Abstract
Ontogenetic development in the snailfish Crystallichthys matsushimae (Jordan and Snyder 1902), including body proportions, pigmentation, tooth pattern and barbels, is described from specimens reared for 180 days after hatching. Newly hatched individuals, obtained 157 and 190 days after artificial fertilization of eggs [4.4 ± 0.22 (mean ± standard deviation) mm in diameter], had a flexed notochord, yolk sac, adhesive pelvic disk, and full complement of counts, including fin rays, gill rakers, and branchiostegal rays. Several dermal tubercles apparent on the nasal, maxillary, and mandibular regions in day 110 (20.7 ± 0.6 mm SL) had increased in length to form barbels by day 180 (33.2 mm SL).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snailfishes of the family Liparidae comprise about 32 genera with 450 species worldwide (Chernova et al. 2021; Murasaki et al. 2021; Orr 2021). The family is widely distributed from the shallowest waters to near the deepest parts of the ocean, essentially limited to cool-temperate and cold waters (Orr et al. 2019). In addition, the fecundity of snailfish adapted to the deep sea tends to be low and eggs correspondingly large, probably being indicative of direct development without a planktonic stage (Chernova et al. 2004). Therefore, available information on early life history stages is very limited due to the rarity of material (Able et al. 1984; Kojima 2014), with morphological development having been described only for the species of Allocareproctus Pitruk and Fedorov 1993, Careproctus Krøyer 1862, Liparis Scopoli 1777, Paraliparis Collett 1879, and Rhinoliparis Gilbert 1896 (Kido and Kitagawa 1986; Ambrose 1996; Fahay 2007; Takami and Fukui 2012; Kojima 2014; Overdick et al. 2014).
The genus Crystallichthys Jordan and Gilbert in Jordan and Evermann 1898, diagnosed by round or subround, large blotches on the body and dorsal and anal fins (not variegated), a single nostril, well-developed sucking disk, and six branchiostegal rays (Kido 1988; Mecklenburg et al. 2002), comprises only four species: Crystallichthys cameliae (Nalbant 1965), Crystallichthys mirabilis Jordan and Gilbert in Jordan and Evermann 1898, Crystallichthys cyclospilus (Gilbert and Burke 1912), and Crystallichthys matsushimae (Jordan and Snyder 1902). Crystallichthys matsushimae, known from continental slopes of the Sea of Japan, southern Sea of Okhotsk (including coasts of Hokkaido, Japan and southeastern Sakhalin, Russia), and the Pacific coast of northern Japan (Nakabo and Kai 2013), is distinguishable from congeneric species by having 20–30 barbels on the snout and both jaws (Kido 1988). Because morphological development in the species of Crystallichthys has at no time been described, a detailed description of such in Cr. matsushimae, based on laboratory-reared samples, is provided herein.
Materials and methods
Parental fishes and egg collection. For observations of early development, eggs were obtained by artificial fertilization utilizing mature individuals [one male, 287.0 mm in standard length (SL), and two females, 305.0 mm and 319.0 mm SL, see Electronic Supplementary Materials (ESM) Fig. S1 and ESM Table S1] collected by commercial gillnets from the Nemuro Strait, southern Sea of Okhotsk, Hokkaido, Japan, at depths of 200–300 m on 25 December 2019. The specimens were kept in a 1,500-l tank (2 m × 1 m, 75 cm depth) maintained at a water temperature of 3.1–4.0 °C (average 3.6 °C). After the abdomens of the females had become bloated, eggs were stripped and artificially fertilized with sperm diluted in seawater, on 17 March 2020. The eggs were subsequently incubated in two aerated 30-l columnar hatching tanks (35 cm diameter × 35 cm depth) at 1.9–3.9 °C (average 2.8 ºC).
Rearing of juveniles. Hatching occurred between 157 and 190 days after fertilization, 150 juveniles being obtained. Two tanks (each tank reared 59 individuals hatching on day 157 and 34 individuals hatching on day 190) were used for rearing observation. For each tank, 50 ml of Artemia sp. nauplii (2–3 individuals/ml) were provided daily from day 3 and 10 g frozen copepods (China cope L: Pacific trading Co., Ltd) daily from day 10, respectively. Incubation and rearing temperatures ranged from 1.9 to 3.9 °C (average 2.8 ºC).
