Abstract
Chelidoperca tosaensis is described as a new species based on 84 specimens from Japan and the Philippines taken from depths of 60–302 m. The species can be distinguished from all known congeners by having the following combination of characters: scale rows between lateral line and base of spinous dorsal fin 3; pored lateral-line scales 37–42 (modally 39); scale rows in longitudinal series 39–43 (modally 40); no longitudinal dark stripe or row of dark blotches on body side. The new species is additionally characterized by having a combination of numerous, scattered, yellow spots on dorsal and anal fins with red streak or cluster of reddish spots over bases of about 4–6th dorsal-fin spines; large ocellated red spot with pinkish white border present on membrane between opercular spines; pelvic fin with middle area yellow with whitish spine, and whitish first, second and fifth soft rays; caudal fin with about three transverse rows of yellow spots centrally and posteriorly, two fan-shaped rows of red blotches on basal third, and a pair of white blotches with a pair of yellow blotches between white blotches on the base. Diagnostic characters of Chelidoperca stella, previously known only from its type locality in the Andaman Sea, are updated based on 12 specimens from Taiwan, the Gulf of Tonkin, Vietnam and the Philippines. These specimens represent the first records of the species from the Pacific Ocean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chelidoperca Boulenger 1895 comprises a group of small, bottom-dwelling Indo-Pacific marine fishes of the family Serranidae. The genus is characterized by having a relatively slender, elongate body; dorsal-fin rays usually X, 10; anal-fin rays usually III, 6; and two flattened strong opercular spines. All nine nominal Indo-West Pacific species are currently recognized as valid (Bineesh et al. 2013; Williams and Carpenter 2015; Matsunuma and Motomura 2016): Chelidoperca hirundinacea (Valenciennes in Cuvier and Valenciennes 1831), Chelidoperca pleurospilus (Günther 1880), Chelidoperca investigatoris (Alcock 1890), Chelidoperca margaritifera Weber 1913, Chelidoperca occipitalis Kotthaus 1973, Chelidoperca lecromi Fourmanoir 1982, Chelidoperca maculicauda Bineesh and Akhilesh in Bineesh et al. 2013, Chelidoperca santosi Williams and Carpenter 2015, and Chelidoperca stella Matsunuma and Motomura 2016. The Atlantic Chelidoperca africanus Cadenat 1960 has been reassigned to the genus Serranus Cuvier 1816 (Heemstra and Anderson 2016).
Eighty-four specimens of an unidentified species of Chelidoperca were collected from numerous localities in the northwestern Pacific Ocean, ranging from the Philippines northward to southern Japan. Examination of these specimens revealed that they represent a new species, described herein.
An additional 12 specimens of Chelidoperca stella, previously known only from its type locality in the Andaman Sea, were collected from Taiwan, the Gulf of Tonkin, Vietnam and the Philippines. Morphometric data are reported for these specimens, which represent the first records of the species from the Pacific Ocean.
Materials and methods
Methods of taking counts and measurements generally follow Matsunuma and Motomura (2016) and Matsunuma (2016). The last two rays of the dorsal and anal fins are counted as single rays, each pair being associated with a single pterygiophore. We use soft rays to refer to segmented rays, but use unsegmented and segmented ray designations for the caudal fin to differentiate the soft unsegmented procurrent rays from the soft segmented caudal rays. The dorsal-most scale in the transverse row beneath the spinous dorsal-fin base is often between about 50 % and 100 % of the height of lower adjacent scales (the tiny and irregularly spaced scales at the bases of the spines are not counted; Fig. 1). In this paper, we refer to the slightly smaller dorsal-most scale as a full-size scale and count it as one scale (Fig. 1). In previous studies (Bineesh et al. 2013; Williams and Carpenter 2015; Matsunuma 2016; Matsunuma and Motomura 2016), the variably sized dorsal-most scale in this row has been counted either as a half- or full-size scale depending on its height relative to the next lower adjacent scale. Counting this scale as full size in all species will minimize confusion for this character. Preopercular, interopercular and subopercular serrae (see Matsunuma and Motomura 2016: fig. 1), including a variety of shapes ranging from small spines to indistinct bony protuberances along the distal margins of these bones, are identified as serrae tips. Posttemporal serrae (see Matsunuma and Motomura 2016: fig. 1) include several serrae tips on the exposed posterior bony margin anterior to the first pored lateral-line scale. Counts and measurements are taken on the left side when possible, except for counts of the pectoral-fin rays and head serrae (counted on both sides). Developed gill rakers on the first arch, herein termed “rakers,” include only those with length greater than their base width. Standard length is abbreviated as SL. In the description of Chelidoperca tosaensis, data for the holotype are given first, followed by other specimen data (if different) in parentheses.
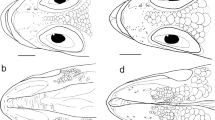
Dorsal portion of body beneath spinous dorsal fin showing scale rows between the lateral line and base of spinous dorsal fin of Chelidoperca tosaensis sp. nov. (a), C. stella (b), C. maculicauda (c) and C. hirundinacea (d). a BSKU 53312, holotype, 82.8 mm SL; b NSMT-P 70495, 68.7 mm SL; c BSKU 95038, 124.1 mm SL; d USNM 306446 (1 of 2 specimens), 114.2 mm SL. Red-outlined scales are included in count of scale rows; blue-outlined small scales are excluded from count
The formula for the configuration of the supraneural bones, anterior neural spines and anterior dorsal pterygiophores follows Ahlstrom et al. (1976). Osteological characters were examined from X-ray photographs of 11 specimens of C. tosaensis (BSKU 52608, 52727, 53312, 54675, 55190, 76443, 76444, 80732, 84452, 121442 and 121443) and six specimens of Chelidoperca stella (FAKU 101095). Meristic data were taken only from specimens marked by an asterisk in the lists of examined specimens. Institutional codes follow Sabaj (2016) with the following additions: Faculty of Fisheries, Nagasaki University (FFNU), Nagasaki; Kazuo Hoshino Private Collection (KHPC), Oita; Kochi Senior High School (HSHS), Kochi; National Museum of Nature and Science (NSMT), Tsukuba; The Museum of Natural and Environmental History (SPMN), Shizuoka; Ichthyology Collection, Museum of Natural Sciences, University of the Philippines Visayas (UPVMI), Miagao; Wakayama Prefectural Museum of Natural History (WMNH), Kainan. Photographs of C. tosaensis referred to in this study for purposes of distributional records are registered in the Image Database of Fishes in KPM (KPM-NR) and uploaded to the Japanese Internet atlas of fishes, WEB sakana-zukan (http://zukan.com/fish/) [see Miyazaki et al. (2014)].
Molecular techniques for DNA extraction and sequencing are those employed at the Smithsonian as described by Carpenter et al. (2017) for genetic analysis of the mitochondrial gene coding for cytochrome c oxidase subunit 1 (COI). Six hundred and fifty-five base-pair fragments from two specimens of the new species [KAUM–I. 86633 (Japan; DDBJ/EMBL/GenBank accession number: MF597717) and USNM 437819 (Philippines; MF597699)], five specimens of Chelidoperca hirundinacea (MF597700–597704), 13 specimens of Chelidoperca santosi [KP150308 (Williams and Carpenter 2015) and MF597705–597716] and one specimen of C. stella (MF597718) were sequenced and compared with the following available sequences (none available for Chelidoperca lecromi, Chelidoperca margaritifera and Chelidoperca pleurospilus) from congeners available in the Barcode of Life Database (BOLD; http://www.boldsystems.org; accessed 22 June 2017) and from DDBJ/EMBL/GenBank accession numbers: C. hirundinacea: JQ681448 and JQ681449 (registered as C. pleurospilus), JQ681476 (Chelidoperca sp.); Chelidoperca investigatoris: JX185305, JX185307, JX185310, JX185312, KP009557–009559; Chelidoperca maculicauda (registered as Chelidoperca sp.; see Bineesh et al. 2013): JX185308, JX185309, JX262929; and Chelidoperca occipitalis: JX185304, JX185306, JX185311, JX185313 [details of sequences used in this study are shown in Electronic Supplemental Material (ESM) Table S1]. Sequence divergence in relation to other known species of Chelidoperca and the full BOLD database of COI sequences was determined by comparing the COI sequences from our two successfully sequenced specimens using tools available on the BOLD database to assist with taxonomic comparisons (including BLAST-Basic Local Alignment Search Tool). Our sequences were compared with all COI sequences in the BOLD database (public and private) to assess sequence similarity/divergence.
Chelidoperca tosaensis sp. nov.
