Abstract
To evaluate the influences of spatial scale on dispersal, the dispersal patterns of masu salmon Oncorhynchus masou masou were investigated at among-river (ca. <43 km) and within-river levels in mid-western Hokkaido, Japan. A genetic differentiation (F ST) and assignment test showed that among-river dispersal was much less common (2.9 % of 339 individuals) than within-river dispersal (7.4 % of 190 individuals). We also found that there was no bias in dispersal at the among-river level, while anadromous males were more likely to disperse at the within-river level, suggesting that the dispersal patterns may be scale dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal is one of the most fundamental life-history traits, because it plays an important role in population dynamics, gene flow, and the colonization of new habitats (Clobert et al. 2001). Salmonid species provide an opportunity to examine the mechanisms and consequences of sex-biased dispersal because of their promiscuous mating system. Generally, such mating systems favor dispersal in the males (Greenwood 1980; Perrin and Mazalov 2000). However, research findings on sex-biased dispersal in salmonids have been controversial (e.g., Consuegra and de Leaniz 2007, reviewed by Hendry et al. 2004). One plausible factor for such ambiguity is life-history polymorphism. In several species of salmon, males exhibit partial migration (i.e., anadromous and resident individuals). The freshwater resident individuals mature at sizes an order of magnitude smaller than anadromous males and large anadromous fish outcompete small resident fish, forcing the resident fish to disperse to seek other mating opportunities (Olsen et al. 2006). Another possible factor for the contradictory findings is the differences in the spatial scale investigated in each study. For instance, at a broader spatial scale (i.e., among rivers), environmental conditions may involve different selective pressures affecting dispersal, and females in particular would be less likely to disperse because homing could lead to an appropriate spawning location (see Hendry et al. 2004). Given the differing selective pressures presumably acting on males versus females, as well as between individuals with different life histories, the dispersal patterns of salmonids could be influenced by species- and population-specific life-history traits associated with spatial scales, leading to wide variability in the dispersal patterns among salmonids (Palstra et al. 2007). Therefore, a pluralistic approach, including both life histories and investigation at different spatial scales, is necessary for the detailed understanding of the mechanisms and consequences of dispersal.
Masu salmon Oncorhynchus masou masou inhabiting mid-western Hokkaido are partially migratory: all females and some males are anadromous, while other males remain in their natal rivers (Sugiwaka and Kojima 1984). In the studied area, after downstream migration, anadromous fish move into the Sea of Okhotsk along the Tsushima Current for feeding and, following one-year in their sea life, return to the natal river to spawn (i.e., natal homing, Kato 1991). Female masu salmon simultaneously mate with multiple males, including both anadromous and resident individuals (Kato 1991). Like other salmonids, masu salmon show the development of distinct populations within local spawning areas due to their homing behavior (Kitanishi et al. 2009). Recently, the dispersal patterns of masu salmon were investigated within the Atsuta River; and the study identified 7.6 % of all individuals as putative dispersers. In addition, the patterns of dispersal differed between the sexes and between life histories, with anadromous males showing higher dispersal tendencies than anadromous females and resident males (Kitanishi et al. 2012), although these findings were limited at within-river level. In this study, to evaluate the influences of spatial scale on dispersal, we investigated the dispersal patterns of masu salmon across sex and life history at the among-river and within-river levels.
Materials and methods
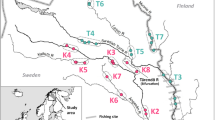
Sampling was conducted in three rivers located in mid-western Hokkaido, Japan (Fig. 1). The rivers were approximately 10 km apart from each other. These rivers have no record of the artificial release of masu salmon and serve as natural spawning grounds for the fish. The Bishabetsu and Gokibiru Rivers are 8.4 and 5.7 km in length, respectively. The Bishabetsu River has an impassable dam at its midstream, while the dam established in the Gokibiru River has a fishway, allowing masu salmon to migrate upstream (Fig. 1; Shimoda and Kawamula 2012). The Atsuta River is approximately 34 km in length and has no artificial migration barriers. In the Atsuta River, sampling was conducted in three tributaries to compare among-river and within-river dispersals. The Atsuta River has been closed to fishing due to fishing regulations, whereas the other two rivers have not been closed. Masu salmon inhabiting these three rivers are naturally reproducing wild fish, and their densities within each river seem to be similar (Shimoda and Kawamula 2012).
