Abstract
Two cyprinodontids, Garmanella pulchra and Cyprinodon artifrons, coexist in a small mangrove floodplain on the Yucatan Peninsula, enabling comparison of male territoriality in two species with similar social structure and resource needs. There were two contrasting situations, one where territorial males of G. pulchra were several times more abundant than those of C. artifrons and one where the reverse was true. In both situations, the roughly circular breeding territories were non-overlapping intraspecifically and showed complete overlap interspecifically. Territories of both species were several times smaller in the situation where they were numerically dominant. In that situation, the territories of G. pulchra were about twice as large as those of C. artifrons and males of both species showed higher conspecific aggression, lower heterospecific aggression, more reproductive activity, lower feeding rates, and lower percentages of body fat. In both situations of relative density, the percentage fat content was orders of magnitude greater in C. artifrons than in G. pulchra, potentially reflecting higher rates of territorial male turnover in the latter. Social behavior in the wild, described for the first time for both species, generally conforms to typical cyprinodontid themes for territorial and reproductive behavior. There was no evidence, in G. pulchra, of the courtship dance, nor the overt, male parental care described for Jordanella floridae, a species once considered a congener.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Territoriality is a central feature of social structure in many fishes (Grant 1997; Maher and Lott 2000). A common pattern, especially in freshwaters (Ah-King et al. 2005), is male breeding territoriality, where the male of the species defends a space for the apparent purpose of attracting females and restricting access to them by other males. When territorial defense persists after egg deposition, the territory also provides a place relatively free of predators on eggs and young, even in “primitively custodial males” (Loiselle 1981, 1983) having no well-defined nest area or overt parental care of eggs and young (Baylis 1981; Paciorek et al. 2014). Further, the territory might be the male’s primary feeding area for extended periods (Thresher 1976; Loiselle 1983; Itzkowitz and Slocum 1994). Reflecting the multifunctional aspect of territoriality, breeding males typically exhibit both conspecific and heterospecific aggression. A general expectation is that competition for both mates and resources (spawning habitat, food, and shelter) might drive interspecific territoriality between closely related species, whereas with more distantly related species it is driven by competition for resources (Drury et al. 2020).
Heterospecific aggression with implications for reproductive success is expected when territorial males of different species with similar breeding systems co-occur in the same habitat (Cowen et al. 2020). In this paper, we describe such a situation for two small-bodied (generally < 45 mm total length) cyprinodontids, Yucatan flagfish Garmanella pulchra, and Yucatan pupfish Cyprinodon artifrons, in a mangrove floodplain on the Yucatan Peninsula, Mexico. Nothing is published on social behavior in the monotypic Garmanella beyond two brief articles in the fish hobbyist literature (Richter 1975; Loiselle 1981). For C. artifrons, behavior is undescribed except for laboratory studies comparing C. artifrons and various members of its sister-group, a small species flock in Lake Chichancanab, Yucatan, with respect to interspecific mate choice (Kodric-Brown and Strecker 2001) and levels of aggressiveness versus shoaling behavior (Plath and Strecker 2008).
Besides adding to what is known about social behavior in two relatively unstudied cyprinodontids, this work describes interspecific interactions between territorial males in localities of co-occupancy with asymmetric densities of the two species. Our purpose is to address three general questions. First, how does territory size and the spatial configuration of territories vary with relative density of the two species? One expectation is that males should be less tolerant of conspecific males (Myrberg and Thresher 1974; Peiman and Robinson 2010). For example, in a situation where male pumpkinseed sunfish Lepomis gibbosus defended territories within a large nesting-male colony of rock bass Ambloplites rupestris, interspecific male-male behaviors resembled conspecific behavior, but the outcome was different: distances between nests of A. rupestris and L. gibbosus were about half those between males of A. rupestris (Gross and Nowell 1980). Similarly, in another field study, territorial male three-spined sticklebacks Gasterosteus aculeatus were spaced more distantly from conspecific males than from male fourspine sticklebacks Apeltes quadracus (Gaudreault and Fitzgerald 1985). These results indicate that, for these species, competition for mates is a stronger driver of male territoriality than competition for resources.
Second, how does relative density of territories of the two species affect activity budgets of the territorial males? Are tradeoffs between agonistic and reproductive or maintenance (resting, feeding) behaviors the same when the relative densities are reversed? Marked differences with impacts on male reproductive success occurred in a somewhat similar study of territorial behavior in the Pecos pupfish Cyprinodon pecosensis and a fundulid, the plains killifish Fundulus zebrinus (Kodric-Brown and Mazzolini 1992). Third, are differences in activity budget reflected in the dietary ecology and body condition of the males? Most studies of reproductive investment in fishes have dealt with females, leaving male investment understudied (Schütz et al. 2010; von Kuerthy et al. 2015). For cyprinodontids, male investment studies have been based on activity budgets (Feldmeth 1983; Kodric-Brown 1986; Leiser and Itzkowitz 2002, 2003), and there have been no direct assessments of body condition. Territorial males of Cyprinodon are intensely active (Barlow 1961; Echelle 1973; Feldmeth 1983; Brannan et al. 2003) in a generally food-limited space, the male’s territory (Loiselle 1983). Therefore, as in other vertebrates (Ord 2021), effects of male density on territory size and the social environment might have detectable effects on the body condition of territorial males, thereby affecting factors important to reproductive success, such as the male’s vigor and ability to maintain territorial activity.