(New Japanese name: Tosa-himekodai; new English name: Red-spot Perchlet) (Figs 1a, 2, 3, 4, 5, 6, 7, 8, 11; Tables 1, 2, 3)
Fresh specimens of Chelidoperca tosaensis sp. nov. a KAUM–I. 150001, paratype, 47.3 mm SL, East China Sea (photo: KAUM); b WMNH 2010 PIS-208, paratype, 66.3 mm SL, Wakayama Prefecture, Japan (photo: Y. Kaji); c BSKU 52608, paratype, 75.3 mm SL, Kochi Prefecture, Japan; d KAUM–I. 150000, paratype, 92.8 mm SL, East China Sea (photo: KAUM); e BSKU 53312, holotype, 82.8 mm SL, Kochi Prefecture, Japan
Distributional map of Chelidoperca tosaensis sp. nov. (stars) and C. stella (circles) in the northwestern Pacific Ocean. Open star indicates type locality of C. tosaensis, Tosa Bay, Kochi Prefecture, Japan. Record of C. tosaensis from Amami-oshima Island, Japan, are based on photographs and literature
Relationships of penultimate soft dorsal-fin ray (a); longest soft dorsal-fin ray (b); penultimate anal-fin soft ray (c); and longest soft anal-fin ray (d) to standard length (mm) in Chelidoperca hirundinacea (squares); C. lecromi (circles with cross); C. occipitalis (crosses); C. pleurospilus (triangles); and C. tosaensis sp. nov. (diamonds). Arrowheads indicate holotype of C. tosaensis
Relationships of numbers of preopercular serrae (a); interopercular serrae (b); and subopercular serrae (c) (counted in both sides) to standard length (mm) in Chelidoperca hirundinacea (squares); C. lecromi (circles with cross); C. maculicauda (plus signs); C. occipitalis (crosses); C. pleurospilus (triangles); C. santosi (asterisks); C. stella (circles); and C. tosaensis sp. nov. (diamonds)
Chelidoperca margaritifera (not Weber): Konishi 2011: 66, unnumbered fig. [off Amami-oshima Island, Ryukyu Islands, Japan; figured photograph also appeared in WEB sakana-zukan (http://zukan.com/fish/leaf16957)].
Holotype. BSKU 53312, 82.8 mm SL, off Tosa Bay, Kochi Prefecture (obtained at Mimase Fishing Port), bottom trawl, 21 Jan. 2001.
Paratypes. 75 specimens, 29.3–92.8 mm SL: JAPAN: Shizuoka Prefecture: MSM-09-194, 76.9 mm SL, off Awa-shima Island, Numazu, Suruga Bay, 20–80 m, H. Kobayashi, line-fishing, 15 Sept. 2009; MSM-09-195, 84.5 mm SL, collected with MSM-09-194, K. Yamada; MSM-17-43, 87.1 mm SL, Suruga Bay, 50 m, S. Mizuno, line-fishing, 25 May 2016; SPMN-PI 40431, 86.6 mm SL, Suruga Bay, 50 m, S. Mizuno, line-fishing, 8 Dec. 2015; SPMN-PI 42124, 83.5 mm SL, collected with MSM-09-194, Y. Itou. Wakayama Prefecture: WMNH 2010 PIS-208, 66.3 mm SL, off Shirasaki, Kii Channel, 60 m, M. Nishiyama, bottom trawl, 11 June 2010. Kochi Prefecture (Tosa Bay): BSKU 52608, 75.3 mm SL, obtained at Mimase Fishing Port, bottom trawl, 28 Nov. 2000; BSKU 52727, 81.7 mm SL, obtained at Mimase Fishing Port, bottom trawl, 1 Dec. 2000; BSKU 54604, 29.3 mm SL, 90 m, RV Toyohata-maru, 4 Apr. 1995; BSKU 54675, 56.2 mm SL, 125 m, RV Kotaka-maru, 14 Mar. 1998; BSKU 55190, 81.9 mm SL, obtained at Mimase Fishing Port, bottom trawl, 18 Oct. 1997; BSKU 76443, 74.8 mm SL, BSKU 76444, 80.3 mm SL, obtained at Mimase Fishing Port, bottom trawl, 23 Nov. 2001; BSKU 80731, 90.9 mm SL, BSKU 80732, 84.4 mm SL, obtained at Kochi City Central Fish Market, 28 Nov. 1992; NSMT-P 129211 (formerly KSHS 590), 76.3 mm SL, obtained at Mimase Fishing Port, bottom trawl, 28 Apr. 1961; NSMT-P 129212 (formerly KSHS 17989), 76.4 mm SL, obtained at Mimase Fishing Port, bottom trawl, 8 Feb. 1979; NSMT-P 129213 (formerly KSHS 18017), 78.8 mm SL, obtained at Mimase Fishing Port, bottom trawl, 8 Mar. 1978; NSMT-P 129214 (formerly KSHS 18058), 61.6 mm SL, obtained at Mimase Fishing Port, bottom trawl, 24 Mar. 1979; NSMT-P 129215 (formerly KSHS 18063), 61.0 mm SL, NSMT-P 129216 (formerly KSHS 18064), 62.4 mm SL, obtained at Mimase Fishing Port, bottom trawl, 23 Mar. 1979; NSMT-P 129217 (formerly KSHS 18469), 73.2 mm SL, NSMT-P 129218 (formerly KSHS 18470), 73.1 mm SL, NSMT-P 129219 (formerly KSHS 18471), 60.6 mm SL, obtained at Mimase Fishing Port, bottom trawl, 3 Apr. 1980; NSMT-P 129220 (formerly KSHS 18505), 75.8 mm SL, NSMT-P 129221 (formerly KSHS 18506), 76.0 mm SL, NSMT-P 129222 (formerly KSHS 18507), 70.8 mm SL, obtained at Mimase Fishing Port, bottom trawl, 5 Apr. 1980; UPVMI 2214 (formerly BSKU 84452), 71.5 mm SL, 125 m, RV Kotaka-maru, 14 Mar. 1998. Yamaguchi Prefecture: NSMT-P 129227 (formerly SNFR 15882), 79.8 mm SL, off Mishima Island, 34°52′30″–53′57″N, 131°01′11″–04′55″E, 87–115 m, RV Tanshu-maru, 25 July 1998. Oita Prefecture: BSKU 121439 (formerly one of KHPC 3590), 52.6 mm SL, BSKU 121440 (formerly one of KHPC 3590), 72.9 mm SL, off Fuka-shima Island (obtained at Kamae Fishing Port), K. Hoshino, bottom trawl, 4 Sept. 2000; BSKU 121441 (formerly KHPC 5268), 72.7 mm SL, off Fuka-shima Island (obtained at Kamae Fishing Port), K. Hoshino, bottom trawl, 24 Sept. 2002; BSKU 121442 (formerly KHPC 4592), 79.9 mm SL, off Fuka-shima Island (obtained at Kamae Fishing Port), K. Hoshino, bottom trawl, 19 Sept. 2001; BSKU 121443 (formerly KHPC 5620), 77.7 mm SL, off Senzaki, Saeki (obtained at Kamae Fishing Port), K. Hoshino, bottom trawl, 16 July 2003; BSKU 121444 (formerly KHPC 5838), 70.7 mm SL, off Senzaki, Saeki (obtained at Kamae Fishing Port), K. Hoshino, bottom trawl, 29 July 2005; BSKU 121445 (formerly KHPC 5914), 73.3 mm SL, off Senzaki, Saeki (obtained at Kamae Fishing Port), K. Hoshino, bottom trawl, 16 July 2003; USNM 440339 (formerly KHPC 3427), 5 specimens, 55.9–80.7 mm SL, off Fuka-shima Island (obtained at Kamae Fishing Port), K. Hoshino, bottom trawl, 5 July 2000. Nagasaki Prefecture: FAKU 58108, 74.9 mm SL, FAKU 58109, 79.5 mm SL, FAKU 58110, 63.6 mm SL, FAKU 58111, 59.1 mm SL, FAKU 58112, 61.3 mm SL, FAKU 58113, 73.6 mm SL, FAKU 58114, 76.7 mm SL, FAKU 58115, 68.5 mm SL, FAKU 58117, 73.5 mm SL, FAKU 58118, 76.5 mm SL, FAKU 58119, 63.4 mm SL, FAKU 58120, 84.5 mm SL, FAKU 58121, 63.9 mm SL, FAKU 58122, 56.1 mm SL, FAKU 58123, 59.0 mm SL, FAKU 58124, 61.2 mm SL, FAKU 58125, 55.9 mm SL, FAKU 58126, 58.4 mm SL, FAKU 58127, 58.7 mm SL, southwest of Tsushima Islands, RV Tanshu-maru, 16 July 1990; NSMT-P 69277, 2 specimens, 44.3–45.5 mm SL, off Tsushima Islands, 34°15′16″N, 129°09′14″E, M. Mitsuhashi, RV Toyoshio-maru, 302 m, 7 Sept. 2003. Japan Sea: FAKU 82646, 65.2 mm SL, Japan Sea (no further data); NSMT-P 129223 (formerly SNFR 11190), 79.1 mm SL, 34°52′45″–54′17″N, 131°01′52″–05′39″E, 80–108 m, RV Tanshu-maru, 16 July 1992; NSMT-P 129224 (formerly SNFR 15117), 80.7 mm SL, 34°31′01″–33′30″N, 130°31′44″–34′27″E, 123 m, RV Tanshu-maru, 11 July 1996; NSMT-P 129225 (formerly SNFR 15753), 4 specimens, 61.3–67.6 mm SL, 34°26′33″–28′49″N, 130°06′55″–09′59″E, 115 m, RV Tanshu-maru, 23 July 1996; NSMT-P 129226 (formerly SNFR 15872), 2 specimens, 74.7–75.6 mm SL, 34°00′55″–57′56″N, 131°00′25″–03′10″E, 102–118 m, RV Tanshu-maru, 25 July 1998. East China Sea: KAUM–I. 86633, 61.9 mm SL, 31°53′00″N, 128°08′43″E, 148–201 m, bottom trawl, 18 June 2014; KAUM–I. 150000, 92.8 mm SL, KAUM–I. 150001, 47.3 mm SL, 31°54′57″N, 128°09′31″E, 152 m, bottom trawl, 11 Dec. 2016. PHILIPPINES: USNM 437819, 69.5 mm SL, off Guimaras and Panay islands (obtained at Iloilo City fish market), tissue sample PHIL-209, J. Williams et al., 3 Sept. 2015.
