Abstract
Many fish species generally migrate among habitats because of ontogenetic changes in their resource requirements; therefore, clarifying linkages among habitats throughout their life cycle is important for the management and conservation of their populations. Although carbon (δ13C) and nitrogen (δ15N) stable isotope ratios are widely used for elucidating fish migrations, information is still scarce how each fish species, including marbled flounder (Pleuronectes yokohamae), change the isotopic composition in their body tissues after they switch to a different diet during migrations. This study clarified isotopic turnover rates of marbled flounder during the juvenile stage using a diet-switch experiment in an aquarium. Furthermore, we statistically compared bulk samples (i.e., whole body without head and viscera) to partial muscle tissue samples to estimate the difference in the turnover rate as an index for diet switches. An exponential model was used to describe the temporal changes in δ13C and δ15N isotopic rates of juvenile marbled flounder. No significant difference was noted between the exponential models for changes in both δ13C and δ15N between muscle tissue and bulk samples, and the half-life values were similar for muscle and bulk samples for both δ13C and δ15N (14.0–18.5 days). These results suggest that bulk samples are a useful substitute for muscle tissues in isotopic analysis of juvenile fish and that stable isotope analysis and the estimated isotopic turnover rates in tissues of marbled flounder can be used to clarify their migration route in coastal areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish species migrate for a variety of purposes, including feeding, predation avoidance, reproduction, and ontogenetic changes in their resource requirements (Dingle and Drake 2007). Loss and fragmentation of suitable habitats in each life stage are the main causes of decline in many species (Caughley 1994). Thus, clarifying migration routes and establishing linkages between suitable habitats is a key issue in biological conservation. However, directly tracking migration routes of freshwater and marine animals under water is difficult. Therefore, tissue assays may be an effective method to estimate the migration route between habitats (Hobson 1999).
Stable isotope analysis is a powerful tool to demonstrate fish migration routes, especially as coastal species use multiple habitats during their life cycles (Cocheret de la Morinière et al. 2003; Frédérich et al. 2012). A fundamental principle in this analysis is based on the fact that stable isotopic signatures in body tissues reflect those of their diets, and thus the signatures’ change occurs as a result of diet switches to isotopically different food sources in each habitat. However, there is a time lag before isotopic changes equilibrate, and information about such time lags is essential for quantitatively analyzing the migration between isotopically different habitats (Hobson 1999).
Marbled flounder (Pleuronectes yokohamae) is an important fishery species in Japan, typically inhabiting coastal areas, including macroalgal meadows, estuaries, and mud flats, during the juvenile stages (Omi et al. 2001; Yamada 2004; Tanda 2008). The adult stocks have decreased for several decades despite stock enhancement by releasing hatchery-reared individuals based on the decline in fishery catches (Kume et al. 2006; Imoto et al. 2007). Recently, it has been thought that the adult stock decline can be attributed to the loss of juvenile habitat or the lack of connectivity among habitats in coastal areas (Fisheries Research Agency 2015). However, habitat use and migration patterns among habitats in coastal areas at the juvenile stage are still poorly understood. As the first step in clarifying the migration route of juvenile marbled flounder, a controlled diet-switch experiment in an aquarium was conducted to empirically determine the turnover rates and fractionations of δ13C and δ15N stable isotopes in the muscle tissue. Bulk or whole body samples are often utilized for stable isotope analysis in larva or juvenile studies (e.g., Herzka et al. 2001; Pease et al. 2006). Therefore, we assessed the turnover rate in bulk samples (i.e., whole body without head and viscera) and compared it with the rate in the muscle tissue. We then tested whether bulk samples can be used as an index for dietary or habitat shifts instead of the muscle tissue samples.
Materials and methods
Diet-switch experiment. Juvenile marbled flounders were obtained from fingerlings hatched at the Kudamatsu City Center for Stock Enhancement (Kudamatsu City, Yamaguchi Prefecture, Japan). Juveniles were raised on a diet of formulated feed (Otohime B2, Marubeni Nisshin Feed Co., Tokyo, Japan) in the hatchery. Juveniles were transported from Kudamatsu City to the National Research Institute of Fisheries and Environment of Inland Sea in Hiroshima Prefecture and allowed to acclimate to an experimental tank for four days while being fed the previous diet. Juveniles were maintained in a 100-L acrylic tank with seawater pumped from Hiroshima Bay and filtered through a 0.2-µm mesh. The water temperature (mean ± standard division; 19.2 ± 1.0 °C) in the experimental tanks was similar to that of the water at about 1 m above the bottom of Hiroshima Bay (H. Hamaoka, unpublished data). The light:dark regime in the aquarium was controlled to 7:17 h.