Materials and methods
Environmental setting
The study site was in a mangrove flood-plain in Sisal, a seaport town in northern Yucatán, México (21° 9′40.73″N, 90° 2′35.96″W) (Fig. 1a). The structure of the mangroves was Rizophora mangle > Laguncularia racemose > Avicennia germinans, from highest to lowest abundance. The flooded area extended southwest to northeast for 280 m from an ocean inlet and had a maximum width of about 100 m and a maximum depth of about 30–40 cm. The central marsh near the ocean inlet was generally deeper with more cover from mangroves and more direct contact with tidal inflows from the ocean, which reach the marsh during the rainy season. The two pupfishes were concentrated in the shallowest waters near the marsh edge. Preliminary observations indicated marked spatial differences in relative abundance, with G. pulchra and C. artifrons several times more abundant than the other in waters, respectively, nearer the ocean inlet and those at the upper end of the marsh. This difference persisted throughout the study.
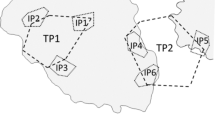
Location of the study area in Yucatán, México (21° 9′40.73″N, 90° 2′35.96″O). a The photographs outside of the general map show the town of Sisal and the mangrove floodplain—red lines show the two study transects in the floodplain; 1 = situation 1 (C. artifrons numerically dominant), 2 = situation 2 (G. pulchra dominant). Images of Sisal and the marsh are from Google Earth; imagery date 1/3/2023; accessed 14/11/2023; Map Data ©2017 Google. b Field photograph showing the two situations of study; upper = C. artifrons numerically dominant, lower = G. pulchra dominant. Dotted lines mark the territory of a male of the less abundant species (determined from videos). The arrows indicate the boundaries of some males of the numerically dominant species; note the accumulation of organic material marking the boundary of some territories of the more abundant species, a result of the intense activity of these males in a small space. Line = 50 cm
Data gathering
Observations were made between 0800 and 1100 h from 17 September to 10 October, late in the wet season (May to November) of 2021. We established two observation transects separated by about 150 m of shoreline. The transects were parallel to the marsh edge with yellow flags positioned in the substrate at 1-m intervals, one (50 m long, ~ 1 m wide) at the northeast end of the marsh where C. artifrons was numerically dominant (situation 1) and one (60 m long, ~ 1 m wide) at the southwest end where G. pulchra was dominant (situation 2) (Fig. 1b). The transects had depths ≤ 5 cm, salinities of 27–35 ppm, and pH values of 8.3–8.6. Both situations had a sand-mud substratum and 1–3 mangrove pneumatophores per meter2. The substrate in situation 2 generally had more filamentous algae and more mangrove cover. At the beginning of the study, we estimated number of territories of the two species in each transect by counting the number of males. Also, before each recording of a focal male, the observer counted the number of breeding males of each species in the roughly 1 m2 of area between the shoreline and a pair of transect flags at the observation point.
We used a focal-animal sampling approach (Martin and Bateson 1986), tracking (from shore) a single male during each trial and avoiding the tracking of males adjacent to a previously recorded male. Once the density was determined at a transect point, a focal male was selected based on ease of observation. Focal observations were made by one of us (ODC) on 30 territorial males of each species per situation. He observed each male for 20 min and recorded the frequency of each behavior listed in Table 1 from a position 1–1.5 m away from the segment of the transect to be observed, and partially hidden in vegetation. Observations began after 10–15 min with little movement by the observer. Behaviors were recorded by hand, using a notebook and pencil with a chart of the behavioral acts. For behaviors occurring in bouts of repeated acts (spawning, feeding), each act was counted separately. For combat between males, which included multiple, often different acts (see results) the entire interaction was counted as a single event. After recording behavioral data, the longest axis of the focal male’s territory was measured with a tape measure.
For each of the two situations, we captured 25 territorial males of each species in late October, immediately after the period of behavioral observations. The fish were sacrificed with an overdose of clove essence, fixed in 4% formalin for 24 h, and preserved in 70% alcohol. We follow the standards for handling and euthanizing animals of the Government of Mexico (NOM-062-ZOO-199), and the guidelines for researchers issued by The International Council for Laboratory Animal Science. In the laboratory, we calculated an index of feeding intensity for 15 of the preserved males. Each male was measured for standard length (SL, nearest mm) and weighed (± 0.0001 g) prior to and after removal of the digestive tract. Following Zacharia (2017), feeding intensity = 10,000 × (digestive tract weight/body weight). Then the gut contents were dissected out and identified to the lowest taxonomic level from keys in Haney et al. (2013). Following Hagmayer et al. (2018), we calculated the proportion body fat in the 10 remaining males from each situation to evaluate the body condition. Each fish was dried at 55 °C for 24 h, weighed for dry mass, then exposed twice (24 h each) to anhydrous diethyl ether to remove triglycerides, dried for another 24 h, and weighed again to obtain lean mass. We calculated the proportion body fat by subtracting lean mass from whole-body dry mass and dividing by whole-body mass. Finally, we calculated the Fulton index of body condition K = (W/L3) × 100, where W = wet body weight (grams) and L = standard length (centimeters).