Non-type specimens: Eight specimens, 53+ – 83.3 mm SL (all from Japan): *FAKU 58116 (head deformed), 53+ mm SL, southwest of Tsushima Island, Nagasaki Prefecture, RV Tanshu-maru, 16 July 1990; KHPC 3438, 78.4 mm SL, off Fuka-shima Island (obtained at Kamae Fishing Port), Oita Prefecture, K. Hoshino, bottom trawl, 5 July 2000; SNFR 14188, 83.3 mm SL, East China Sea, 31°54′57″–54′58″N, 128°09′48″–11′46″E, 152 m, RV Kumamoto-maru, 19 May 2008; SNFR 15195, 5 specimens, 64.1–81.6 mm SL, southwestern Japan Sea, 35°04′22″–06′58″N, 131°00′25″–03′10″E, 130 m, RV Tanshu-maru, 13 July 1994.
Diagnosis. A species of Chelidoperca distinguished from other members of the genus by the following combination of characters: pectoral-fin rays 14–16 (modally 15); pored lateral-line scales 37–42 (39); scale rows in longitudinal series 39–43 (40); scale rows between lateral line and base of sixth dorsal-fin spine 3 (Fig. 1a); preopercular serrae 15–40, interopercular serrae 3–19; subopercular serrae 5–20; interorbital scales reaching to or extending slightly beyond mid-orbit level but not extending beyond anterior margin of orbit (Fig. 2a); scales on ventral surface of lower jaw restricted to angular, no scales on dentary (Fig. 2b); relatively long dorsal- and anal-fin soft rays, penultimate dorsal-fin soft ray 15.9–25.7 (21.1) % of SL, longest dorsal-fin soft ray 16.4–25.7 (21.4) % of SL, penultimate anal-fin soft ray 18.2–26.4 (22.0) % of SL and longest anal-fin soft ray 18.2–26.4 (22.0) % of SL; dorsal-fin with numerous small yellow spots scattered over fin in irregularly diagonal rows, several small to large red blotches forming reddish diagonal streak from middle of 3rd or 4th spine to base of 5th or 6th spine; anal fin with small yellow spots scattered basally and centrally over posterior half of fin (Fig. 3); caudal fin with about 3 irregular columns of small yellow spots alternating with columns of whitish spots on central and distal portions of fin, about 2 narrow columns of red spots on interradial membranes of basal portion of lower lobe of fin, and a pair of large poorly defined white blotches on fin base with a pair of small yellow blotches between them; large ocellated red spot located on opercular membrane; side of body with longitudinal row of about 4 dark red elongate blotches.
Description. Morphometric and meristic values are summarized in Tables 1, 2, 3. Pelvic-fin rays I, 5. Body fusiform, slightly elongated; snout pointed, dorsal profile of snout forming angle of about 40° to horizontal axis of head and body; caudal peduncle relatively long (Figs 3, 4, 5). Orbit large, its dorsal margin included in dorsal contour of head. Mouth large, slightly oblique; posterior margin of maxilla extending beyond a vertical through mid-orbit, but not reaching a vertical through posterior margin of orbit; maxilla expanded posteriorly, with low lateral ridge along dorsal margin; lower jaw slightly protruding beyond upper jaw when mouth closed. Upper jaw with band of about 7 rows (in anterior portion) of small, sharp-tipped conical teeth, tooth band becoming narrow posteriorly, outermost row of teeth enlarged, anteriorly projecting antrorse canines; lower jaw with band of about 4 rows (in anterior portion) of small, sharp-tipped conical teeth, innermost and outermost rows of teeth enlarged canines, band of small teeth narrowing posteriorly to 1 or 2 rows; vomer with V-shaped band of about 5 rows of small conical teeth, with several large canines in posterior row directed posteriorly; palatine with relatively long band of 4 rows of small, sharp-tipped conical teeth. Anterior nostrils situated at middle of snout, with small rounded flap rising from posterior rim; posterior nostril with elliptical opening at anterior border of orbit. Posterior margins of preopercle, interopercle and subopercle finely serrated, serrae on preopercle 26 (31 on right side) (15–40), interopercle 11 (10) (3–19) and subopercle 8 (10) (5–20); number of serrae increasing with growth. Opercle with 2 flat, prominent spines, upper spine slightly longer than lower; angle of upper and lower spines about 40°. Posttemporal with 2 (3 on right side) (2–7) serrae tips at beginning of lateral line, number of serrae increasing with growth.
Body covered with ctenoid scales; lateral line slightly arched over pectoral fin before gradually descending, terminating at caudal-fin base (rarely with one pored lateral-line scale on caudal fin). Uppermost row of body scales along dorsal-fin base always about half size of adjacent lower body scales. Caudal-fin base covered with ctenoid scales (slightly elongate cycloid scales in posterior portion), extending onto fin over about basal two-thirds length of fin. Pectoral fin with ctenoid basal scales, small elongate cycloid scales extending onto fin ventrally. Basal scales absent on dorsal fin, but membranes between 1st and 3rd soft rays covered with row of elongate poorly developed ctenoid or cycloid scales (easily lost). Anal fin without basal scales, but membrane between 2nd and 3rd spines covered with a row of elongate cycloid scales. Pelvic-fin base and membrane without scales. Head generally covered with ctenoid scales, but snout and maxilla naked; scales on ventral surface of lower jaw present on angular, dentary naked; interopercle, subopercle and opercle with ctenoid scales (based on paratype), dorsal portion of opercle with several cycloid scales; interorbital scales mostly cycloid, reaching or slightly extending beyond mid-orbit level, but not extending to anterior margin of orbit. A pair of interorbital canals with numerous small pores along outer margin of interorbital region, canals diverging outward anteriorly and reaching to a point between anterior and posterior nasal pores; small pores of interorbital canal forming about 4 rows (about 2 rows on each side; Fig. 2a). Lower jaw with 1 pore anteriorly on each side of dentary symphysis), followed on each side by 2 pore positions along dentary sensory canal, each position with 2 (sometimes 1) minute pore openings, and a fourth slit-like or rounded pore at angular–dentary junction (Fig. 2b).
Dorsal-fin origin above pectoral-fin base, 4th spine longest, 9th (or rarely 10th) spine shortest; all soft rays branched, subequal in length, 9th (or sometimes 8th, rarely 7th) longest. Anal-fin origin below base of 1st dorsal-fin soft ray, 3rd spine longest; all soft rays branched, 5th (or rarely 4th) ray longest. Posterior tip of dorsal and anal fins slightly extending beyond a vertical through caudal-fin base when fins adpressed. Pectoral fin with uppermost 2 rays unbranched, remaining rays branched, 9th (or 8th) longest, its posterior tip reaching a vertical through anal-fin origin. Pelvic-fin origin below pectoral-fin base; spine entirely covered with skin; all soft rays branched, 2nd longest, elongate, slightly expanded distally, its tip reaching anus when adpressed. Caudal fin truncate, but posterior margin slightly rounded; upper lobe with pointed tip, slightly longer than lower lobe; upper lobe with 6–8 unbranched unsegmented procurrent rays, 2 or 3 unbranched segmented rays and 8 branched segmented rays; lower lobe with 4–7 unbranched unsegmented procurrent rays, 2–4 unbranched segmented rays and 7 branched segmented rays; 19–22 segmented rays in total.
Formula for configuration of supraneural bones, anterior neural spines and anterior dorsal pterygiophores 0/0/0 + 2/1 + 1/1; vertebrae 10 + 14.