To determine the initial δ13C and δ15N stable isotope ratios of juveniles prior to the diet switch, six juveniles were sampled from the experimental tank and killed. The remaining fish received a diet of commercially purchased krill, Euphausia superba (Hamaichi, Wakayama, Japan), which had substantially different isotopic values from the previous hatchery feed. Juveniles were fed twice daily (approximately, 15 % body weight per day) and were killed at 1, 3, 7, 14, 28, 42, 56, 70, 84, and 98 days from the start of the diet switch. The total length (TL) and wet weight (WW) of each juvenile were measured, and samples were immediately frozen at −20 °C until preparation for stable isotope analysis. A subsample of the krill diet was also frozen for analysis. A control treatment group under a hatchery feed was not set up in this experiment, because it is indicated that isotopic values in fish body under a constant diet do not change (e.g., Watanabe et al. 2005).
Stable isotope analysis. Dorsal white muscle was excised from frozen juveniles for stable isotope analysis. After muscle excising, head and viscera were removed from the body and bulk sample, which includes white muscle, axial skeleton, anal fin and skin, was excised from the ventral part of the intact body for stable isotope analysis. All samples were dried at 60 °C overnight and ground to a homogeneous fine powder using an agate pestle and mortar. Samples were immersed in a chloroform:methanol (2:1) solution for 24 h to remove lipids (Bligh and Dyer 1959). Following lipid extraction, samples were again dried at 60 °C overnight. The dried samples were wrapped in a tin capsule and their δ13C and δ15N stable isotope ratios were measured using a mass spectrometer (ANCA-GSL; Europa Science Inc., UK). The stable isotope ratios were expressed in δ notation and defined as the ‰ deviation from the standard as follows:
where R represents 13C/12C or 15N/14N. The standards were Pee Dee Belemnite (PDB) limestone carbonate for δ13C and atmospheric nitrogen (N2) for δ15N. The analytical precision was 0.2 ‰ for δ13C and 0.3 ‰ for δ15N.
Statistical analysis. An exponential model (Tieszen et al. 1983; Hesslein et al. 1993) was fitted to the stable isotope data:
where δ t is the mean value of the stable isotope ratio of juveniles at t days after the initial day, m is the metabolic turnover constant, δ f is the expected mean value of the stable isotope ratios of juveniles when completely equilibrated to the new diet, and δ i is the mean value of the initial isotope ratios of juveniles. The specific growth rate constant, k, was estimated by fitting an exponential growth model (Ricker 1979) to the available data:
where W f is the final mean WW of juveniles when sampled on t days after the initial day and W i is the mean WW of six randomly selected juveniles sampled on the initial day. The k, m, and δ f parameters were estimated by nonlinear regression with program R (R Development Core Team 2012).
The fractionation of δ13C and δ15N between the diet and each fish (Δtissue) was defined as follows:
where δ d is the mean stable isotope ratio of the diet (Minagawa and Wada 1984). The time needed to achieve an α percent turnover of δ13C and δ15N was calculated (Tieszen et al. 1983) as follows:
Half-life (T 0.5) was obtained when α was 50 %.
A Student’s t test was used to test m against a null hypothesis of m = 0, which indicates that metabolism has no contribution to isotopic turnover rate in the model (Zar 2010). An analysis of the residual sum of squares (ARSS) was employed to compare the parameters from each model of tissue types (muscle and bulk sample). Procedures of the ARSS were as follows: the residual sum of squares (RSS) and an associated degree of freedom (DF) of the exponential model was calculated for each sample. The resultant RSS and DF of each sample were summed, and the data of all samples were pooled to calculate the RSS and DF of a total exponential model. The F-statistic was calculated (Chen et al. 1992) as follows:
where RSSp is the RSS of the exponential model fitted by pooling all data, RSSs is the sum of the RSS for each exponential model fitted to muscle or bulk sample data, N is the total sample size, and K is the number of models in the comparison. The calculated F-statistics were compared with the critical F, where the DFs of the numerator and denominator were equal to 3(K-1) and N-3K in equation (6).
Results
The initial body size (mean ± standard deviation) of juvenile marbled flounder was 46.3 ± 0.6 mm TL and 1.45 ± 0.54 g WW (n = 6) and 88.3 ± 10.1 mm TL and 8.82 ± 4.52 g WW (n = 3) after the 98-day experiment. Some individuals died after jumping out of the experimental tank, but a total of 93 juveniles were included in the experiment. Juvenile marbled flounder grew well during the experiment (Fig. 1), and the specific growth rate (k) was 0.021.