Observations and measurements. Seven fertilized eggs and nine newly hatched individuals (corresponding to day 0) were sampled, five having hatched on day 157 following fertilization (see above) and the remainder on day 190. Subsequently, hatched juveniles on day 157 were sampled on days 10, 20, 30, 40, 50, 60, 85, 110, 150 and 180, being preserved in 10% formalin for a few days and thereafter transferred to 50% isopropyl alcohol. For comparison, a wild juvenile (20.0 mm SL), collected by diving at a depth of 28 m (water temperature −0.2 °C) off the coast of Rausu, Hokkaido, Japan on 19 March 2021, was also examined. The fertilized eggs and juveniles were deposited in the Marine Science Museum, Fukushima Prefecture, Aquamarine Fukushima (AMF) (see ESM Table S1).
Counts, measurements and descriptive terminology followed Stein et al. (2001). Adults were measured with digital calipers to the nearest 0.1 mm. Body depth was measured from the posterior margin of the pelvic disk to the base of the dorsal fin. Counts and measurements [using a micrometer under a stereomicroscope (SteREO Discovery.V12; ZEISS, Germany)] were made on seven eggs and 2–4 juveniles sampled periodically as above, totaling 39 juveniles (10.0–34.4 mm SL). The juveniles were stained in Aniline Blue (Wako Chemicals) to observe fin rays and head sensory pores, and sketched using a stereomicroscope. For teeth observations and vertebral counts, day 0 YS juveniles (n = 4; 10.2–12.0 mm SL, AMF-116-3–5, AMF-117-4) were stained with alcian blue 8 GX and alizarin red (Wako Chemicals), following Kawamura and Hosoya (1991), and radiographs taken of parental individuals (n = 3).
For observations of barbel development, ventral lower jaw views of each of the specimens collected on day 110 (21.4 mm SL), 150 (25.3 mm SL), and 180 (33.2 mm SL) were photographed.
Result and discussion
Adults. Counts and measurements of the parental individuals are shown in Table 1. Body somewhat compressed, gradually tapering posteriorly; nostril single; snout of male protruded (ESM Fig. S1a); teeth trilobed with small lateral cusps; snout and both jaws with 25 or 26 barbels in total; cephalic lateralis pores: nasal pores 2, maxillary pores 5, preoperculo-mandibular pores 6, suprabranchial pore 1 (cephalic pore pattern 2-5-6-1); head and body with round reddish markings (ESM Fig. S1).
Remarks. Tohkairin et al. (2014) reported two color morphotypes of Crystallichthys matsushimae, a yellow morphotype distributed in southern Sea of Japan and red morphotype in Sea of Okhotsk, northern Sea of Japan, and Pacific coast of northern Japan. The parental individuals (AMF-128, -129 and -130) used in this study conformed to the red morphotype of Tohkairin et al. (2014), owing to the rounded reddish markings and collection locality in the southern Sea of Okhotsk (ESM Fig. S1).
Eggs. A total of 2,335 eggs was obtained from the 2 females. The eggs were demersal, almost spherical in shape, transparent and unpigmented; the yolk was pale yellow with 350–660 oil globules (Fig. 1). Egg size was 4.4 ± 0.2 mm (mean ± SD, n = 7) in diameter, with small perivitelline space (ca. 0.1 mm).
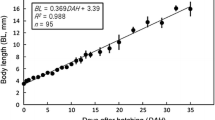
Juveniles. Individuals at hatching possessed a flexed notochord tip and full complement of fin rays, although still retaining yolk [yolk-sac (YS) juveniles] (Fig. 2a). Standard lengths of juveniles from day 0 to 180 are shown in Fig. 3, with counts and measurements of YS juveniles (on day 0 and day 40) and measurements of juveniles (on day 50) shown in Table 1.