Sampling locations of masu salmon Oncorhynchus masou masou on the Atsuta, Gokibiru, and Bishabetsu Rivers. For abbreviations, see Table 1
From 2007 to 2011, 339 masu salmon (anadromous males: 107; anadromous females: 133; resident males: 99) were collected during the spawning season using nonlethal electrofishing (Table 1). The sex and life history of each individual were readily distinguished by secondary sexual characters and body size. Genomic DNA was extracted from an adipose fin using Chelex 100 (Bio-Rad, Hercules, CA, USA). Individual genotypes were obtained at 15 microsatellite loci combined in six multiplexes. Detailed information about polymerase chain reaction (PCR) experiments including each primer is given in Electronic Supplementary Material (ESM) Table S1. The PCR products were analyzed using an ABI PRISM 3730 Genetic Analyser and Genemapper ver. 4.0 (Applied Biosystems, Foster City, CA, USA).
Allelic richness (Ar) was calculated using FSTAT 2.9.3 (Goudet 2001). The expected heterozygosity (H E) and the deviation from Hardy–Weinberg equilibrium (HWE) and linkage disequilibrium (LD) were evaluated using GENEPOP 3.4 (Raymond and Rousset 1995). Significance levels of the tests were assessed following the false discovery rate (FDR; Benjamini and Yekutieli 2001). The presence of null alleles was assessed using MICRO-CHECKER (Van Oosterhout et al. 2004). To infer the effects of sex and life history on dispersal, the extent of genetic differentiation between samples that were grouped by sex and life history were quantified by F ST using GenAlEx 6.5 (Paekall and Smouse 2012). The statistical significance was obtained by 10,000 permutations and adjusted according to the FDR.
To infer whether each individual originated from the tributary in which it was sampled or not (i.e., a disperser), we used a Bayesian estimator of dispersal and estimated the number of dispersers presented in the current generation using GENECLASS 2 (Piry et al. 2004). In this approach, a disperser is defined as an individual that was not born into the tributary from which it was sampled, and each putative disperser is assigned to the most likely source population at a specific confidence level (Paetkau et al. 2004). In this study, the ratio L home and a Bayesian statistical approach (Rannala and Mountain 1997) with the Monte Carlo resampling method of Paetkau et al. (2004) were used as the statistical criteria (Paetkau et al. 2004), since these approaches are more powerful to identify individuals as dispersers under the condition in which all putative source populations are not sampled (Cornuet et al. 1999; Paetkau et al. 2004). The analysis was conducted with a simulation of 10,000 independent individuals (α = 0.05).
Results
The 15 microsatellite loci were moderately to highly polymorphic in each population, with mean allelic richness (Ar) within the population ranging from 6.9 to 8.3 (Table 1, ESM Table S2). The mean expected heterozygosity (H E) was similar for each population (Table 1). The exact test of HWE showed that only one population (MIG) and two loci (One 110 and OMM1316) deviated from HWE after FDR. These deviations were not caused by a specific trend in a particular locus, because Kendall’s test of concordance showed that the ranks of all loci, as estimated by F IS, were not consistent across populations (Kendall’s W = 0.264, P > 0.05). Tests for LD indicated significant associations between loci in four of 105 pairwise comparisons after FDR (between One110 and OMM1325, between OMM1325 and OMM1375, between One110 and OMM1402, and between OMM1347 and OMM1404). These four cases of potential disequilibrium were further examined by testing for LD within populations. No case of LD was detected systematically for the same pair of loci across different populations. In addition, some loci showed no evidence of LD in previous studies (Kitanishi et al. 2009, 2012). Therefore, we treated them as being independent. No null allele was found at any locus. Significant genetic differentiation (F ST) was observed in all samples that were grouped by sex and life history, with the global F ST of all individuals, anadromous males, anadromous females, and resident males being 0.0206, 0.0407, 0.0306, and 0.0340, respectively (P < 0.001 in all cases). Most of the pairwise comparisons between rivers, except for the comparison between the Gokibiru and Bishabetsu Rivers, showed significant differentiation (ESM Table S3). In contrast, there was no significant differentiation in comparisons among tributary samples (ESM Table S3). At both among-river and within-river levels, the degrees of genetic differentiation were similar for each sex and life history. Similar trend of genetic differentiations were observed when three tributary populations in the Atsuta River were pooled (ESM Table S4).
Using the Bayesian dispersal estimator, 24 out of 339 individuals (7.1 % of all individuals) were identified as putative dispersers (Table 2). At the within-river level, the number of dispersers was 14 (7.4 % of 190 individuals); anadromous males tended to disperse more than anadromous females and resident males, with the numbers of anadromous male, anadromous female, and resident male dispersers being 6 (9.7 % of 62 individuals), 4 (5.8 % of 69 individuals), and 4 (6.8 % of 59 individuals), respectively. At the among-river level, the rate of dispersal was much lower than that at the within-river level (χ 2 = 4.955, P < 0.05), and 10 out of 339 individuals (2.9 %) were identified as putative dispersers. No dispersal patterns were observed, with the numbers of anadromous male, anadromous female, and resident male dispersers being 3 (2.8 % of 107 individuals), 3 (2.3 % of 133 individuals), and 4 (4.0 % of 99 individuals), respectively (Table 2).