Fresh coloration. Based on color photographs of the following specimens, including holotype when fresh or alive (Figs 3, 4): BSKU 52608, BSKU 53312, KAUM–I. 86633, KAUM–I. 150000, KAUM–I. 150001, KHPC 3438, MSM-17-43, SPMN-PI 40431, SNFR 14188, USNM 437819 and WMNH 2010 PIS-208. Head and body reddish pink, becoming whitish ventrally; 5 irregular, broad slightly darker pinkish red bands extend from dorsal profile to about midbody, each band with contrasting dark red, longitudinally rectangular blotch (height of about one scale) at or slightly above lateral midline; about 10–12 small white spots along lateral line; row of 8–10 poorly defined white spots midlaterally and 10 vertically elongate bright white blotches alternating with 10 irregular yellowish orange to reddish orange blotches along ventral portion of body from pectoral-fin axil to base of caudal fin. Upper lip reddish at anterior tip flanked by white (sometimes dusky yellow) bar, followed by about two alternating reddish orange and dusky yellow bars distally on premaxilla; maxilla with triangular red area dorsally and yellow streak extending through length of maxilla ventral to red triangular area; lower jaw with red tip flanked by white bar, then red blotch and yellow from mid jaw to beneath ventral tip of maxilla. Cheek pinkish red with posteroventrally directed diagonal white stripe from posterior border of maxilla toward pelvic-fin base; opercle pinkish red above with white ventrally, large ocellated red spot with pinkish white border present between opercular spines. Dorsal-fin membrane translucent dusky whitish with numerous small yellow spots scattered over fin in irregularly diagonal rows, several small to large red blotches forming reddish diagonal streak from middle of 3rd or 4th spine to base of 5th or 6th spine; soft dorsal fin with narrow whitish distal margin. Anal fin dusky white, with narrow yellow distal margin and about 10 (4–12) small yellow spots with 1st spot at about base of middle rays followed by 1 or 2 rows of yellow spots with 2 or 3 yellow spots along latter two rays from base to about three-quarters length of rays. Number of yellow spots on dorsal and anal fins increasing with growth. Pectoral fin translucent or with pale yellowish tint, small yellowish orange to red blotch basally on middle rays. Pelvic fin with bright white anterior and posterior streaks surrounding yellow area over middle rays, base of fin white. Caudal fin dusky with 3–5 small red or yellowish red spots along dorsal margin and small yellow spots on rays arranged in about 3 irregular columns of small yellow spots alternating with columns of whitish spots on central and distal portions of fin, about 2 narrow columns of red spots on interradial membranes of basal portion of lower lobe of fin, a pair of large poorly defined white blotches on fin base with a pair of small yellow blotches between them, dusky band along distal margin.
Preserved coloration. Based on all examined specimens (Fig. 5). Head and body tan, scales on nape and along dorsal quarter of body with dark distal margins; dusky yellow blotches on upper jaw retained as bands of melanophores. Red and yellow markings on fins retained as dark markings but obscured in long-term preserved specimens, including holotype (Fig. 5b). Small dark blotch on base of 1st dorsal-fin spine (Fig. 5c); 3 (or 4) small dark spots along dorsal margin of caudal fin (Fig. 5a) almost always retained.
Etymology. The specific name tosaensis is derived from the name of the type locality of the species, Tosa Bay, Kochi Prefecture, Japan. The English common name, Red-spot Perchlet, refers to the distinctive ocellated red spot located on the opercular membrane.
Distribution. The species is currently recorded from Japan, including the northern East China Sea, southern Japan Sea and the Pacific coast of central and southern Japan, and the Philippines (around Guimaras and Panay islands) in depths of 60–302 m (Fig. 6). A photograph of a hook-and-line captured individual of Chelidoperca from off Amami-oshima Island in the Ryukyu Islands, Japan, at 210 m provided by a sport fisherman via WEB sakana-zukan was identified here as C. tosaensis. The photograph of the Amami-oshima specimen was also figured by Konishi (2011) as Chelidoperca margaritifera. An adult specimen (KPM-NR 91866) was photographed from Suruga Bay, Shizuoka Prefecture, on the Pacific coast of central Japan at 11 m depth and is also identified here as C. tosaensis.
Comparisons. Meristic character differences are: Chelidoperca tosaensis has 3 scales rows between the lateral line and the middle of the spinous dorsal-fin base (Fig. 1a), whereas there are 4 scale rows in Chelidoperca hirundinacea, Chelidoperca lecromi, and Chelidoperca pleurospilus (Fig. 1d); and 3 scale rows in Chelidoperca investigatoris, Chelidoperca maculicauda, C. margaritifera, Chelidoperca occipitalis, Chelidoperca santosi and Chelidoperca stella (Figs. 1b and c). Chelidoperca tosaensis has 37–42 (modally 39) pored lateral-line scales [vs. 33–36 (usually 34 or 35) in C. stella, 40–43 (43) in C. pleurospilus; and 42 or more in C. hirundinacea, C. investigatoris, C. lecromi, C. maculicauda, C. margaritifera, C. occipitalis and C. santosi] and scale rows in the longitudinal series [39–43 (40) vs. 34–38 (35 or 36) in C. stella, 42–47 (45) in C. pleurospilus, 43–45 (44) in C. occipitalis and 44 or more in C. hirundinacea, C. lecromi and C. maculicauda] (Bineesh et al. 2014; this study) (Table 2). Chelidoperca tosaensis has pectoral-fin rays 14–16 (modally 15), while C. hirundinacea has 15–17 (modally 16), although one specimen (OMNH 13827) of C. hirundinacea abnormally possesses 14 and 16 pectoral-fin rays in the left and right sides, respectively. Chelidoperca tosaensis has modally fewer upper gill rakers, including all rudiments 4–6 (modally 5) [vs. 6–8 (modally 7) in C. maculicauda]; fewer developed gill rakers 6–8 (modally 7) [vs. 7–9 (modally 8) in C. hirundinacea and C. pleurospilus; 9 or 10 in C. maculicauda; and 8 or 9 (modally 8) in C. occipitalis]; and total gill rakers, including all rudiments 14–19 (modally 16) [vs. 20–22 (modally 20) in C. maculicauda] (this study).
Morphometric character differences: C. tosaensis has relatively long dorsal- and anal-fin soft rays. Chelidoperca tosaensis differs from its congeners, except for C. margaritifera, C. maculicauda, C. investigatoris and C. santosi, in having a slightly longer penultimate dorsal-fin soft ray 15.9–25.7 % (mean 21.1 %)of SL [vs. 14.4–18.7 % (16.7 %) of SL in C. hirundinacea; 13.7–19.8 % (18.1 %) of SL in C. pleurospilus]; longest dorsal-fin soft ray 16.4–25.7 % (21.4 %) of SL [vs. 16.0–18.7 % (17.7 %) of SL in C. hirundinacea; 16.6–19.8 % (18.5 %) of SL in C. pleurospilus]; penultimate anal-fin soft ray 18.2–26.4 % (22.0 %) of SL [vs. 15.9–23.2 % (18.7 %) of SL in C. hirundinacea; 15.6–16.5 % (16.1 %) of SL in C. lecromi; 17.7–19.2 % (18.3 %) of SL in C. occipitalis; and 16.6–19.9 % (17.6 %) of SL in C. pleurospilus]; and longest anal-fin soft ray 18.2–26.4 (22.0) % of SL [vs. 17.4–23.4 (19.2) % of SL in C. hirundinacea; 16.2–16.7 (16.5) % of SL in C. lecromi; 17.7–19.2 (18.3) % of SL in C. occipitalis; and 16.6–19.2 (17.6) % of SL in C. pleurospilus] (Fig. 7). Chelidoperca tosaensis differs from C. maculicauda and C. occipitalis in having relatively shorter postorbital length 17.4–20.6 % (mean 19.1 %) of SL [vs. 22.6–23.6 % (23.2 %) of SL in C. maculicauda and 22.3–23.6 % (23.0 %) of SL in C. occipitalis]. Chelidoperca tosaensis is not known to exceed 93 mm SL (large adults of C. hirundinacea, C. lecromi and C. pleurospilus reach an SL exceeding 93 mm).
The number of serrae on some bones of the head has been regarded as diagnostic characters for some species of Chelidoperca [see Akazaki (1972), Matsunuma (2016) and Matsunuma and Motomura (2016)]. Although the number of serrae on preopercle, interopercle and subopercle tends to increase with growth and broadly overlap at similar sizes for most species (Fig. 8), C. tosaensis differs from C. stella in having relatively fewer preopercular serrae 15–40 (mean 29.5) at 29.3–92.8 mm-SL [vs. 25–52 (38.9) in 39.0–72.3 mm-SL C. stella] and subopercular serrae 5–20 (11.1) [vs. 14–45 (24.9) in C. stella].
