzyme level were assumed to be equally affected in randomly sampled eels. This assumption supports the conclusion that stress enzyme levels are effective in distinguishing yellow and silver eels.
When the silver–yellow separation methods based on the study were evaluated in four items (observation, morphological parameters, body condition data, and enzyme levels), it was revealed that the most accurate distinction was made mathematically, especially with digestive enzyme activities, with the multiple logistic regression model. From this point of view, we determined the samples of our country as yellow and silver with digestive enzyme levels and created two different decision trees to make them usable in practical applications with the morphological and body condition data of individuals. For this reason, we think that it would be useful to distinguish between yellow and silver by using these measurements that can be made in the field, especially in the studies of the eel population in the region. This sample size only reflects the results of this study. However, we suggest producing a cutoff value for easily observable variables using the physiological variables including digestive and stress enzyme levels probably in larger sample sizes in particular geographic scales. Based on our belief that this decision tree study, which is created with morphological parameters and body condition data, will create a practical method in eel research, stock estimation, and declining population management plans, we think that it will pave the way for similar decision trees to become widespread in different parts of the world.
References
Aarestrup K, Økland F, Hansen MM, Righton D, Gargan P, Castonguay M, Bernatchez L, Howey P, Sparholt H, Pedersen MI, McKinley RS (2009) Oceanic spawning migration of the European eel (Anguilla anguilla). Science 325:1660
Ahmad I, Fatima M, Athar M, Khan NZ, Raisuddin S (1998) Responses of circulating fish phagocytes to paper mill effluent exposure. Bull Environ Contam Toxicol 61:746–753
Ahmad I, Pacheco M, Santos MA (2003) Naphthalene-induced differential tissue damage association with circulating fish phagocytes induction. Ecotoxicol Environ Saf 54:7–15
Ahmad I, Pacheco M, Santos MA (2004) Enzymatic and nonenzymatic antioxidants as an adaptation to phagocyte-induced damage in Anguilla anguilla L. following in situ harbor water exposure. Ecotoxicol Environ Saf 57:290–302
Ahmad I, Pacheco M, Santos MA (2006) Anguilla anguilla L. oxidative stress biomarkers: an in situ study of freshwater wetland ecosystem (Pateira de Fermentelos, Portugal). Chemosphere 65:952–962
Akaike HA (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723
Amérand A, Mortelette H, Belhomme M, Moisan C (2017) Silvering and swimming effects on aerobic metabolism and reactive oxygen species in the European eel. Respir Physiol Neurobiol 235:40–44
Arrington DA, Davidson BK, Winemiller KO, Layman CA (2006) Influence of life history and seasonal hydrology on lipid storage in three Venezuelan fish species. J Fish Biol 68:1–16
Balm SP, Durif C, van Ginneken V, Antonissen E, Boot R, van Den Thillart G, Verstegen M (2007) Silvering of European eel (Anguilla anguilla L.): seasonal changes of morphological and metabolic parameters. Anim Biol 57:63–77
Berrar D (2019) Performance measures for binary classification. Encycl Bioinform Comput Biol 1:546–560
Bertin L (1956) Eels, a biological study. Cleaver-Hume Press, London
Böetius I, Böetius J (1967) Studies in the European eel Anguilla anguilla (L.). Experimental induction of the male sexual cycle, its relation to temperature and other factors. Meddr Danm Fisk Havunders 4:339–405
Böetius I, Böetius J (1980) Experimental maturation of female silver eels, Anguilla anguilla. Estimates of fecundity and energy reserves for migration and spawning. Dana 1:1–28
Böetius J, Böetius I, Hemmingsen AM, Brunn AF, Moller-Christensen L (1962) Studies of ovarial growth induced by hormone injections in the European and American eel (Anguilla anguilla L. and Anguilla rostrata Le seur). Meddr Danm Fisk Huvunders 3:183–198
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35
Carliske DB, Denton EJ (1959) On the metamorphosis of the visual pigments of Anguilla anguilla (L.). J Mar Biol Assoc U K 38:97–102
Caruso G, Maricchiolo G, Micale V, Genovese L, Caruso R, Denaro MG (2010) Physiological responses to starvation in the European eel (Anguilla anguilla): effects on hematological, biochemical, non-specific immune parameters and skin structures. Fish Physiol Biochem 36:71–83
Clairborne, A., Greenwald, R. A. (1985). Catalase Activity. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 283–284