General morphology. Head and body somewhat compressed, moderately elongate and thin posteriorly. Skin relatively thick, with gelatinous tissue. Body depth greatest at position of yolk sac or at posterior margin of pelvic disk, becoming gradually shallower posteriorly. Snout deep, rounded. Mouth horizontal, subterminal, posterior margin below center of pupil. Eye rounded; dorsal margin of eye at level of upper pectoral-fin base; suborbital distance (least distance between eye and upper jaw) ca. 1/2 eye diameter at hatching. Conical teeth arranged in two (upper jaw) or three (lower jaw) rows at hatching (10.2–13.0 mm SL), becoming shouldered by day 85 (20.3 mm SL) and distinctly trilobed by day 180 (33.2 mm SL), being arranged in three (upper jaw) or four (lower jaw) rows. A single nostril horizontally level with center of eye, with distinct tube. Counts of nasal, maxillary, preoperculo-mandibular and suprabranchial pores 2-5-6-0 in newly hatched YS juveniles (Fig. 2b, c), attaining full adult counts (2-5-6-1) in day 10 YS juveniles (10.0–11.4 mm SL). Gill slit small, lower margin slightly lower than origin of pectoral-fin base. Origin of anus position vertically below twelfth dorsal-fin ray and moved more anteriorly to ninth dorsal-fin ray with development until day 50 (16.4–17.8 mm SL). Indistinct tubercles appearing on ventral aspects of snout, maxillary and mandibular regions by day 110 (21.4 mm SL, Fig. 4a). Tubercles evident by day 150 (25.3 mm SL, Fig. 4b), ten on ventral aspect of snout, three on maxillary, eight on mandibular regions (Fig. 4b); increasing in size forming barbels by day 180 (33.2 mm SL), 10 on ventral side of snout, three on maxillary, and eight on mandibular regions (Fig. 4c).
Barbel development in juvenile Crystallichthys matsushimae. Ventral view of nasal, maxillary and mandibular regions. 10 on ventral aspect of snout (yellow arrows), three on maxillary (red arrows), eight on mandibular regions (black arrows). a AMF-125-3, day 110 (21.4 mm SL); b AMF-126-3, day 150 (25.3 mm SL); c AMF-127-2, day 180 (33.2 mm SL). Horizontal bars 1 mm
Dorsal, anal, pectoral and caudal fins with full adult fin ray complement in newly hatched YS juveniles (Table 1). Dorsal-fin origin vertically above or slightly behind level of gill slit, anal-fin origin below eleventh to twelfth dorsal-fin rays. Posteriormost rays of dorsal and anal fins continuous with fin membrane of caudal-fin rays. Pectoral fin slightly notched, upper lobe extending vertically to eighth dorsal-fin ray; lower lobe slightly elongate, fifth or sixth ray from ventral longest, extending to center margin of pelvic disk. Pelvic disk pear-shaped (not round), flat with broad margin. Yolk sac occupying most of abdominal cavity in newly hatched YS juveniles (10.8–11.4 mm SL), absorbed entirely in juveniles by day 50 (16.4–17.8 mm SL). Gill rakers (0 + 8–9) and branchiostegal rays (6) (full complements attained in newly hatched YS juveniles).
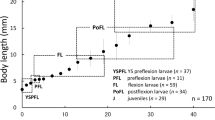
Body proportions (Fig. 5). Head length to standard length (HL/SL) initially 22.1–25.0% SL in newly hatched YS juveniles (10.2–13.0mm SL), subsequently decreasing slightly to 21.9% SL on day 20 (12.2–12.7 mm SL), increasing thereafter to ca. 35% SL on day 150 (24.5–25.3 mm SL, Fig. 5a). Snout length to standard length (SNL/SL) initially 5.8–8.3% SL in YS juveniles (10.0–14.9 mm SL, days 0–30), increasing to ca.10% SL until day 85 (19.3–20.3 mm SL, Fig. 5b). Body depth to standard length (BDA/SL) varying from 21.6 to 27.6% SL in all YS juveniles and juveniles (10.0–34.4 mm SL, Fig. 5c). Lower jaw tip to anus distance to head length (LJT/HL) 109.7–155.6% HL in YS juveniles (10.0–11.4 mm SL, days 0–10), thereafter gradually decreasing to ca. 90% HL (Fig. 5d). Pre-anal length to standard length (PAL/SL) varying from 35.5 to 45.9% SL in all YS juveniles and juveniles (10.0–34.4 mm SL, Fig. 5e). Eye diameter to standard length (ED/SL) varying from 6.2–9.2% SL in all YS juveniles and juveniles (10.0–34.4 mm SL, Fig. 5f). Pelvic disk length to standard length (DL/SL) varying from 8.7 to 13.5% SL in all YS juveniles and juveniles (10.0–34.4 mm SL, Fig. 5g).