Head squamation is useful for distinguishing some of the species of Chelidoperca, as stated by Akazaki (1972), Matsunuma (2016) and Matsunuma and Motomura (2016). In C. tosaensis, the anteriormost interorbital scales almost reach, or extend slightly beyond, the level of the mid-orbit (Fig. 2a), but in C. hirundinacea, C. investigatoris and C. maculicauda they extend far beyond the level of the mid-orbit, reaching the level of the anterior margin of the orbit (Akazaki 1972; Bineesh et al. 2014: fig. 3; Matsunuma 2016: fig. 3A; this study), and in C. lecromi they reach the posterior nasal pores (Fourmanoir 1982; this study). Chelidoperca tosaensis has lower jaw scales on the ventral surface of the angular, but lacks scales on the dentary at all sizes (Fig. 2b), whereas at least some scales are usually present posteriorly on the dentary in C. hirundinacea, C. pleurospilus, C. santosi, C. maculicauda, C. margaritifera and C. stella (Williams and Carpenter 2015; Matsunuma and Motomura 2016: fig. 3; Matsunuma 2016: fig. 3B; this study).
In coloration, C. tosaensis can be distinguished from C. investigatoris, C. occipitalis and C. pleurospilus by having no longitudinal stripe or dashed line of black blotches midlaterally along the body, which are present in the latter species (Bineesh et al. 2014; Matsunuma 2016; this study). The combination of markings on the head, body and fins of C. tosaensis is unique among the species of Chelidoperca. Chelidoperca tosaensis also differs from C. hirundinacea and C. maculicauda by having an almost truncate upper caudal-fin lobe, elongate and pointed in C. hirundinacea and rounded in C. maculicauda (Bineesh et al. 2013; Matsunuma 2016; this study). Chelidoperca maculicauda and C. santosi also differ in having broad red bands (Bineesh et al. 2013; Williams and Carpenter 2015) on the body compared to the irregular pinkish red bands with red rectangular blotches midlaterally on C. tosaensis.
Identifications of C. margaritifera and C. santosi. Chelidoperca margaritifera was originally described by Weber (1913) based solely on the holotype from the Ceram Sea between Misool Island and New Guinea. This area is currently in the region of the Bird’s Head Peninsula Marine reserve, an area known for its high level of endemism (Allen and Erdmann 2009). Fowler (1928) and Weber and de Beaufort (1931) referred to the original description of the species. Akazaki (1972), subsequently, reviewed the species of Chelidoperca in Japan and identified two specimens from off Tanega-shima Island, Kagoshima Prefecture, southern Japan as C. margaritifera. Katayama (1984) and Senou (2013) followed Akazaki (1972) and included C. margaritifera among Japanese serranid fishes. Although some of Akazaki’s (1972) specimens of Chelidoperca are now deposited at the Maizuru Fisheries Research Station, Kyoto University (FAKU), the two specimens of C. margaritifera listed by Akazaki (1972) were not found in the FAKU collection and are apparently missing. The species subsequently was recorded from Vietnam (Orsi 1974), the Philippines (Fourmanoir 1985), the Andaman Sea (Satapoomin 2011) and New Caledonia (Fricke et al. 2011), but only in their checklists. Yamakawa (1985) identified his specimens as C. margaritifera and described them based on three specimens from the Okinawa Trough in the East China Sea. Yamada and Horikawa (1999) identified a single specimen from the East China Sea as C. margaritifera and provided a color photograph of the fresh specimen for the first time. Yamada et al. (2007) also recorded the species from the East China Sea and described the fresh coloration. No other records of C. margaritifera with specimen descriptions have previously been reported. All extralimital specimens previously identified as C. margaritifera that we have examined have proven to be referrable to C. tosaensis, C. santosi or C. stella. This is not surprising as these three species had not been described when the earlier studies were published, so there were no other names available for these specimens when they were misidentified. We have not examined any specimens of Chelidoperca that share the diagnostic characters possessed by the holotype of C. margaritifera, and no additional specimens of Chelidoperca have been collected from the Misool region that might provide evidence for character variation. Although we have not been able to examine all of the specimens previously identified as C. margaritifera, to avoid confusion we tentatively consider all of these extralimital records to be misidentifications until confirmed identifications are made or additional C. margaritifera are collected from the Misool region.
Williams and Carpenter (2015) described Chelidoperca santosi based on the holotype and two paratypes from the Philippines. Matsunuma (2017) also recorded and figured C. santosi from Panay Island in the Philippines. Williams and Carpenter (2015) stated that C. margaritifera differs from C. santosi in having a yellow stripe and small white spots below the stripe on the body side, whereas such markings are absent in the latter when fresh or preserved. Their color description of C. margaritifera was based on Weber (1913). The color description of the species provided by Weber (1913) was based solely on the relatively freshly preserved holotype and only known specimen and some colors had probably faded before Weber’s examination. Photographs of the preserved holotype of C. margaritifera (ZMA 101029, 51.3 mm SL) indicate that it possesses a pale stripe along the ventrolateral portion of the body from the posterior tip of the opercle to the caudal peduncle, but the rows of white spots above and below the stripe, originally described by Weber (1913), are no longer evident. Specimens of C. santosi lack this stripe in both fresh and preserved conditions (Williams and Carpenter 2015). The second author’s examination of the holotype of C. margaritifera, in combination with examination of high-resolution images provided to the authors by Ronald de Ruiter and Mao-Ying Lee, resulted in recognition of several additional characters that differ between C. margaritifera and C. santosi. Chelidoperca santosi is diagnosed as having two pairs of small dark spots anteriorly on the snout and a pair of small dark spots on the chin [see Williams and Carpenter (2015: fig. 1); Fig. 9a, b], with the melanophores of these dark spots retained on preserved specimens, whereas these melanophores are absent on the holotype of C. margaritifera, although other melanophores are present on the body (Fig. 10a–c). Chelidoperca santosi also differs from C. margaritifera in having more posttemporal serrae 3–12 [usually 5–7 (rarely 3; the smallest 39.5 mm SL specimen has 2–4, but is not included in range)] on each side [the holotype of C. margaritifera (51.3 mm SL) with 2 serrae on each side] and in having an outer row of anteriorly directed, antrorse canine teeth in the upper jaw and an outer row of dorsally directed enlarged canines in the lower jaw (Fig. 9c) [no outer row of canines in either jaw of C. margaritifera, but there are several mesially directed enlarged canines posteriorly in the lower jaw. (Fig. 10c, d)]. The existence of enlarged canines in the jaws is useful as a diagnostic character for the species of Chelidoperca. Chelidoperca hirundinacea lacks an outer row of enlarged canines in either jaw similar to C. margaritifera, and has several rows of small interorbital scales extending to posterior nostrils (scales extend in a single row almost to anterior margin of orbit, Fig 10b, c, on C. margaritifera).
Based on these morphological differences between C. margaritifera and C. santosi, the photographs of the East China Sea specimens recorded by Yamada and Horikawa (1999) and Yamada et al. (2007) as C. margaritifera are consistent with C. santosi, as the specimens possess the diagnostic features of C. santosi. An additional 29 non-type specimens listed as comparative material from several localities from the northwestern Pacific Ocean, including specimens from the East China Sea and the Philippines, previously identified by Matsunuma and Motomura (2016) and Matsunuma (2016) as C. margaritifera, are all consistent with C. santosi. Among them, three specimens from the East China Sea (KAUM–I. 81603, 81604 and 81683; Fig. 9) are also identical with C. santosi based on COI sequence data. Therefore, the above-mentioned previous records of C. margaritifera from Japanese waters are referable to C. santosi. Akazaki’s (1972) specimens of C. margaritifera are not available for this study and a definitive identification of the specimens is not possible based on his brief description. As no confirmed specimens of margaritifera have been captured from Japanese waters, Akazaki’s specimens are almost certainly misidentifications and his records should be disregarded until his specimens can be examined to determine their correct identification. Additional specimens of C. margaritifera from the Bird’s Head Peninsula region are needed before the species can be more thoroughly redescribed.
Chelidoperca tosaensis can be distinguished from C. margaritifera and C. santosi by several morphometric and meristic characters as well as by coloration. Chelidoperca tosaensis can be distinguished from the holotype of C. margaritifera (51.3 mm SL) by having relatively narrower interorbital width 2.3–3.3 (mean 2.9) % of SL (vs. 3.9 % of SL in the latter) and fewer interopercle and subopercle serrae 3–10 and 5–12, respectively, in 50–60 mm-SL specimens [vs. 14 (10 on right side) and 16, respectively]. Although there is overlap, C. tosaensis has modally fewer pored lateral-line scales 37–42 (modally 39; see Table 1), compared to the holotype of C. margaritifera with 42 pored lateral-line scales. Chelidoperca tosaensis and C. santosi have similar dentition, but differ by having relatively fewer pored lateral-line scales 37–42 (modally 39) [vs. 42–46 (modally 44) in the latter]; circumpeduncular scale rows 17–20 (18) [vs. 19–22 (modally 20)]; preopercular serrae 15–40 (modally 30 for specimens over 60 mm SL) [vs. 29–60 (modally 45 for specimens over 60 mm SL)]; no dark spot on the snout and chin (vs. 2 pairs of dark spots on the snout and a pair of spots on the chin); and the dorsal-fin soft-rayed portion dusky whitish with numerous yellow spots (vs. pale yellow with a longitudinal row of translucent spots dorsally) (Williams and Carpenter 2015; this study; Figs 3, 9; Table 2).