Body weight increase of juvenile marbled flounder (Pleuronectes yokohamae) juveniles throughout the experiment. Diamonds and vertical lines represent mean wet weight and standard deviation. Estimated growth trajectory was based on an exponential growth model (Ricker 1979)
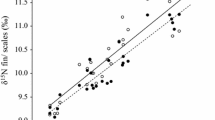
The mean δ13C and δ15N values of the hatchery feed, experimental diet (i.e., krill), and muscle and bulk samples collected on day 1 are provided in Table 1. Both the δ13C and δ15N values of the muscle and bulk samples at the initial day of the experiment were close to the values of hatchery feed, and changed toward the more depleted values and converged on the asymptotic values reflecting the experimental diet (Fig. 2). The exponential model provided a good fit for changes in both δ13C and δ15N values of muscle and bulk samples. The metabolic turnover contributed significantly to isotopic changes for both muscle tissue and bulk samples (δ13Cmuscle: t = 4.77, P < 0.05; δ13C bulk: t = 4.60, P < 0.05; δ15Nmuscle: t = 2.97, P < 0.05; Δδ15Nbulk: t = 11.06, P < 0.05).
Changes in mean δ13C and δ15N of juvenile marbled flounder (Pleuronectes yokohamae) tissues during the diet-switch experiment. Circles and vertical lines represent mean values and standard deviations of bulk (a, c) and muscle (b, d) tissue samples. The trajectory indicates the fitted model for isotopic values of each tissue type. The horizontal dashed and dotted lines indicate mean isotopic value for the experimental diet and the hatchery feed
The Δtissue values of δ13C were 1.5 ‰ for both muscle and bulk samples (Table 2). The Δtissue of δ15N muscle samples was 3.5 ‰ and the bulk sample was 3.7 ‰ (Table 2). The T 0.5 values for the muscle tissue and bulk samples were 17.2 and 17.4 days for δ13C, and 18.5 and 14.0 days for δ15N (Table 2). There were no significant differences between the muscle tissue and bulk sample exponential models for changes in δ13C and δ15N (δ13C: F 1,14 = 2.85, P = 0.08; δ15N: F 1,14 = 2.52, P = 0.10).
Discussion
Fish growth is affected by both dietary conditions and density of individuals within a culturing facility (Nash and Geffen 2004). In our diet-switch experiment, daily supplied rations were lower than daily food consumption of juvenile marbled flounder under typical aquarium conditions (approximately, 30 %; Yamamoto et al. 2005), and the juvenile density was probably higher than that under natural conditions. However, most juveniles in our experiment grew well, with the body weight increasing approximately sevenfold by the final day of the experiment. It has been previously reported that the isotopic values of the muscle and fin tissues of moderate-growing fishes, such as Japanese temperate bass (Lateolabrax japonicus), change as a result of growth and metabolic turnovers (Suzuki et al. 2005). Further, isotopic values reach equilibrium after the body mass increases by several fold for juveniles (Suzuki et al. 2005). Therefore, the experimental design in our study was probably appropriate for analyzing the turnover rate at the juvenile stage.
Despite large variation in the T 0.5 among fish species, life stages, different growth, and different tissue types (e.g., Tominaga et al. 2003; Suzuki et al. 2005), it has been reported that T 0.5 values range between 1 and 25 days for both δ13C and δ15N in juveniles (Bosley et al. 2002; Suzuki et al. 2005). Although slight differences were noted in the half-life (T 0.5) of δ15N between the muscle and bulk samples in our study, the T 0.5 for both δ13C and δ15N were identical between the muscle and bulk samples at approximately 14–18 days, conforming to the range of published half-life times. Fractionations of δ15N for the muscle tissue and bulk samples (Δδ15N: 3.53 and 3.71 ‰) were roughly similar to the mean value of 3.4 ‰, which has been previously reported (Minagawa and Wada 1984; Post 2002). Although fractionations of δ13C in the muscle tissue and bulk samples (Δδ13C: 1.52 and 1.53 ‰) were slightly more enriched than the traditionally assumed values of 0–1 ‰ (Post 2002; McCutchan et al. 2003), our results conformed to the broad bounds of published fractionations for rapidly growing fishes (0.2–3.6 ‰ for the whole body or fin: Herzka and Holt 2000; Suzuki et al. 2005; 0.71–4.79 ‰ for the muscle tissue: Suzuki et al. 2005; Trueman et al. 2005; Buchheister and Latour 2010).