Statistical analysis
We performed a principal component analysis (PCA) on the numbers of behavioral units (log transformed) recorded for the males of both species to identify the main components of variation in situations 1 and 2. To test the significance of differences between the two situations, we computed the pseudo-F test statistic from a permutational multivariate analysis of variance (Anderson 2005). Also, we computed Bray–Curtis dissimilarities (Bray and Curtis 1957) from log-transformed behavioral units, with 9999 random permutations. We performed a similarity percentage (SIMPER) analysis (Clarke 1993) on the dissimilarity matrix to determine the percentage contribution of each behavior to the dissimilarity. To identify specific differences in frequencies of behavioral acts and body condition between ecological situations, we applied ether Kruskal–Wallis/Wilcoxon/Mann–Whitney test, or a t-test, depending on whether the Shapiro–Wilk test of normality was, respectively, significant (= non-normality) or not. All statistical computations were done in PAST v4.03 (PAleontological Statistics; Hammer et al. 2001).
Results
The fishes in the floodplain adjacent to the mangrove forests were G. pulchra, C. artifrons, Yucatan gambusia Gambusia yucatana, sailfin molly Poecilia velifera, and giant killifish Fundulus grandisimus. Aggressive interactions occurred between G. pulchra and C. artifrons and between them and G. yucatana and P. velifera. In contrast, the typically much larger (up to 20 cm TL) F. grandisimus displayed predation behavior directed at G. pulchra and C. artifrons.
Estimated numbers of territorial males in the 50-m situation-1 transect was ~ 360 C. artifrons and ~ 50 G. pulchra. The respective densities at points of observation for focal males averaged 6.2 ± 1.0 and 1.2 ± 0.4/m2 (Fig. 2a). Estimated numbers in the 60-m situation-2 transect was ~ 440 G. pulchra and ~ 55 C. artifrons. The respective densities at points of observation for focal males averaged 7.4 ± 1.0 and 1.0 ± 0.4/m2. The differences between situations were statistically significant for both species (Fig. 2a).
Territoriality
The territories of both species were exclusive of conspecific males defending neighboring territories, but, interspecifically, there was broad overlap with territories of the less abundant species encompassing or overlapping several territories of the more abundant (Fig. 1b). In both situations of relative density, territory sizes were smaller for the more abundant species, about twice as small for G. pulchra in situation 2 and about five times smaller for C. artifrons in situation 1 (Fig. 2). In general, the relatively small territories of the more abundant species were more tightly circular than those of the less abundant species, which were more irregular in shape (Fig. 1b).
Territorial males of both species were conspicuous because of their hyperactivity and coloration—orange on the fins, head, and body of G. pulchra and, in C. artifrons, iridescent blue dorsolaterally, partially black dorsal and anal fins, black pectoral fins, and terminal black band on the caudal fin. In both species, the males have a black eye-bar, and in G. pulchra there is a black lateral spot on the body. The males of both species attempted to exclude all intruding fish, although they usually fled from the large F. grandissimus.
Agonistic behavior involving conspecific territorial males typically occurred near the boundary of adjacent territories. These encounters varied in intensity and duration and could involve one or more of the following: reciprocal lunging across the territory boundary, lateral displays (pelvic and median fins erect, bodies vibrating in head-to-tail orientation), circling (each fish chasing the other in a tight circle little more than a body length in diameter), and, less frequently in both species, tail-beating (one male slaps its caudal fin at the other), and mouth-fighting (tumbling about with jaws interlocked). Bouts involving one or more of these acts were grouped as Combat in our data. Although individual acts of combat were not quantified separately, there were notable differences between the species. Circling was less tight (greater diameter) in Garmanella, and there was less tail-beating and mouth-fighting. Conflicts between territorial males of the two species differed from that of conspecific males in primarily consisting of attempting to drive the opponent away with a charge and biting attempts.
Courtship and spawning
In both species, females entering the territory were either immediately chased away by the male or he initiated courtship. Courtship was very brief in C. artifrons, consisting of the pair making a rapid spiral to the substratum. As in other species of Cyprinodon, the female, just before spawning, tilted head down and nipped the substrate as the male attempted to sidle alongside and wrap his anal fin around her venter, appearing to press her onto the substrate, their bodies parallel, bent into an S-shape, and vibrating. The vibrating apparently is associated with the release of sperm and, as in other cyprinodontids, a single egg. The encounter can end with one spawning act, or it can be followed with additional spawnings (with or without courtship spiraling). Occasionally, during the spawning clasp on the substrate, a smaller, cryptically colored male (sneaker) quickly entered the territory, aligned himself to the female, usually on the side opposite the territorial male, and spawned in a triad with the pair, after which the territorial male chased him away. In courtship and spawning the male retained his conspicuous coloration, including the black eye-bar, which appeared less pronounced in the female.