Relationships between standard length (SL) and selected body proportions in laboratory-reared juveniles (closed circles) and parental fishes (open circles). a Head length to standard length (HL/SL); b snout length to standard length (SNL/SL); c body depth at posterior margin of pelvic disk to standard length (BDA/SL); d lower jaw tip to anus length to head length (LJT/HL); e pre-anal length to standard length (PAL/SL); f eye diameter to standard length (ED/SL); g disk length to standard length (DL/SL)
Pigmentation. Cluster melanophore presented directly below eye, between eye and gill slit, and at the base of maxillary, increasing in number to form some bands radiating from around eye by day 50 (17.8 mm SL) or later (Fig. 6b–g). Two dark spots above gill slit in day 0 YS juveniles (11.4 mm SL, Figs. 2a, 6a), increasing in number and size (Fig. 6b–e) to form a dark band by day 150 (24.6 mm SL) or later (Fig. 6f–g). One or two dark spots appearing on pectoral-fin base in day 50 and 60 juveniles (17.8–18.2 mm SL, Fig. 6b–c), subsequently forming a dark vertical band (Fig. 6d–g). Three dark spots on distal margin of third, ninth, and sixteenth dorsal-fin ray, respectively, and four dark spots on basal parts of seventh, fourteenth, twenty-fifth, and forty-first dorsal-fin ray, respectively, in day 0 YS juveniles (11.4 mm SL, Figs. 2a, 6a), increasing in number and size in day 50 and 60 juveniles (Fig. 6b–c), subsequently forming vertical bands as spots coalesce (Fig. 6d–g). Three dark spots on distal margin of fifth, thirteenth, and twenty-second anal fin and five dark spots on basal parts of seventh, fourteenth, nineteenth, twenty-eighth, and thirty-first anal fin in day 0 YS juveniles (11.4 mm SL, Figs. 2a, 6a), increasing in number and size in day 50 and 60 juveniles (17.8–18.2 mm SL, Fig. 6b–c), subsequently forming vertical bands as spots coalesce (Fig. 6d–g). Many dark spots on body in day 50 juveniles (17.8 mm SL, Fig. 6b), becoming larger in day 60–110 juveniles (18.2–20.4 mm SL, Fig. 6c–e), subsequently forming dark markings and vertical bands (Fig. 6f–g). Minute melanophores were covering the surface of snout, head, dorsal fin, anal fin, body, above upper lobe pectoral fin; melanophores were absent on the pelvic fin, caudal fin, lower lobe pectoral fin, below upper lobe pectoral fin and yolk sac (Figs. 2a, 6a).
Body coloration and markings in reared juvenile (except h) Crystallichthys matsushimae (preserved condition). a Newly hatched yolk-sac juvenile, AMF-116-2, day 0 (11.4 mm SL); b AMF-122-3, day 50 (17.8 mm SL); c AMF-123-2, day 60 (18.2 mm SL); d AMF-124-3, day 85 (20.3 mm SL); e AMF-125-2, day 110 (20.4 mm SL); f AMF-126-2, day 150 (24.6 mm SL); g AMF-127-2, day 180 (33.2 mm SL); h AMF-131, 20.0 mm SL, wild individual, off Rausu, depth 28 m (−0.2 °C water temperature)
Remarks. Within the family Liparidae, the dorsal, anal, pectoral and caudal fins, and pelvic disk are underdeveloped in early stage larval Liparis (Able et al. 1984; Ambrose 1996; Fahay 2007; Kojima 2014). In contrast, the larvae of Paraliparis dipterus (Kido 1988) and Rhinoliparis barbulifer (Gilbert 1896) had adult median fin ray complements, and the tip of notochord upturned (Kido and Kitagawa 1986; Takami and Fukui 2012). Similarly, newly hatched juveniles of Careproctus melanurus (Gilbert 1892) and Careproctus reinhardti (Krøyer 1862) had fully developed fin ray complements and a flexed notochord tip, while retaining the yolk (Ambrose 1996; Fahay 2007). Compared with the Careproctus, Paraliparis and Rhinoliparis genera, the newly hatched individuals of Cr. matsushimae were similar development like fully developed fin ray complements and a flexed notochord tip, while retaining the yolk to the species of Careproctus. In fact, Cr. matsushimae was placed in a monophyletic group with some species of Careproctus in a molecular phylogenetic tree (Orr et al. 2019). It should also be noted that newly hatched Cr. matsushimae yolk-sac juveniles were adhered to the walls of the rearing tank by the pelvic disk (observed during the present study) (ESM Fig. S2), suggesting that the pelvic disk is functional at hatching.