Turnover and fractionation of δ13C and δ15N are suggested to differ according to tissue type (e.g., Suzuki et al. 2005; Guelinckx et al. 2007; Buchheister and Latour 2010) due to tissue-specific metabolic activity and growth (Bosley et al. 2002). Watanabe et al. (2005) reported that δ13C in the caudal fin, including the fin ray and membrane, changes more quickly than muscle tissue because of the difference in metabolic activity between the fin and the muscle. Therefore, uniform tissue samples (e.g., white muscle, liver, and blood tissue) are required for analysis. However, bulk samples are often used for analyses of fish larva or juvenile studies (e.g., Herzka and Holt 2000; Bosley et al. 2002; Witting et al. 2004), because the body size is sometimes too small to excise uniform tissue samples. In our results, the m values in δ13C and δ15N turnover models were similar between muscle tissue and bulk samples, representing little difference in metabolic activity between the muscle tissue and bulk samples. There were also no significant differences between turnover models in muscle tissue and bulk samples for both δ13C and δ15N, indicating the potential for using bulk samples to investigate diet switch or migrations at the juvenile stage.
Our results encourage the application of stable isotope analysis for studying diet switches of juvenile marbled flounder over the time scales of several days to weeks. Stable isotopes may also be useful indicators of the migration among isotopically different habitats through time (Herzka et al. 2001). In Seto Inland Sea, juvenile marbled flounder occurs in various ecological components (macroalgal meadows, estuaries, and mud flats) (Omi et al. 2001; Sano and Ariyama 2001; Tanda 2008) that are isotopically different (e.g., Yokoyama 2008). Juveniles migrate among these costal area habitats until finally reaching offshore habitats necessary for maturation. Our results suggest that the body tissue of juvenile marbled flounder, post-migration, would reflect the isotopic characteristics of the coastal migration route. Furthermore, our results indicate that bulk samples can be used instead of muscle tissue samples to reduce the processing time of juvenile fish.
References
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bosley KL, Witting DA, Chambers RC, Wainright SC (2002) Estimating turnover rates of carbon and nitrogen in recently metamorphosed winter flounder Pseudopleuronectes americanus with stable isotopes. Mar Ecol Prog Ser 236:233–240
Buchheister A, Latour RJ (2010) Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Can J Fish Aquat Sci 67:445–461
Caughley G (1994) Directions in conservation biology. J Anim Ecol 63:215–244
Chen Y, Jackson DA, Harvey HH (1992) A comparison of von Bertalanffy and polynomial functions in modelling fish growth data. Can J Fish Aquat Sci 49:1228–1235
Cocheret de la Morinière E, Pollux BJA, Nagelkerken I, Hemminga MA, Huiskes AHL, Van der Velde G (2003) Ontogenetic dietary changes of coral reef fishes in the mangrove-seagrass-reef continuum: Stable isotope and gut-content analysis. Mar Ecol Prog Ser 246:279–289
Dingle H, Drake VA (2007) What is migration? BioScience 57:113–121
Fisheries Research Agency (2015) Technological development in the restoration of ecological and habitat networks of abalone, littleneck clam and flatfish species toward sustainable coastal fisheries. 2013–2014 report on the project in Ministry of Agriculture, Forestry and Fisheries, Fisheries Research Agency, Yokohama
Frédérich B, Colleye O, Lepoint G, Lecchini D (2012) Mismatch between shape changes and ecological shifts during the post-settlement growth of the surgeonfish, Acanthurus triostegus. Front Zool 9:8 doi:10.1186/1742-9994-9-8
Guelinckx J, Maes J, Van Den Driessche P, Geysen B, Dehairs F, Ollevier F (2007) Changes in δ13C and δ15N in different tissues of juvenile sand goby Pomatoschistus minutus: a laboratory diet-switch experiment. Mar Ecol Prog Ser 341:205–215
Herzka SZ, Holt GJ (2000) Changes in isotopic composition of red drum (Sciaenops ocellatus) larvae in response to dietary shifts: potential applications to settlement studies. Can J Fish Aquat Sci 57:137–147
Herzka SZ, Holt SA, Holt GJ (2001) Documenting the settlement history of individual fish larvae using stable isotope ratios: model development and validation. J Exp Mar Biol Ecol 265:49–74
Hesslein RH, Hallard KA, Ramlal P (1993) Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can J Fish Aquat Sci 50:2071–2076