D’Ancona U (1929) Sui fenomeni di circolazione che accompagnano l’ingrandimento dell’occhio nella maturita sessuale dell’Anguilla. Boletin de la Sociedad Española de Biología 27:1–8
D’Ancona U (1927) Richerche sull’ingrandimento dell’occhio dell’Anguilla in rapporto alla maturita sessuale e considerazioni sul suo significata biologico. R C Accad Lincei 5(Ser 6):360–363
Dave G, Johansson-Sjöbeck ML, Larsson Å, Lewander K, Lidman U (1975) Metabolic and hematological effects of starvation in the European eel, Anguilla anguilla L.-I. Carbohydrate, lipid, protein and inorganic ion metabolism. Comp Biochem Physiol A Physiol 52:423–430
Dekker W (2000) A Procrustean assessment of the European eel stock. ICES J Mar Sci 57:938–947
Durif C, Dufour S, Elie P (2005) The silvering process of Anguilla anguilla: a new classification from the yellow resident to the silver migrating stage. J Fish Biol 66:1025–1043
Durif C, Gosset C, Rives J, Travade F, Elie P (2003) Behavioral study of downstream migrating eels by radio-telemetry at a small hydroelectric power plant. In: Dixon DA (ed) Biology, management, and protection of catadromous eels. American Fisheries Society Symposium 33. American Fisheries Society, Bethesda, MD, pp. 343–356
Durif C (2003) La migration d’avalaison de l’anguille europe´enne Anguilla anguilla: caracterisation des fractions de´valantes, phenomene de migration et franchissement d’obstacles. The`se de doctorat, Universite´ Paul Sabatier, Toulouse
Facey DE, Helfman GS (1985) Reproductive migrations of American eels in Georgia. Proc Annu Conf Southeast Assoc Fish Wildl Agencies 39:132–138
Fatima M, Ahmad I, Sayeed I, Athar M, Raisuddin S (2000) Pollutant-induced overactivation of phagocytes is concomitantly associated with peroxidative damage in fish tissues. Aquat Toxicol 49:243–250
Feunteun E, Acou A, Lafaille P, Legault A (2000) The European eel: prediction of spawner escapement from continental population parameters. Can J Fish Aquat Sci 57:1627–1635
Flohé L, Ötting F (1984) Superoxide dismutase assay. Methods Enzymol 105:93–104
Folch J, Lees M, Sloan-Stanley GH (1957) A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fontaine YA (1994) L’argenture de l’anguille: me´tamorphose, anticipation, adaptation. Bull Fr Pêche Piscic 335:171–186
Freeman EA, Moisen GG (2008) A comparison of the performance of threshold criteria for binary classification in terms of predicted prevalence and kappa. Ecol Modell 217:48–58
Frost WE (1945) The age and growth of eels (Anguilla anguilla) from the Windermere catchment area, Part II. J Anim Ecol 14:106–124
Han YS, Tzeng WN, Huang YS, Liao IC (2001) Silvering in the eel: changes in morphology, body fat content, and gonadal development. J Taiwan Fish Res 9:119–127
Hansen RA, Eversole AG (1984) Age, growth, and sex ratio of American eels in brackish-water portions of a South Carolina river. Trans Am Fish Soc 113:744–749
Helfman GS, Bozeman EL, Brothers EB (1984) Size, age, and sex of American eels in a Georgia River. Trans Am Fish Soc 113:132–141
Holmgren K, Wickstrom H, Clevestam P (1997) Sex-related growth of European eel, Anguilla anguilla, with focus on median silver eel age Can J Fish Aquat Sci 54:2775–2781
ICES (2020) Joint EIFAAC/ICES/GFCM Working Group on eels (WGEEL). ICES Scientific Reports, vol 2, issue 85. ICES, Copenhagen
ICES (2021) Workshop on the Future of Eel Advice (WKFEA). ICES Scientific Reports, vol 3, issue 13. ICES, Copenhagen
Jacoby D, Gollock M (2014) Anguilla anguilla. The IUCN Red List of Threatened Species 2014: e.T60344A45833138. http://dx.doi.org/https://doi.org/10.2305/IUCN.UK.2014-1.RLTS.T60344A45833138.en
Küçük F, Gümüşi E, Gülle İ (2005) Determination of Entrance Seasons of Elvers (Anguilla anguilla L., 1766) in Gözlen Creek and Assessment of Different Catching Methods. Turk J Vet Anim Sci 29:1061–1066
Larsson Å, Lewander K (1973) Metabolic effects of starvation in the eel Anguilla anguilla L. Comp Biochem Physiol A Mol Integr Physiol 44A:367–374
Métais P, Bieth J (1968) Determination of alpha-amylase by a microtechnic. Annales de biologie clinique 26:133–142
Moriarty C, Dekker W (1997) Management of the European eel. Marine Institute, Dublin
Mortelette H, Amérand A, Sébert P, Belhomme M, Calvès P, Moisan C (2010) Effect of exercise training on respiration and reactive oxygen species metabolism in eel red muscle. Respir Physiol Neurobiol 172:201–205
Okamura A, Yamada Y, Mikawa N, Horie N, Tsukamoto K (2012) Effect of starvation, body size, and temperature on the onset of metamorphosis in Japanese eel (Anguilla japonica). Can J Zool 90:1378–1385
Oliveira K (1999) Life history characteristics and strategies of the American eel, Anguilla rostrata. Can J Fish Aquat Sci 56:795–802