On the other hand, the diagnostic barbels of Cr. matsushimae, being important morphological characters, are not fully developed at hatching, appearing first in day 110 juveniles (20.3–21.4 mm SL). Notably, the standard length of individuals began to increase rapidly with the development of barbels (Fig. 3), which can be attributed to their use for more effective feeding behavior. In fact, day 300 (ca. 80 mm SL) juveniles were apparently searching for food with barbels in the aquarium (digital video image: http://www.momo-p.com/index.php?movieid=momo210710cm01b&embed=on). Other three species of larvae of the same genus have not yet been reported, but similar morphological juvenile need to be investigated this after. Larval morphology of Liparidae possessing a flexed notochord tip while retaining the yolk, in addition to a full complement of fin rays and pelvic disk is known for Ca. melanurus, Ca. reinhardti (Ambrose 1996; Fahay 2007). However, the cluster melanophore patterns on the head and body of Cr. matsushimae differ in those species.
A wild juvenile of 20.0 mm SL (Fig. 6h) corresponded to reared day 85–110 juveniles (20.3–20.4 mm SL, Fig. 6d–e) (estimated from standard length). Because the body coloration of wild and reared individuals was similar, the developmental pattern of markings described herein appear to reflect the natural condition. In the present study, the number of specimens of larger than 25 mm SL is small. The morphological changes in specimens over 25 mm SL will need future study based on larger number of specimens.
References
Able KW, Markle DF, Fahay MP (1984) Cyclopteridae: development. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richardson SL (eds) Ontogeny and systematics of fishes. Special Publication 1. American Society of Ichthyologists and Herpetologists, Lawrence, pp 428–437
Ambrose DA (1996) Cyclopteridae: snailfish and lumpsuckers. In: Moser HG (ed) The early stages of fishes in the California current region. CalCOFI atlas 33. CalCOFI, La Jolla, pp 860–871
Chernova NV, Stein DL, Andriashev AP (2004) Family Liparidae Scopoli 1777, snailfishes. Calif Acad Sci Annotated Checklists of Fishes 31:1–72
Chernova NV, Vedischeva EV, Datskii AV (2021) A new species of snailfishes (Liparidae) of the genus Careproctus from the northern slope of the Aleutin Basin (Bering Sea). J Ichthyol 61:375–383
Collett R (1879) Fiske fra Nordhavs-expeditionens sidste togt, sommeren 1878. Forhandl Vidensk-selsk Christiania 14:1–106
Fahay MP (2007) Early stage of fishes in the Western North Atlantic Ocean, vol 2. Northwest Atlantic Fisheries Organization, Nova Scotia
Gilbert CH (1892) Descriptions of thirty-four new species of fishes collected in 1888 and 1889, principally among the Santa Barbara Islands and in the Gulf of California. Proc U S Natl Mus 14:539–566
Gilbert CH (1896) The ichthyological collections of the steamer Albatross during the years 1890 and 1891. Rep U S Fish Comm 19:393–476
Gilbert CH, Burke CV (1912) Fishes from Bering Sea and Kamchatka. Bull Bureau Fish 30:31–96
Jordan DS and Evermann BW (1898) The fishes of North and Middle America: a descriptive catalogue of the species of fish-like vertebrates found in the waters of North America north of the Isthmus of Panama. Part III. Bull U S Natl Mus 47:2183–3136
Jordan DS, Snyder JO (1902) A review of the discobolous fishes of Japan. Proc U S Natl Mus 24:343–351
Kawamura K, Hosoya K (1991) A modified double staining technique for making a transparent fish-skeletal specimen. Bull Natl Res Inst Aquaculture 20:11–18
Kido K (1988) Phylogeny of the family Liparidae, with taxonomy of the species found around Japan. Mem Fac Fish Hokkaido Univ 35:125–256