Discussion
This study revealed that the level of among-river dispersal was much lower than that of within-river dispersal. This might simply be dependent on the geographic distance. Previous studies have reported that the number of dispersers is negatively correlated with geographic distance (Hendry et al. 2004; Keefer and Caudill 2014). Our results suggest that this trend is also true for masu salmon. Some studies have found significant genetic differentiation in masu salmon even between neighboring tributaries, suggesting that they have precise homing behavior (Okazaki 1986; Kitanishi et al. 2009). A study using physical marking (e.g., fin clips and otolith marks) also reported that dispersal might be rare and suggested that masu salmon exhibit precise homing even between neighboring tributaries (Mayama et al. 1988; Miyakoshi et al. 2012). Furthermore, based on a rough comparison, the rate of dispersal observed here is at the lower end of the range found in earlier studies using physical marking and genetic techniques (e.g., >10 % of chum or pink salmon, 3–36 % of chinook salmon, 1–17 % of coho salmon, reviewed by Keefer and Caudill 2014), indicating the higher homing ability of masu salmon. Dispersal among distant populations might be much less likely to occur, so the reduction might be clearer in masu salmon.
In addition, compared to within-river dispersal, among-river dispersal would incur additional costs that reduce the reproductive success of individuals, such as increased mortality and/or increased energy expenditure (e.g., Kinnison et al. 2003; Jonsson and Jonsson 2006), leading to a reduction in dispersal among rivers. Moreover, the differences of the number of chances of dispersal between among-river and within-river dispersal could be simply tied to the occurrence of dispersal. At the within-river dispersal, there are many opportunities to move into different tributaries, because individuals can move to these during a longer period (i.e., throughout the spawning period), while the chance of dispersal to a different river happens only once, at the time of returning from the sea. Finally, the larger spatial extent may involve a variety of selective pressures that may reduce dispersal, especially in anadromous females because females’ reproductive success may link to environmental conditions (Hendry et al. 2004). However, the spatial extent focused on in this study was small (ca. <43 km), and it is unlikely that environmental conditions associated with selection acting on dispersers differed among these three rivers. Further research at a broader spatial scale is necessary for the detailed understanding of the patterns of dispersal and the processes leading to it.
At the within-river level, anadromous males might tend to disperse more than anadromous females and resident males. The results of this study support the previous study (Kitanishi et al. 2012) and the prediction that male-biased dispersal is favored in a promiscuous mating system (Perrin and Mazalov 2000). In contrast to within-river dispersal, no biases were observed in among-river dispersal, suggesting that patterns of dispersal may be scale dependent. Conversely, in other salmonids, a sex bias in dispersal has been documented at broader spatial extents (e.g., Hard and Heard 1999), rather than at a more local scale (e.g., Neville et al. 2006). Such a discrepancy could be attributed to some mutually nonexclusive factors. First is the precise homing of masu salmon. Because the precise homing of masu salmon has been suggested from both direct and indirect estimations (see above), there might be too few dispersers to show any biases in dispersal. Second, selective pressures acting on the dispersers of each sex may vary depending on the spatial scale, and the spatial extent of this study may not include sufficient environmental variation to create any bias in the dispersal pattern. Finally, the fact that the sex ratio of anadromous populations is biased toward females (Kato 1991) could simply increase the dispersal tendency of females and/or reduce the dispersal tendency of anadromous males. Accordingly, no dispersal patterns were found at the among-river level.