Hobson KA (1999) Tracing Origins and Migration of Wildlife Using Stable Isotopes: A Review. Oecologia 120:314–326
Imoto Y, Kimura H, Yoshioka N, Zenitani H (2007) Fisheries management of mud dab Pleuronectes yokohamae in Suo-Nada, Seto Inland Sea based on spawning per recruit. Nippon Suisan Gakkaishi 73:684–692
Kume G, Horiguchi T, Goto A, Shiraishi H, Shibata Y, Morita M, Shimizu M (2006) Seasonal distribution, age, growth, and reproductive biology of marbled sole Pleuronectes yokohamae in Tokyo Bay, Japan. Fish Sci 72:289–298
McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. OIKOS 102:378–390
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochem Cosmochem Acta 48:1135–1140
Nash RDM, Geffen AJ (2004) Age and growth. In Flatfishes: Biology and Exploitation. Edited by Gibson RN, Blackwell Science, Oxford
Omi H, Nabeshima Y, Kusakabe T (2001) Seasonal change of species of larval and juvenile fishes occurrence in the estuary in the innermost area of Osaka Bay. Bull Osaka Pref Fish Exp Stat 13:61–72
Pease AA, Davis JJ, Edwards MS, Turner TF (2006) Habitat and resource use by larval and juvenile fishes in an arid-land river (Rio Grande, New Mexico). Freshw Biol 51:475–486
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ricker WE (1979) Growth rates and models. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology. Academic Press, New York, pp 677–743
Sano M, Ariyama H (2001) Juvenile flounders captured at the mouth of the Yodo River. Bull Osaka Pref Fish Exp Stat 13:72–77
Suzuki KW, Kasai A, Nakayama K, Tanaka M (2005) Differential isotopic enrichment and half-life among tissues in Japanese temperate bass (Lateolabrax japonicus) juveniles: implications for analyzing migration. Can J Fish Aquat Sci 62:671–678
Tanda M (2008) Fisheries and ecological studies on marbled sole Pleuronectes yokohamae in the Seto Inland Sea of Hyogo Prefecture. Bull Hyogo Pref Tech Cent Agr Forest Fish 40:1–96
Tieszen LL, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: Implications for δ13C analysis of diet. Oecologia 57:32–37
Tominaga O, Uno N, Seikai T (2003) Influence of diet shift from formulated feed to live mysids on the carbon and nitrogen stable isotope ratio (δ13C and δ15N) in dorsal muscles of juvenile Japanese flounders, Paralichthys olivaceus. Aquaculture 218: 265–276
Trueman CN, McGill RAR, Guyard PH (2005) The effect of growth rate on tissue-diet isotopic spacing in rapidly growing animals. An experimental study with Atlantic salmon (Salmo salar). Rapid Commun Mass Spectrom 19:3239–3247
Watanabe Y, Seikai T, Tominaga O (2005) Estimation of growth and food consumption in juvenile Japanese flounder Paralichthys olivaceus using carbon stable isotope ratio δ13C under laboratory conditions. J Exp Mar Biol Ecol 326:187–198
Witting DA, Chambers RC, Bosley KL, Wainright SC (2004) Experimental evaluation of ontogenetic diet transitions in summer flounder (Paralichthys dentatus), using stable isotopes as diet tracers. Can J Fish Aquat Sci 61:2069–2084
Yamada H (2004) Variations in species composition of fishes in Kushida River Estuary in Ise Bay. Bull Fish Res Div Mie Pref 11:33–48
Yamamoto S, Sugino H, Cyuuriki K, Masunari N, Kondou M (2005) Developmental changes in diel feeding activity and estimation of daily food consumption in hatchery-reared larval and juvenile marbled sole Pleuronectes yokohamae. Suisanzoshoku 53:157–165
Yokoyama H (2008) Food sources of consumers in temperate estuaries and costal waters: Achievements and potential problems of isotopic studies. Jpn J Ecol 58:23–36
Zar JH (2010) Biostatistical analysis. 5th ed. Prentice-Hall, Upper Saddle River, New Jersey
Acknowledgments
We are grateful to Mr. M. Itohara, Kudamatsu City Center for Stock Enhancement, and Dr. T. Baba, Yamaguchi Prefectural Fisheries Research Center Inland Sea Division, for supplying marbled flounder juveniles. We thank Dr. A. Sano for her help with the experiments. We thank the two anonymous reviewers for insightful readings and constructive suggestions. This work was supported by the Ministry of Agriculture, Forestry and Fisheries of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hamaoka, H., Shoji, J. & Hori, M. Turnover rates of carbon and nitrogen stable isotopes in juvenile marbled flounder Pleuronectes yokohamae estimated by diet switch. Ichthyol Res 63, 201–206 (2016). https://doi.org/10.1007/s10228-015-0488-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-015-0488-1