Courtship in Garmanella was more complex. When a receptive female entered a male’s territory, he swam rapidly around her in circles (as described by Richter 1975) or looped back and forth in front of her, at a distance of about two to four body lengths. The female appeared to select the spawning spot by nipping at the substrate (mangrove pneumatophores, filamentous algae, or the bottom substrate). The spawning act closely resembled that in C. artifrons except the dorsal and anal fins were both wrapped (respectively, dorsally and ventrally) around the female, not just the anal fin (also described by Richter 1975). Several spawning acts might occur in succession, with or without additional courtship. In courtship and spawning, both sexes retained the black lateral spot on the body. Sneaking behavior by another male was not observed.
Maintenance behavior
Resting, as we define it, consisted of remaining immobile on the bottom substrate of the territory. Presumptive body cleaning behavior occurred when the fish scraped its body by swimming quickly against the substrate or surfaces such as rocks or mangrove pneumatophores. In G. pulchra, feeding consisted of nibbling elements in the territory, primarily filamentous algae, rocks, mangrove pneumatophores, and, to a lesser extent, the bottom substrate of the territory. Feeding activity in C. artifrons mainly involved nipping and digging in the substrate with the head and pectoral fins, apparently in search of food items. In avoiding potential predators (mainly birds and F. grandissimis), the males of C. artifrons typically fled from the territory, whereas those of G. pulchra tended to hide under filamentous algae within or near the territory. This difference might reflect habitat differences because most fleeing events for both species occurred in their areas of greatest abundance and cover in the form of filamentous algae was more available in the situation of high abundance for G. pulchra.
Behavior at asymmetric densities of the two species
High percentages of the variance in behavior of male C. artifrons and G. pulchra were explained by the first two principal component (PC) axes for situations 1 (40.3 and 17.9%) and 2 (41.0 and 12.2%). For both situations, PC1 separated the two species, with positive scores for the more abundant species and negative scores for the less abundant (Fig. 3). Behaviors with positive loadings on PC1 for both situations involved aggression toward conspecifics (chases of juveniles, females, and breeding males, and combat with territorial males), spawning, and courtship (Fig. 3a). Behaviors with negative PC1 loadings in both situations were associated with feeding, body cleaning, fleeing from a predator, and aggression toward heterospecific species, including P. velifer and G. yucatana and territorial males of the numerically dominant species. The variance explained by PC2 was associated with individual variation in behavior independent of the relative densities of territorial male G. pulchra and C. artifrons.
Principal component axes for behavior of territorial male of C. artifrons (black circles) and G. pulchra (open circles) in two situations of relative abundance. a C. artifrons most abundant, b G. pulchra most abundant. Thin lines indicate relative loadings of behaviors on PCA1 (horizontal axis) and PCA2 (vertical axis)
For both species, the differences in behavior between situations 1 and 2 were visually detectable (Fig. 3b, Table 1), and statistically significant (C. artifrons pseudo-F = 107.3, p < 0.001; G. pulchra 80.4; p < 0.001). When numerically dominant, territorial males of both species showed significantly higher frequencies of chases against conspecific territorial males, more combat in territorial disputes, and higher frequencies of courtship (Table 1); both species also showed higher frequencies of spawning, although this was not statistically significant for G. pulchra. When numerically inferior, the males of both species exhibited greater numbers of feeding acts and chases of heterospecific territorial males; C. artifrons also rested more frequently (Table 1).
The SIMPER analysis reveals the relative importance (%) of each behavioral variable to the behavioral dissimilarity indexes between the two situations, which were 0.38 and 0.59, respectively for C. artifrons and G. pulchra. This summary gives the behaviors with ≥ 5% contribution to the dissimilarity; s1 and s2 signify higher frequency in, respectively, situation 1 (C. artifrons dominant) and situation 2 (G. pulchra dominant). For C. artifrons, the most important variable by far was chases of heterospecific breeding male (G. pulchra) (s2) at 71.3%, followed by chases of conspecific juveniles and females (s1), chases of conspecific territorial males (s1), and combat with conspecific males (s1) at, respectively, 6.4, 5.9, and 4.7%. For G. pulchra, importance was more evenly distributed among variables. At 25.2%, chases of heterospecific breeding male (C. artifrons) (s1) was again the most important variable, followed by chases of conspecific territorial males (s2), combat with conspecific males (s2), feeding (s1), and courtship (s2) at, respectively, 14.1, 12.9, 12.9, and 8.9%.