Chelidoperca sp. 2 described by Sainsbury et al. (1985) from the Timor Sea is primarily distinguished from C. tosaensis by having 45–57 pored lateral-line scales (vs. 37–42 in the latter) and a broad yellow longitudinal band along the body side (vs. absent). Chelidoperca sp. 1 recorded by Gloerfelt-Tarp and Kailola (1984) from off Indonesia and northwestern Australia is also readily distinguished from C. tosaensis by having 46 or 47 pored lateral-line scales (vs. 37–42 in the latter) and 2 or 3 rows of dark olive streaks along the dorsal fin (vs. absent). Chelidoperca sp. 1 and Chelidoperca sp. 2 described by Sainsbury et al. (1985) and Gloerfelt-Tarp and Kailola (1984), respectively, from off northwestern Australia are most similar to C. stella in having 35 or 36 pored lateral-line scales [see “Remarks” for C. stella and Matsunuma and Motomura (2016)].
Morphological changes with growth. The numbers of serrae on the head tend to increase with growth in C. tosaensis, although the number of serrae on each bone is highly variable (see Fig. 8). The smallest examined specimen (BSKU 54604, 29.3 mm SL) of C. tosaensis possesses 15 preopercular serrae, 3 interopercular serrae, 6 subopercular serrae and 2 posttemporal serrae, whereas there are 33, 12, 10 and 5, respectively, in the largest examined specimen (KAUM–I. 150000, 92.8 mm SL). In morphometric measurements, relative lengths (% of SL) of orbit diameter, maxilla depth and pelvic-fin spine tend to decrease with growth (Fig. 11a), whereas suborbital depth, lengths of penultimate dorsal- and anal-fin soft rays, and lengths of longest dorsal- and anal-fin soft rays all increase with growth (Fig. 11b) in C. tosaensis. Similar trends were also recognized in the examined specimens of C. stella. No other significant relationships were recognized between the other morphometric characters and standard length in C. tosaensis. Small specimens less than about 50 mm SL of C. tosaensis differ from large specimens in having a faint dusky yellowish stripe with about four red blotches formed where the stripe crosses the irregular body bands extending along mid-body from the posterior tip of the opercle to the caudal-fin base (usually retained in preserved specimens) (Fig. 3a), whereas this stripe is broken into dark red rectangular blotches in larger specimens (Fig. 3b–e).
Molecular analysis results. A comparison of the COI sequences from the Philippine and Japanese specimens against the BOLD database using the BLAST technique revealed that these two sequences are nearly identical, diverging from each other by about 0.3 %. The next most similar sequences are a group of three specimens (private dataset in BOLD) from Vanuatu that are not identified to species, but are likely C. lecromi based on the locality, and these diverge from C. tosaensis by almost 10 % sequence divergence. The next most similar group is slightly more than 10 % divergent and is identified as C. hirundinacea [26 nearly identical samples ranging from China and the Philippines to northwestern Australia; most are private and five specimens (USNM 437778, 437790, 437792, 437794, 437834) from the Philippines are confirmed to be this species by the third author]. Other species of Chelidoperca that are shown to be more than 10 % divergent from C. tosaensis based on the BOLD analysis (BLAST against all public and private sequences in BOLD with GenBank sequences included) include C. hirundinacea, C. investigatoris, C. maculicauda, C. occipitalis, C. santosi and C. stella.
Chelidoperca stella Matsunuma and Motomura 2016
(Figs 1b, 6, 8, 11, 12; Tables 1, 2, 4)
Chelidoperca stella Matsunuma and Motomura 2016: 388, figs 1–7 (type locality: off Thailand, Andaman Sea).
Material examined. Twelve specimens, 39.0–72.3 mm SL: TAIWAN: NMMB-P 22749, 2 specimens, 66.0–72.3 mm SL, off Kaohsiung, H.-C. Ho, 11 Feb. 2015. GULF OF TONKIN: FAKU 101095, 6 specimens, 39.0–71.3 mm SL, 31 May–6 Aug 1957; NSMT-P 63938, 48.7 mm SL, Sanya Bay, south coast of Hainan Island, China, 24 Nov. 1997. VIETNAM: NSMT-P 70495, 68.7 mm SL, Nha Trang, 7 Oct. 2004. PHILIPPINES: KAUM–I. 69436, 42.0 mm SL, off Iloilo, Panay Island (obtained at Miagao Market, Iloilo), 24 Feb. 2015; NSMT-P 110259, 59.0 mm SL, Philippines (no further locality), 24 Mar. 1975.
Diagnosis. A species of Chelidoperca distinguished from other members of the genus by the following combination of characters: pectoral-fin rays 15 or 16 (modally 15); pored lateral-line scales 33–36 (34 or 35); scale rows in longitudinal series 34–38 (34 or 35); scale rows between lateral line and sixth dorsal-fin spine base 3; preopercular serrae 24–52; interopercular serrae 5–24; subopercular serrae 14–45; interorbital scales slightly extending anteriorly, almost reaching or slightly beyond level of mid-orbit; scales on ventral surface of lower jaw covering the angular; relatively wide interorbital region, its width 2.7–3.7 (mean 3.2) % of SL; length of longest anal-fin soft ray moderate, 16.4–21.6 (18.5) of % SL; 2 longitudinal rows of white blotches, containing numerous melanophores, along trunk; pelvic fin white with several yellow spots or bands; no longitudinal dark stripe of blotches laterally on body sides.
Remarks. Morphometric and meristic values for the new records are listed in Tables 1, 2 and 4. Other meristic and osteological characters for these specimens are as follows: pelvic-fin rays I, 5; formula for configuration of supraneural bones, anterior neural spines and anterior dorsal pterygiophores 0/0/0 + 2/1 + 1/1/1 (0/0/0 + 2/1 + 1/1 + 1 /1 in one of six examined specimens); vertebrae 10 + 14 (11 + 13 in one of six examined specimens). The present specimens from the northwestern Pacific Ocean (Fig. 6) were identified as C. stella by having the diagnostic characters of the species described in the original description. As the species was previously known only from its type locality in the Andaman Sea, eastern Indian Ocean, the present specimens represent the first records for the species from the Pacific Ocean. Matsunuma and Motomura (2016) stated that Chelidoperca sp. 1 briefly described by Sainsbury et al. (1985) from off northwestern Australia in the southeastern Indian Ocean may be identical to C. stella. A photograph of Chelidoperca sp. 2 recorded by Gloerfelt-Tarp and Kailola (1984) from off northwestern Australia is the same as that of Sainsbury et al.’s (1985) Chelidoperca sp. 1. Chelidoperca stella is probably widely distributed in the eastern Indian and western Pacific oceans.
The present specimens from the northwestern Pacific Ocean slightly differ from the Andaman Sea specimens in having relatively larger orbit diameter (11.5–14.2 % of SL in the former vs. 10.3–11.4 % of SL in the latter) and darker pelvic fins. Such differences are tentatively regarded herein as geographic variation within the species. Specimens from a geographic distributional gap between the presently known distributional ranges (the Arafura and Timor seas and the southern South China Sea) may provide overlapping values between geographically disjunct known populations.
Newly recognized diagnostic characters. The diagnostic characters of C. stella stated in the original description are enhanced by these additional specimens. Excellent photographs of specimens from Vietnam and the Philippines clearly show the fresh coloration of the species. The Vietnamese specimen (NSMT-P 70495, 68.7 mm SL; Fig. 12b) has about three very narrow red stripes midlaterally from just above and beneath the pectoral fin reaching posteriorly to about the caudal peduncle; it also has two longitudinal rows of poorly defined small white blotches along the ventral portion of the trunk. The upper row is located along the mid-body from the upper portion of the pectoral-fin base to the middle of the caudal-fin base. The lower row is located along the ventral portion of the trunk from the abdomen to the lower portion of the caudal-fin base. The white blotches on the trunk contain numerous minute melanophores (retained in the preserved specimens). In a small specimen from the Philippines (KAUM–I. 69436, 42.0 mm SL; Fig. 12a), most of the body scales are missing, but it has about three very narrow red stripes midlaterally from just above and beneath the pectoral fin reaching posteriorly to about the caudal peduncle. Although C. stella was originally diagnosed as having the pelvic fin with several small yellow spots on the basis of a photograph of the fresh paratype (Matsunuma and Motomura 2016: fig. 4), the photographs of the additional fresh specimens reveal that the species possess 2 or 3 short yellow bands alternating with similar sized whitish bands on the pelvic fin, rather than yellow spots (Fig. 12).