References
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188
Clobert J, Danchin E, Dhondt AA, Nichols JD (2001) Dispersal. Oxford University Press, Oxford
Consuegra S, de Leaniz CG (2007) Fluctuating sex ratios, but no sex-biased dispersal, in a promiscuous fish. Evol Ecol 21:229-245
Cornuet JM, Piry S, Luikart G, Estoup A, Solignac M (1999) New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics 153:1989-2000
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). http://www2.unil.ch/popgen/softwares/fstat.htm. Accessed 15 November 2013
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Hard JJ, Heard WR (1999) Analysis of straying variation in Alaskan hatchery chinook salmon (Oncorhynchus tshawytscha) following transplantation. Can J Fish Aquat Sci 56:578-589
Hendry AP, Castric V, Kinnison MT, Quinn TP (2004) The evolution of philopatry and dispersal. In: Hendry AP, Stearns SC (eds) Evolution Illuminated: salmon and their relatives. Oxford University Press, New York, pp 52–91
Jonsson B, Jonsson N (2006) life-history effects of migratory costs in anadromous brown trout. J Fish Biol 69:860-869
Kato F (1991) Life histories of masu and amago salmon (Oncorhynchus masou and Oncorhynchus rhodurus). In: Groot C, Margolis L (eds) Pacific Salmon life histories. UBC Press, Vancouver, pp 447–520
Keefer ML, Caudill CC (2014) Homing and straying by anadromous salmonids: a review of mechanisms and rates. Rev Fish Biol Fish 24:333–368
Kinnison MT, Unwin MJ, Quinn TP (2003) Migratory costs and contemporary evolution of reproductive allocation in male chinook salmon. J Evol Biol 16:1257–1269
Kitanishi S, Yamamoto T, Higashi S (2009) Microsatellite variation reveals fine-scale genetic structure of masu salmon, Oncorhynchus masou, within the Atsuta River. Ecol Freshw Fish 18:65-71
Kitanishi S, Yamamoto T, Koizumi I, Dunham JB, Higashi S (2012) Fine scale relationships between sex, life history, and dispersal of masu salmon. Ecol Evol 2:920-929
Mayama H, Nomura T, Ohkuma K (1988) Seaward migration and adult return of the marked masu salmon, Oncorhynchus masou, released in late fall before wintering. Sci Rep Hokkaido Fish Hatchery 42:21–36
Miyakoshi Y, Takahashi M, Ohkuma K, Urabe H, Shimoda K, Kawamula H (2012) Homing of masu salmon in the tributaries of the Shiribetsu River evaluated by returns of marked fish. Sci Rep Hokkaido Fish Res Inst 81:125–129
Neville HM, Isaak DJ, Dunham JB, Thurow RF, Rieman BE (2006) Fine-scale natal homing and localized movement as shaped by sex and spawning habitat in Chinook salmon: insights from spatial autocorrelation analysis of individual genotypes. Mol Ecol 15:4589-4602
Okazaki T (1986) Genetic variation and population structure in masu salmon Oncorhynchus masou of Japan. Bull Jpn Soc Sci Fish 52:1365–1376
Olsen JB, Wuttig K, Fleming D, Kretschmer EJ, Wenburg JK (2006) Evidence of partial anadromy and resident-form dispersal bias on a fine scale in populations of Oncorhynchus mykiss. Conserv Genet 7:613-619
Paekall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539
Paetkau D, Slade R, Burden M, Estoup A (2004) Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Mol Ecol 13:55-65
Palstra FP, O’Connell MF, Ruzzante DE (2007) Population structure and gene flow reversals in Atlantic salmon (Salmo salar) over contemporary and long-term temporal scales: effects of population size and life history. Mol Ecol 16:4504–4522
Perrin N, Mazalov V (2000) Local competition, inbreeding, and the evolution of sex-biased dispersal. Am Nat 155:116-127
Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A (2004) GENECLASS2: A software for genetic assignment and first-generation migrant detection. J Hered 95:536-539
Rannala B, Mountain JL (1997) Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci USA 94:9197-9201
Raymond M, Rousset F (1995) Genepop (Version-1.2): Population-Genetics Software for Exact Tests and Ecumenicism. J Hered 86:248-249
Shimoda K, Kawamula H (2012) Distribution of masu salmon spawning redds in the Gunbetsu River, Bishabetsu River and Gokibiru River in Hokkaido. Sci Rep Hokkaido Fish Hatchery 81:145–148
Sugiwaka K-i, Kojima H (1984) Influence of individual density on smoltification in wild juvenile masu salmon (Oncorhynchus masou) in the Atsuta River. Sci Rep Hokkaido Fish Hatchery 39:19–37
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Acknowledgments
We are grateful to H. Omiya, R. Tanaka, T. Endo, C. Uehara, and T. Suzuki for their assistance in collecting the samples. Yoshiaki Kai and two anonymous reviewers also provided comments that helped to improve the manuscript. This research was supported in part by the JSPS KAKENHI (24710278) to S. K. and the River Fund in charge of The River Foundation, Japan (231215023 and 241215024) to S. K. This study was conducted with the permission of the Hokkaido Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kitanishi, S., Yamamoto, T., Ishii, H. et al. Dispersal patterns of anadromous and freshwater resident masu salmon at different spatial scales in mid-western Hokkaido, Japan. Ichthyol Res 64, 111–115 (2017). https://doi.org/10.1007/s10228-016-0525-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-016-0525-8