Olivereau M, Olivereau JM (1997) Long-term starvation in the European eel: general effects and responses of pituitary growth hormone-(GH) and somatolactin-(SL) secreting cells. Fish Physiol Biochem 17:261–269
Pacheco M, Santos MA (2002) Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol Environ Saf 53:331–347
Pankhurst NW (1982) Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla (L.). J Fish Biol 21:127–140
Pearce J, Ferrier S (2000) Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Modell 133:225–245
Post JR, Parkinson EA (2001) Energy allocation strategy in young fish: allometry and survival. Ecology 82:1040–1051
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rad F, Baris M, Bozaoglu SA, Temel GO, Üstündag M (2013) Preliminary investigation on morfometric and biometric characteristics of female silver and yellow, Anguilla anguilla, from eastern mediterranean (Göksu Delta/Turkey). J Fishscicom 7:253–265
Regoli F, Winston GW, Gorbi S, Frenzilli G, Nigro M, Corsi I, Focardi S (2003) Integrating enzymatic responses to organic chemical exposure with total oxyradical absorbing capacity and DNA damage in the European eel Anguilla anguilla. Environ Toxicol Chem 22:2120–2129
Ribeiro CO, Vollaire Y, Sanchez-Chardi A, Roche H (2005) Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the Eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat Toxicol 74:53–69
Santos MA, Pacheco M, Ahmad I (2004) Anguilla anguilla L. antioxidants responses to in situ bleached kraft pulp mill effluent outlet exposure. Environ Int 30:301–308
Schmidt J (1923) Breeding places and migration of the eel. Nature 111:51–54
Schmidt J (1925) The breeding places of the eel. Annu Rep Smithson Inst 1924:279–316
Schmidt J (1935) Danish eel investigations during 25 years (1905–1930). The Carlsberg Foundation’s Oceanographical Expedition round the World 1928–1930 and previous Danish Oceanographical Expeditions under the leadership of late Professor Johannes Schmidt. The Carlsberg Foundation, Copenhagen
Shanubhogue A, Gore A (1987) Using logistic regression in ecology. Curr Sci 56:933–935
Stramke D (1972) Veranderungen am auge des Europaischen Aales (Anguilla anguilla L.) wahrend der Gelb-und Blankaalphase. Arch Fischereiwiss 23:101–117
Svedäng H, Wickstrom H (1997) Low-fat contents in female silver eels: indications of insufficient energetic stores for migration and gonadal development. J Fish Biol 50:475–486
Svedäng H, Neuman E, Wickstrom H (1996) Maturation patterns in female European eel: age and size at the silver eel stage. J Fish Biol 48:342–351
Tseng HC, Grendell JH, Rothman SS (1982) Food, duodenal extracts, and enzyme secretion by the pancreas. Am J Physiol 243:G304–G312
van den Thillart G, van Ginneken V, Körner F, Heijmans R, van der Linden R, Gluvers A (2004) Endurance swimming of European eel. J Fish Biol 65:312–318
van der Oost R, Goksøyr A, Celander M, Heida H, Vermeulen NP (1996) Biomonitoring of aquatic pollution with feral eel (Anguilla anguilla) II. Biomarkers: pollution-induced biochemical responses. Aquat Toxicol 36:189–222
Versaw WK, Cuppett SL, Winters DD, Williams LE (1989) An improved colorimetric assay for bacterial lipase in nonfat dry milk. J Food Sci 54:1557–1558
Vøllestad LA (1992) Geographic variation in age and length at metamorphosis of maturing European eel: environmental effects and phenotypic plasticity. J Anim Ecol 61:41–48
Wendel A (1980) Glutathione peroxidase. In: Jakoby WB (ed) Enzymatic basis of detoxication. Vol 1. Academic Press, New York, pp 333–353
Worthington TM (1982) Pepsin. Enzymes and related biochemicals. Worthington Diagnostic System Inc., Freehold, New Jersey
Yalçın Özdilek Ş, Solak K (2007) The feeding of European eel (Anguilla anguilla L.) in the River Asi, Turkey. J Ichthyol 1:26–35
Zambonino Infante JL, Cahu CL (1994) Influence of diet on pepsin and some pancreatic enzymes in sea bass (Dicentrarchus labrax) larvae. Comp Biochem Physiol A Physiol 109:209–212
Acknowledgments
The authors would like to acknowledge support by COMU- BAP (FBA-2019-2906 coded Project). This study was completed with the permission of COMÜ Animal Ethics Committee (07.03.2019/2019-2/2019-1900037141/2019/02-10). The specimens were collected by eel monitoring program and we thank all the collaborators of the Republic of Turkey Ministry of Agriculture and Forest, General Directorate of Fisheries and Aquatic Resources and local administrators and officers. We also thank Vedat Çalışkan who supplied Asi River specimens.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ertürk Gürkan, S., Mestav, B. & Yalçin Özdilek, Ş. Evaluation of the role of enzyme activities in the silvering process of European eels (Anguilla anguilla). Ichthyol Res 70, 132–141 (2023). https://doi.org/10.1007/s10228-022-00870-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-022-00870-5