Foods and body condition
For both species, the index of feeding intensity and the variety and abundance of food items were greater in the situation where the species was less abundant (Fig. 4a, Table 2). This is reflected in the frequency of fish with empty digestive tracts: 9 C. artifrons, all in situation 1, and 3 G. pulchra, all in situation 2. Interspecific differences in food items were statistically significant in both situations (situation 1, pseudo-F = 25.1, p < 0.001; situation 2, 21.0, p < 0.001). The primary difference was in the frequency of two kinds of algae, type I being abundant in G. pulchra and virtually absent in C. artifrons, type II being common in C. artifrons and absent in G. pulchra (Table 2). Also, there was no evidence of fish remains in C. artifrons, but in G. pulchra, fish scales were found in both situations and fish eggs in situation 1. Sand was more abundant in C. artifons, occurring in 5 and 12 fish in, respectively, situations 1 and 2. Sand occurred in 8 G. pulchra, all in situation 1. The food items in both C. artifrons and G. pulchra differed significantly between situations 1 and 2 (respectively, pseudo-F = 21.3 and 18.4; p < 0.0001), primarily reflecting quantitative differences rather than kinds of food items eaten.
Indexes of feeding activity, body size, and condition of territorial male C. artifrons (Ca) and G. pulchra (Gp) in two situations of relative abundance. a feeding intensity index, b standard length, c wet weight, d body condition index, e dry weight of fat content in grams expressed as the logarithm plus 4, and f dry weight of fat as a percentage of total body dry weight. Values are means (± SD)
In our samples of breeding males, C. artifrons in situation 1 (Ca dominant) were somewhat larger than in situation 2 (Gp dominant), having higher averages for standard length 2 (albeit non-significant; Fig. 4b, U = 23, p = 0.11) and body weight (Fig. 4c, t = 2.58, p = 0.04). The condition factor, K, was also greater in situation 1 (Fig. 4d, U = 5, p = 0.14). In contrast, dry fat content was significantly higher in situation 2 (Gp dominant), in both amount (Fig. 4e; t = 7.90, p < 0.001) and percentage of total dry weight (Fig. 4f, t = 3.78, p = 0.01), which averaged about twice that in situation 1. For G. pulchra, males in the two situations were similar in length (t = 0.70, p = 0.51) and body weight (U = 6.0, p = 0.67), but dry fat content was higher in situation 1 (Ca dominant) both in amount (t = 11.37, p < 0.001) and percentage of total dry weight (U = 31, p = 0.003). The difference in body condition (K) for G. pulchra in the two situations (higher in situation 1; t = 1.40, p = 0.02) paralleled the results for body fat. Between species, C. artifrons was significantly larger than G. pulchra in situation 1 (SL, t = 6.83, p < 0.001; weight, t = 4.37, p < 0.001), but not in situation 2 (SL, t = 0.35, p = 0.73; weight, t = 0.75, p = 0.48). Percentage fat in C. artifrons was one and two orders of magnitude greater than in G. pulchra, in, respectively, situations 1 and 2.
Discussion
The breeding systems and behaviors for C. artifrons and G. pulchra fit the general cyprinodontid pattern with males congregating in lek-like situations (Leiser et al. 2015) in shallow water where they defend mutually exclusive, contiguous territories, with frequent border-conflicts against conspecific males (Barlow 1961; Foster 1967; Kaill 1967; Liu 1969; Echelle 1973; Itzkowitz and Minckley 1969; Kodric-Brown 1977; Leiser and Itzkowitz 2004). The behaviors described here for both C. artifrons and G. pulchra are common in cyprinodontids (reviewed by Echelle and Echelle 2020). Regarding whether G. pulchra is the sister taxon to the flagfish Jordanella floridae (Parenti 1981; Costa 1997) or not (Echelle and Echelle 1993; Parker and Kornfield 1995), the former showed nothing resembling the peculiar courtship dance nor the overt, male parental care characteristic of Jordanella (Mertz and Barlow 1966; St Mary et al. 2001).
Territoriality in breeding male G. pulchra and C. artifrons conformed to the theory (Brown 1964; Davies and Houston 1984) in showing an inverse relationship between territory size and density of breeding males. Contraction at times of high territorial male densities and intense breeding activity has also been observed in wild populations of desert pupfish Cyprinodon macularius (Barlow 1958) and Pecos pupfish C. pecosensis (Kodric-Brown 1978), and experiments on sheepshead minnow Cyprinodon variegatus found smaller territories in tanks with more breeding males (Itzkowitz 1977). At times of intense competition, smaller territories should be more economically feasible regarding the balance between resources committed to defense and those committed to reproduction (Brown 1964).