Comparative material examined. Chelidoperca hirundinacea (57 specimens, including 2 syntypes, 17.6–145.0 mm SL): JAPAN: Shizuoka Prefecture: SPMN-PI 40109, 113.6 mm SL, off Yaizu; SPMN-PI 41879, 116.9 mm SL, Lake Hamana; SPMN-PI 42122, 135.2 mm SL, Suruga Bay, off Awa-shima Island, Numazu. Aichi Prefecture: FAKU 132267, 145.0 mm SL, off Isshiki; FAKU 136519, 144.1 mm SL, Mikawa Bay. Wakayama Prefecture: OMNH 15475, 83.4 mm SL, OMNH 15478, 120.5 mm SL, north of Tomoga-shima Island; OMNH 13826, 132.3 mm SL, OMNH 13827, 129.4 mm SL, OMNH 13828, 114.3 mm SL, off Arita. Kochi Prefecture (Tosa Bay): BSKU 54603, 17.6 mm SL; *BSKU 75108, 98.9 mm SL; BSKU 78672, 47.7 mm SL; *BSKU 78227, 133.5 mm SL; *BSKU 89706, 128.9 mm SL; *BSKU 90605, 127.5 mm SL; *BSKU 90913, 128.7 mm SL; *BSKU 92710, 130.1 mm SL; *BSKU 94228, 71.9 mm SL; *BSKU 94297, 117.1 mm SL; *BSKU 95038, 124.1 mm SL; *BSKU 95470, 112.9 mm SL; KAUM–I. 81742, 49.6 mm SL; KAUM–I. 81743, 45.3 mm SL; KAUM–I. 81744, 43.1 mm SL; KAUM–I. 81756, 52.4 mm SL. Kyoto Prefecture: FAKU 131856, 114.1 mm SL, off Kyoga-misaki; FAKU 132439, 105.5 mm SL, off Ine; FAKU 135373, 99.3 mm SL, off Kammuri-jima Island, Wakasa Bay. Nagasaki Prefecture: FAKU 134652, 112.6 mm SL, northeast of Tsushima Islands; *FFNU P-881, 2 specimens, 30.2–49.4 mm SL, off Fukue-jima Island; *FFNU P-1210, 3 specimens, 96.2–128.7 mm SL. Japan Sea: SNFR 15879, 5 specimens, 46.7–86.7 mm SL. TAIWAN: NSMT-P 115036, 139.5 mm SL, off Pingtung. PAPUA NEW GUINEA: ASIZP 73871, 76.9 mm SL, off Lae. Other 15 specimens were listed in Matsunuma and Motomura (2016). Chelidoperca lecromi (2 specimens, including the holotype, 111.8–125.3 mm SL): listed in Matsunuma and Motomura (2016). Chelidoperca maculicauda (8 specimens, 85.3–114.2 mm): listed in Matsunuma (2016). Chelidoperca margaritifera: ZMA 101029, holotype, 51.3 mm SL, between Misool and New Guinea islands, Indonesia (some measurements and meristics only taken). Chelidoperca occipitalis (6 specimens, 65.9–89.9 mm SL): listed in Matsunuma (2016). Chelidoperca pleurospilus (48 specimens, 16.5–128.2 mm SL): JAPAN: Shizuoka Prefecture MSM-10-584, 113.9 mm SL, Suruga Bay. Kochi Prefecture (Tosa Bay): BSKU 30185, 36.9 mm SL; *BSKU 36114, 128.2 mm SL; *BSKU 36148, 122.7 mm SL; *BSKU 39625, 100.0 mm SL; *BSKU 39646, 85.8 mm SL; *BSKU 40023, 107.5 mm SL; *BSKU 40024, 95.3 mm SL; BSKU 41921, 42.3 mm SL; BSKU 43439, 16.5 mm SL; *BSKU 51438, 113.0 mm SL; *BSKU 53315, 116.9 mm SL; *BSKU 62639, 113.2 mm SL; *BSKU 62640, 128.2 mm SL; BSKU 64448, 36.7 mm SL; BSKU 64449, 39.0 mm SL; *BSKU 68295, 116.0 mm SL; *BSKU 68644, 128.0 mm SL; *BSKU 80094, 86.7 mm SL; *BSKU 80098, 92.5 mm SL; BSKU 120632, 29.8 mm SL; BSKU 120634, 27.6 mm SL; BSKU 120636, 22.8 mm SL; BSKU 120637, 30.7 mm SL; BSKU 120638, 20.4 mm SL; BSKU 120640, 25.3 mm SL; BSKU 120641, 24.8 mm SL; BSKU 120642, 28.7 mm SL. Kagoshima Prefecture: FAKU 26194, 112.4 mm SL, off Tanega-shima Island, Kagoshima Prefecture, Japan. Okinawa Prefecture: URM 31205, 100.9 mm SL. EAST CHINA SEA: FAKU 81431, 122.0 mm SL; FAKU 85502, 107.0 mm SL. NO DATA: FAKU 24478, 65.8 mm SL; FAKU 76583, 115.1 mm SL. Other 13 specimens were listed in Matsunuma and Motomura (2016). Chelidoperca santosi (35 specimens, 52.6–95.5 mm SL): JAPAN: Kochi Prefecture: BSKU 69837, 92.7 mm SL, BSKU 92585, 54.0 mm SL, FAKU 136360, 85.6 mm SL, Tosa Bay. Oita Prefecture: KHPC 6018, 61.7 mm SL, off Senzaki, Saeki. EAST CHINA SEA: BSKU 27428, 77.1 mm SL, BSKU 27429, 66.9 mm SL, Okinawa Trough; KAUM–I. 35776, 89.0 mm SL; KAUM–I. 81603, 87.1 mm SL; KAUM–I. 81604, 77.0 mm SL; KAUM–I. 81684, 95.5 mm SL; KAUM–I. 81685, 93.7 mm SL; KAUM–I. 87048, 83.0 mm SL; KAUM–I. 88497, 77.7 mm SL; SNFR 1645, 84.8 mm SL; SNFR 12277, 81.4 mm SL; SNFR 14074, 85.2 mm SL; SNFR 12295, 70.8 mm SL; SNFR 16454, 90.2 mm SL; SNFR 16455, 70.7 mm SL; URM-P 35684, 80.4 mm SL. TAIWAN: NMMB-P 21104, 68.5 mm SL, off Donggang, Pingtung; NMMB-P 22803, 52.6 mm SL, off Kaohsiung; URM-P 23108, 86.4 mm SL, off Tainan. SOUTH CHINA SEA: BSKU 17276, 84.7 mm SL, BSKU 17277, 75.0 mm SL, ca. 300 km north of Natuna Islands. PHILIPPINES: PNM 15190, holotype, 69 mm SL, vicinity of Palawan; UPVMI 1169, 84.3 mm SL, UPVMI 1170, 87.3 mm SL, UPVMI 1171, 87.0 mm SL, UPVMI 1172, 86.8 mm SL, UPVMI 1173, 78.1 mm SL, UPVMI 1174, 77.6 mm SL, UPVMI 1175, 75.7 mm SL, UPVMI 1176, 65.4 mm SL, off Antique, Panay Island; USNM 427531, paratype, 81.6 mm SL, off Mindanao, Bohol Sea.