Kido K, Kitagawa D (1986) Development of larvae and juveniles of Rhinoliparis barbulifer. In: Uyeno T, Arai R, Taniuchi T, Matsuura K (eds) Indo-Pacific fish biology: proceedings of the second international conference on Indo-Pacific fishes. Ichthyological Society of Japan, Tokyo, pp 697–702
Kojima J (2014) Liparidae. In: Okiyama M (ed) An atlas of early stage fishes in Japan. Tokai University Press, Hadano, pp 1093–1098
Krøyer HN (1862) Nogle Bidrag til Nordisk ichthyologi. Naturhist Tidsskr (Kjøbenhavn) (Ser 3) 1:233–310
Mecklenburg CW, Mecklenburg TA, Thorsteinson LK (2002) Fishes of Alaska. American Fisheries Society, Maryland
Murasaki K, Kai Y, Endo H, Fukui A (2021) Osteodiscus abyssicola, a new snailfish (Cottoidei: Liparidae) collected off the Pacific coast of northern Japan. Zootaxa 5032:136–142
Nakabo T, Kai Y (2013) Liparidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species. Tokai University Press, Hadano, pp 1093–1098
Nalbant TT (1965) Careproctus cameliae, new species of sea-snail from the Bering Sea (Pisces, Liparidae). Senckenb Biol 46:271–273
Orr JW (2021) Three new small snailfishes of the genus Careproctus (Teleostei: Cottiformes: Liparidae) from the Aleutian Islands, Alaska. Ichthyol Herpetol 109:456–466
Orr JW, Spies I, Stevenson DE, Longo GC, Kai Y, Ghodes S, Hollowed M (2019) Molecular phylogenetics of snailfshes (Liparidae: Cottoidei) based on mtDNA and RADseq genomic analyses, with comments on selected morphological characters. Zootaxa 4642:1–79
Overdick AA, Busby MS, Blood DM (2014) Descriptions of eggs of snailfishes (family Liparidae) from the Bering Sea and eastern North Pacific Ocean. Ichthyol Res 61:131–141
Pitruk DL, Fedorov VV (1993) Allocareproctus gen. novum (Scorpaeniformes, Liparidae)—a new genus of snailfishes from the Northwest Pacific Ocean. V Ikhtiol 33:16–20
Scopoli GA (1777) Introductio ad historiam naturalem, sistens genera lapidum, plantarum et animalium hactenus detecta, caracteribus essentialibus donata, in tribus divisa, subinde ad leges naturae. Wolfgang Gerle, Pragae
Stein DL, Chernova NV, Andriashev AP (2001) Snailfishes (Pisces: Liparidae) of Australia, including description of thirty new species. Rec Aust Mus 53:341–406
Takami M, Fukui A (2012) Ontogenetic development of a rare liparid, Paraliparis dipterus, collected from Suruga Bay, Japan, with notes on its reproduction. Ichthyol Res 59:134–142
Tohkairin A, Kai Y, Ueda Y, Hamatsu T, Ito M, Nakabo T (2014) Morphological divergence between two color morphotypes of Crystallichthys matsushimae (Cottoidei: Liparidae). Ichthyol Res 62:145–155
Acknowledgments
We express our sincere thanks to Shigeki Fujimoto and Shigetada Fujimoto, Akihiro Yorozuya and Kazuto Takeda (Fisheries Cooperative Association of Rausu) for their help in collecting parental fishes. We are grateful to Katsunori Seki, Tomoyuki Aoyagi, and Riki Niikura (Shiretoko Diving Service) for collecting juveniles in the wild and valuable information. Our appreciation is also extended to Yoshitaka Abe (former Executive Director, AMF) and Takeshi Furukawa (Executive Director, AMF) for their helpful advice and encouragement, Mai Hibino and Rintaro Ishii (AMF) for their assistance during the study, and Graham S. Hardy (Ngunguru, New Zealand) for his critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Matsuzaki, K., Mori, T., Kai, Y. et al. Morphological development of laboratory-reared Crystallichthys matsushimae (Cottoidei: Liparidae). Ichthyol Res 69, 505–512 (2022). https://doi.org/10.1007/s10228-021-00853-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-021-00853-y