Within species, the males of G. pulchra and C. artifrons rigorously maintained largely non-overlapping territories, but interspecifically there was complete overlap in both situations of relative density. Territories of the less common species were larger and generally overlapped several territories of the more common species. At low relative abundance of males, territory densities and sizes for the two species were similar at about, respectively, 1.2/m2 and 70 cm (longest dimension). At high densities, however, the territories of G. pulchra averaged about twice as large (in longest dimension) as those of C. artifrons (36 vs 15 cm), despite occurring at slightly higher densities (7 vs 6/m2). Larger territories at high densities probably helps explain the higher frequency of acts of aggression (combat and chases) between breeding males in G. pulchra—about 110 versus 45 per 20 min in C. artifrons. A possibility is that the lower bound of territory size in G. pulchra is larger than that of C. artifrons and, consequently, requires more active defense. Another possibility is that the dear enemy effect (reduced aggression toward territorial neighbors), which is well demonstrated for the Leon Springs pupfish Cyprinodon bovinus (Leiser and Itzkowitz 2003; Leiser et al. 2006), was more pronounced in C. artifrons than in G. pulchra, resulting in more territorial intrusions in the latter. The dear enemy effect breaks down in the presence of breeding females (Leiser 2003; Leiser et al. 2006), and accordingly, in males at high density, the frequency of courtship in G. pulchra averaged nearly four times that of C. artifrons (15 vs 4 per 20 min).
Another marked difference in territoriality of the two species was that C. artifrons was much less tolerant of heterospecific intrusions. In situations 1 and 2, the frequency of aggression toward heterospecific individuals was, respectively, 20 and 10 times greater in C. artifrons than in G. pulchra. This was true for aggression toward territorial males of the other species as well as toward the poeciliids, P. velifer and G. yucatana. Aggression toward heterospecific (mainly territorial) cyprinodontid males primarily involved simple chases like those directed toward poeciliids encountered as foraging individuals or small shoals. Such aggression likely reduces egg predation and competition for the limited food resources in the male’s territory. These functions are not unrelated because territorial male cyprinodontids (Foster 1967; Loiselle 1983; Klug and St Mary 2005) consume some of their own eggs, potentially allowing males to persist longer in their food-limited territories (but see Klug et al. 2005 for caveats based on tests with Jordanella). The observed heightened frequency of heterospecific aggression in C. artifrons relative to G. pulchra might reflect an innate difference, although this requires further research. Males of various species of Cyprinodon are known to attack and attempt to drive all intruders except receptive females from their territories (Barlow 1961; Echelle 1973; Itzkowitz 1974). For C. variegatus, there is evidence of reduced aggression toward surface-oriented poeciliids compared with bottom-oriented species (Itzkowitz 1974), but our study sites were so shallow (≤ 5 cm deep) that position in the water column likely was not a factor.
Activity budgets of C. artifrons and G. pulchra in the two situations of relative abundance involved marked shifts reflective of apparent tradeoffs affecting frequencies of reproductive and maintenance activities. Both species showed higher heterospecific aggression in situations of low conspecific male density, as quantified for C. pecosensis in two situations with different relative abundances of the pupfish and Fundulus zebrinus (Kodric-Brown and Mazzolini 1992). In that study, as in ours, courtship and spawning were less frequent in the situation of reduced conspecific density, likely reflecting disturbance caused by the greater frequency of heterospecific acts of aggression, as well as the lower abundance of receptive females.
Another conspicuous shift in behavior from low to high density of conspecific males was a marked decrease in maintenance activities (rest, body cleaning, and feeding). This was most pronounced for feeding rate, which at high density was 9 and 28 times lower in, respectively, G. pulchra and C. artifrons. Lower feeding activity in territorial males at high densities is not surprising given their hyperactivity in territorial defense, courtship, and spawning. In a similar situation, male Amargosa pupfish Cyprinodon nevadensis spent 98% of their active time patrolling the territory and chasing intruders, leaving only 2% for other activities (Feldmeth 1983).
The level of feeding by territorial males is context dependent. Feeding acts averaged 29 and 58% of all behaviors in, respectively, C. artifrons and G. pulchra when they were at low intraspecific densities and, by contrast, only 1 and 4%, respectively, in males at high densities. Correspondingly, the indexes of feeding intensity and fat content were much higher in males at low densities. This paralleled the body condition factor (K) in G. pulchra, but not in C. artifrons where K was smaller at the lower density. This likely reflects a difference in body shape resulting from the larger average size of male C. artifrons sampled from the situation of higher density. Male Cyprinodon show pronounced positive allometry in body depth with increasing SL (e.g., Collyer et al. 2005), thus body weight relative to SL would be greater in larger males, resulting in higher K values.
Because of reduced feeding activity, hyperactive territorial defense, and low food availability (Loiselle 1983), individual pupfish males in periods of high breeding intensity probably have an energy deficit for as long as they are on their territories. In C. rubrofluviatilis, males tend to remain on territories day and night except during temperature extremes, although activity is low at night, when they usually are found resting on the bottom substrate (Echelle 1973). In Cyprinodon, defense of the same territory can extend over multiple days in succession. For example, a male C. pecosensis defended the same territory for ≥ 8 days (Kodric-Brown 1978) and a male Sonoyta pupfish Cyprinodon eremus defended the same space for 28 days (Cox 1966). At southern latitudes, the breeding season for cyprinodontids is effectively year-round (Echelle and Echelle 2020), suggesting that turnover of territorial males must be a prominent feature of the reproductive biology.