References
Ahlstrom EH, Butler JL, Sumida BY (1976) Pelagic stromateoid fishes (Pisces, Perciformes) of the eastern Pacific: kinds, distributions, and early life histories and observations on five of these from the northwest Atlantic. Bull Mar Sci 26:285–402
Akazaki M (1972) A critical study of the serranid fishes of the genus Chelidoperca found in Japan. Jpn J Ichthyol 19:174–282
Allen GR, Erdmann MV (2009) Reef fishes of the Bird’s Head Peninsula, West Papua, Indonesia. Check List 5:587–628
Bineesh KK, Akhilesh KV, Abdussamad EM, Pillai NGK (2013) Chelidoperca maculicauda, a new species of perchlet (Teleostei: Serranidae) from the Arabian Sea. Aqua 19:71–78
Bineesh KK, Akhilesh KV, Abdussamad EM, Pillai NGK, Thiel R, Jena JK, Gopalakrishnan A (2014) Redescriptions of Chelidoperca investigatoris (Alcock, 1890) and Chelidoperca occipitalis Kotthaus, 1973 (Perciformes: Serranidae) from the southwest coast of India. Indian J Fish 61:117–122
Bineesh KK, Mohitha C, Vineesh N, Basheer VS, Joselet M, Pillai NKG, Jena JK, Gopalakrishnan A (2015) Molecular identification of three deepsea fish species of the genus Chelidoperca (Perciformes: Serranidae) from Indian water. Indian J Fish 62:104–108
Carpenter KE, Williams JT, Santos MD (2017) Acanthurus albimento, a new species of surgeonfish (Acanthuriformes: Acanthuridae) from northeastern Luzon, Philippines, with comments on zoogeography. J Ocean Sci Found 25:33–46
Fourmanoir P (1982) Trois nouvelles espèces de Serranidae des Philippines et de la Mer du Corail Plectranthias maculatus, Plectranthias barroi, Chelidoperca lecromi. Cybium 6:57–64
Fourmanoir P (1985) Poissons. Liste et description de cinq espèces nouvelles (MUSORSTOM II). Memoir Mus Natl Hist Nature Ser A Zool 133:31–54
Fowler HW (1928) The fishes of Oceania. Mem Bernice P Bishop Mus 10:i–iii, 1–540, pls 1–49
Fricke R, Kulbicki M, Wantiez L (2011) Checklist of the fishes of New Caledonia, and their distribution in the southwest Pacific Ocean (Pisces). Stuttg Beitr Naturkunde A New Ser 4:341–463
Gloerfelt-Tarp T, Kailola PJ (1984) Trawled fishes of southern Indonesia and northwestern Australia. Australian Development Assistance Bureau (ADAB), Directorate General of Fisheries, Indonesia (DGF), and German Agency for Technical Cooperation (GTZ), Jakarta
Heemstra PC, Anderson WD (2016) Serranidae. Groupers (seabass, hinds, creolefish, combers, anthiines, soapfish). In: Carpenter KR, De Angelis N (eds) The living marine resources of the Eastern Central Atlantic. Vol 4. Bony fishes pt 2 (Perciformes to Tetradontiformes) and sea turtles. FAO species identification guide for fishery purposes. FAO, Rome, pp 2365–2413
Katayama M (1984) Chelidoperca margaritifera. In: Masuda H, Amaoka K, Araga C, Uyeno T, Yoshino T (eds) The fishes of the Japanese Archipelago. Tokai University Press, Tokyo, p 130, pl 348-H
Konishi H (2011) Atlas of 1400 fishing fish species. Enterbrain, Tokyo
Matsunuma M (2016) Records of Chelidoperca maculicauda and C. occipitalis (Serranidae) from the Arabian Sea, with comments on diagnostic characters. Spec Divers 21:161–170
Matsunuma M (2017) Chelidoperca santosi. In: Motomura H, Alama UB, Muto N, Babaran RP, Ishikawa S (eds) Commercial and bycatch market fishes of Panay Island, Republic of the Philippines. The Kagoshima University Museum, Kagoshima, University of the Philippines Visayas, Iloilo, and Research Institute for Humanity and Nature, Kyoto, p 83
Matsunuma M, Motomura H (2016) Chelidoperca stella, a new species of perchlet (Perciformes: Serranidae) from the Andaman Sea, eastern Indian Ocean. Zootaxa 4092:388–400
Miyazaki Y, Murase A, Shiina M, Naoe K, Nakashiro R, Honda J, Yamaide J, Senou H (2014) Biological monitoring by citizens using Web-based photographic databases of fishes. Biodivers Conserv 23:2383–2391
Orsi JJ (1974) A check list of the marine and freshwater fishes of Vietnam. Publ Seto Mar Biol Lab 21:153–177
Sabaj MH (2016) Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Version 6.5 (16 August 2016). American Society of Ichthyologists and Herpetologists, Washington, DC. http://www.asih.org/. Accessed 30 August 2016
Sainsbury K, Kailola PJ, Leyland GG (1985) Fishes of northern and north-western Australia. CSIRO, Canberra
Satapoomin U (2011) The fishes of southwestern Thailand, the Andaman Sea–A review of research and a provisional checklist of species. Phuket Mar Biol Cent Res Bull 70:29–77
Senou H (2013) Serranidae. Groupers, basslets and soapfishes. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species. 3rd edn. Tokai University Press, Hadano, pp 757–802, 1960–1971
Wang Z-D, Guo Y-S, Liu X-M, Fan Y-B, Liu C-W (2012) DNA barcoding South China Sea fishes. Mitochondrial DNA 23:405–410
Weber M (1913) Die fische der Siboga-Expedition. E. J. Brill, Leiden
Weber M, de Beaufort LF (1931) The fishes of the Indo-Australian Archipelago. VI. Perciformes (continued). E. J. Brill, Leiden
Williams JT, Carpenter KE (2015) A new fish species of the subfamily Serraninae (Perciformes, Serranidae) from the Philippines. Zootaxa 3911:287–293
Yamada U, Horikawa H (1999) Minami-himekodai. Chelidoperca margaritifera Weber. Seikai Natl Fish Res Inst News 96:1
Yamada U, Tokimura M, Horikawa H, Nakabo T (2007) Fishes and fisheries of the East China and Yellow seas. Tokai University Press, Hadano
Yamakawa T (1985) 249 Chelidoperca margaritifera Weber. In: Okamura O (ed) Fishes of the Okinawa Trough and the adjacent waters I–II. Japan Fisheries Resource Conservation Association, Tokyo, pp 470–471, 669
Acknowledgments
We are especially grateful to S.-P. Huang (ASIZP), Y. Kai, F. Tashiro and N. Nakayama (FAKU), A. Yamaguchi and N. Yagishita (FFNU), H. Motomura (KAUM), P. Pruvost, R. Causse, Z. Gabsi, C. Ferrara and P. Béarez (MNHN), S. Tomiyama (MSM), M. Takami and T. Tamai (Tokai University, Shizuoka), H.-C. Ho (NMMB), K. Matsuura, G. Shinohara, M. Nakae and K. Kuriiwa (NSMT), K. Hatooka (formerly OMNH), K. Shibukawa (SPMN) and Y. Kaji (WMNH) for their kind hospitality during the first author’s visit to their institution; K. Hoshino (Oita Marine Palace Aquarium Umitamago, Oita), C. Aungtonya (PMBC) and U. Satapoomin (formerly PMBC), K. Hoshino (SNFR) and T. Yoshino (formerly URM) also provided opportunities to examine specimens. We especially thank M.-Y. Lee (NTU) and R. de Ruiter (RMNH) for providing data on and photographs of the holotype of C. margaritifera; H. Motomura (KAUM), S. Mizuno, T. Suzuki and K. Tsuchiya (Izu Mito Sea Paradise, Numazu), M. Nakae (NSMT) and K. Shibukawa (formerly NSMT), and M. Okamoto (formerly SNFR) and Y. Kaji (WMNH) for providing specimens and photographs of Chelidoperca; Y. Kaji (WMNH) for also providing photographs of the new species; U. Alama (UPVMI) for registration of paratype of C. tosaensis; H. Motomura and Y. Fukui (KAUM) for their help during the first author’s research in Paris; Y. Miyazaki (Shiraume Gakuen College, Kodaira) for help with references on WEB source; students and volunteers of BSKU and KAUM for curatorial assistance and sampling of specimens; and C. Baldwin (USNM) and anonymous reviewers for providing constructive comments on this study. This study was supported in part by a Grant-in-Aid from JSPS Research Fellow (PD: 16J00047). The specimen collected by the third author in the Philippines was part of an ongoing (2011 to the present) multi-partnered Memorandum of Agreement between the Department of Agriculture, Bureau of Fisheries and Aquatic Resources-National Fisheries Research and Development Institute (BFAR-NFRDI), Department of Agriculture, Philippines, and the National Museum of Natural History of the Smithsonian Institution-Department of Vertebrate Zoology (USNM), USA (Title: Collaboration on the Inventory and DNA Barcoding of Commercial Fishes of the Philippines for Food Safety and Biodiversity), designed to obtain specimen-vouchered tissue samples of the commercial fishes found in the Philippines. We thank Jon Deeds (United States Food and Drug Administration) for supporting this food safety project. We are particularly grateful to M. D. Santos of BFAR-NFRDI and K. E. Carpenter of Old Dominion University for their collaboration and support in all aspects of the project. We thank C. Baldwin and G. D. Johnson (USNM) for their ongoing support of the third author during the project. D. Pitassy, E. Wilbur, S. Smith, K. Murphy and S. Raredon of the Division of Fishes (USNM) assisted with preparations for the trip and processing specimens. D. Dumale (the Philippine National Museum) assisted in curation and field collections and provided access to the facilities at the Philippine National Museum for packing and storage of specimens. L. Weigt, A. Driskell, K. Macdonald and J. Hunt of the Laboratories of Analytical Biology (Smithsonian Institution) provided support for and assistance with logistics and molecular analysis of samples throughout the project. A. M. Lizano, A. Macaspac, T. Potenciana, M. Mendiola, P. Jessele, J. Dicdiquin and N. A. Flores worked tirelessly and diligently as administrative and field assistants. D. Carpenter, N. Minsalan V and N. Minsalan VI provided collection support on Panay and Negros.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as 3FFEF7A3-3CEC-422F-BA46-5EB1E5BE9CF1.
This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Matsunuma, M., Yamakawa, T. & Williams, J.T. Chelidoperca tosaensis, a new species of perchlet (Serranidae) from Japan and the Philippines, with geographic range extension of C. stella to the northwestern Pacific Ocean. Ichthyol Res 65, 210–230 (2018). https://doi.org/10.1007/s10228-017-0604-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-017-0604-5