Extremely low feeding activity and markedly reduced fat content in males of C. artifrons at high densities suggest that they might be capital breeders (Schütz et al. 2010) that consume little energy and rely primarily on stored reserves for territorial activity. This suggests limits to time spent on territories, as suggested for two capital breeding African cichlids (Munthali 1996). Do energy depleted males abandon their territories? If so, do they return after restoring their reserves? Male C. artifrons and G. pulchra in less dense situations with higher feeding activity and higher fat content appear shifted toward the income-breeding strategy (Schütz et al. 2010), where there is greater reliance on feeding to support territorial activities. Regardless, higher fat content at low densities indicates a potential to persist longer on the territories, perhaps compensating for reduced spawning opportunities in the short term. Like C. artifrons, male G. pulchra at high density had lower fat percentages than at low density, but, strikingly, in those situations it was, respectively, 45 and 6 times lower than in C. artifrons, possibly reflecting greater turnover rates of males on territories. This might not hold, however, if male G. pulcra are income breeders that, despite low fat content, consume sufficient energy to maintain territorial activity. Such questions represent an area of investigation that begs further investigation.
Conclusions
It is well established that aggressive interference can account for differences in abundance between species pairs in a wide variety of animals (Grether et al. 2013; Eurich et al. 2018), and this might help explain the dramatic, situation-dependent variation in density and behavior of territorial males in G. pulchra and C. artifrons. In both study situations, males of the more abundant species defended smaller, more densely packed territories and were more active in territorial defense and reproductive behavior. A tradeoff benefit for males of the less abundant species was higher resting and feeding rates and greater energy stores, which, in terms of individual fitness, might compensate for a reduced spawning rate by allowing them to persist longer on their territories, accruing added reproductive gain. Further understanding of patterns described in this study could be achieved by (1) assessing seasonal and between-year variation at our study site, (2) mesocosm studies of behavior and fat content at, for example, different experimental densities of the two species, and (3) studies in other situations of sympatry between G. pulchra and C. artifrons, for example, the Rio Lagartos lagoon in northern Yucatan (Vega-Cendejas and Santillana 2004).
A particularly difficult problem deals with why the relative densities of C. artifrons and G. pulchra showed such a dramatic shift over a short distance (150 m), with C. artifrons dominant at the upper end of the marshy study area and G. pulchra dominant at the lower end near the ocean inlet. At the time of our study, there was a little difference between the two areas, although the transect dominated by G. pulchra did have a little more mangrove cover and the associated central marsh was somewhat deeper. It would be premature to speculate on this question, which requires further study. The short generation times (< 1 year) and hardiness characteristic of cyprinodontids (Echelle and Echelle 2020) makes them especially amenable to in situ, field-based experimentation, for example, the months-long enclosure experiments employed in a study of interspecies population dynamics in Cyprinodon in the wild (Martin and Wainwright 2013).
Availability of data
The data are available upon request to the senior author.
References
Ah‐King M, Kvarnemo C, Tullberg BS (2005) The influence of territoriality and mating system on the evolution of male care: a phylogenetic study on fish. J Evolution Biol 18:371–382. https://doi.org/10.1111/j.1420-9101.2004.00823.x
Anderson JM (2005) PERMANOVA. Department of Statistics, University of Auckland, Permutational multivariate analysis of variance. A computer program
Barlow GW (1958) Daily movements of desert pupfish, Cyprinodon macularius, in shore pools of the Salton Sea, California. Ecology 39:580–587. https://doi.org/10.2307/1931598
Barlow GW (1961) Social behavior of the desert pupfish, Cyprinodon macularius in the field and in the aquarium. Am Midl Nat 65:339–359. https://doi.org/10.2307/2422959
Baylis JR (1981) The evolution of parental care in fishes, with reference to Darwin’s rule of male sexual selection. Environ Biol Fish 6:223–251. https://doi.org/10.1007/BF00002788
Bray JR, Curtis TJ (1957) An ordination of upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349. https://doi.org/10.2307/1942268
Brannan DK, Brannan CR, Lee TE Jr (2003) Reproductive and territorial behavior of Comanche Springs pupfish (Cyprinodon elegans) in San Solomon Spring Pool, Balmorhea State Park, Reeves County, Texas. Southwest Nat 48:85–88
Brown JL (1964) The evolution of diversity in avian territorial systems. Wilson Bull 76:160–169
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Collyer ML, Novak JM, Stockwell CA (2005) Morphological divergence of native and recently established populations of White Sands pupfish (Cyprinodon tularosa). Copeia 2005:1–11. https://doi.org/10.1643/cg-03-303r1
Costa WJEM (1997) Phylogeny and classification of the Cyprinodontidae revisited (Teleostei: Cyprinodontiformes): are Andean and Anatolian killifishes sister taxa? J Comp Biol 2:1–17. https://doi.org/10.1111/j.1463-6409.2007.00314.x
Cowen MC, Drury JP, Grether GF (2020) Multiple routes to interspecific territoriality in sister species of North American perching birds. Evolution 74:2134–2148. https://doi.org/10.1101/843516
Cox TJ (1966) A behavioral and ecological study of the desert pupfish (Cyprinodon macularius) in Quitobaquito Springs, Organ Pipe National Monument, Arizona. Dissertation, University of Arizona, Tucson
Davies NB, Houston AI (1984) Territory economics. In: Krebs JR, Davies NB (eds) Behavioural Ecology: an Evolutionary Approach. Blackwell Scientific, Oxford, pp 148–169
Drury JP, Cowen MC, Grether GF (2020) Competition and hybridization drive interspecific territoriality in birds. P Nat Acad Sci USA 117:12923–12930. https://doi.org/10.1073/pnas.192138011
Echelle AA (1973) Behavior of the pupfish, Cyprinodon rubrofluviatilis. Copeia 1973:68–76. https://doi.org/10.2307/1442359
Echelle AA, Echelle AF (1993) Allozyme variation and systematics of the New World cyprinodontines (Teleostei: Cyprinodontidae). Biochem Syst Ecol 21:583–590. https://doi.org/10.1016/0305-1978(93)90057-x
Echelle AA, Echelle AF (2020) Cyprinodontidae: Pupfishes. In: Warren ML, Burr BM, Echelle AA, Kuhajda BR, Ross ST (eds) Freshwater Fishes of North America, vol 2. Characidae to Poeciliidae. Johns Hopkins University Press, Baltimore, Maryland, pp 609–673
Eurich JG, McCormick MI, Jones GP (2018) Habitat selection and aggression as determinants of fine-scale partitioning of coral reef zones in a guild of territorial damselfishes. Mar Ecol Prog Ser 587:201–215. https://doi.org/10.3354/meps12458
Feldmeth CR (1983) Costs of aggression in trout and pupfish. In: Aspey WP, Lustick SI (eds) Behavioral energetics: the cost of survival in vertebrates. Ohio State University Press, Columbus, pp 117–138
Foster NR (1967) Comparative studies on the biology of killifishes (Pisces, Cyprinodontidae). Dissertation, Cornell University, Ithaca, New York
Gaudreault A, Fitzgerald GJ (1985) Field observations of intraspecific and interspecific aggression among sticklebacks (Gasterosteidae). Behaviour 94:203–211. https://doi.org/10.1163/156853985x00181
Grant JWA (1997) Territoriality. In: Godin JGJ (ed) Behavioural ecology of teleost fishes. Oxford University Press, Oxford, pp 81–103
Grether GF, Anderson CN, Drury JP, Kirschel AN, Losin N, Okamoto K, Peiman KS (2013) The evolutionary consequences of interspecific aggression. Ann NY Acad 1289:48–68
Gross MR, Nowell WA (1980) The reproductive biology of rock bass, Ambloplites rupestris (Centrarchidae), in Lake Opinicon, OntarioMart. Copeia 1980:482–494. https://doi.org/10.2307/1444526
Hagmayer A, Furness AI, Reznick DN, Pollux BJ (2018) Maternal size and body condition predict the amount of post-fertilization maternal provisioning in matrotrophic fish. Ecol Evol 8:12386–12396. https://doi.org/10.1002/ece3.4542
Hammer Ø, Harper DA, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Haney JF, Aliberti MA, Allan E, Allard S, Bauer DJ, Beagen W, Doan U (2013) An image-based key to the zooplankton of North America. University of New Hampshire Center for Freshwater Biology
Itzkowitz M (1974) The effects of other fish on the reproductive behaviour of the male variegated pupfish, Cyprinodon variegatus (Pisces: Cyprinodontidae). Behaviour 48:l–22. https://doi.org/10.1163/156853974x00228
Itzkowitz M (1977) Interrelationships of dominance and territorial behaviour in the pupfish, Cyprinodon variegatus. Behav Ecol 2:383–391. https://doi.org/10.1016/0376-6357(77)90008-0
Itzkowitz M, Minckley WL (1969) Qualitative behavior of a pupfish (Cyprinodon atrorus) in differing environments. Great Basin Nat 29:169–180
Itzkowitz M, Slocum CJ (1994) Is the amount of algae related to territorial defense and reproductive success in the beaugregory damselfish? Mar Behav Physiol 24:243–250. https://doi.org/10.1080/10236249509378899
Kaill WM (1967) Ecology and behavior of the cyprinodontid fishes Jordanella floridae Goode and Bean, Floridichthys carpio (Günther) and Cyprinodon variegatus (Lacepede). Dissertation, Cornell University, Ithaca, New York
Klug H, St Mary CM (2005) Reproductive fitness consequences of filial cannibalism in the flagfish, Jordanella floridae. Anim Behav 70:685–691. https://doi.org/10.1016/j.anbehav.2004.12.015
Klug H, Chin A, St Mary CM (2005) The net effects of guarding on egg survivorship in the flagfish, Jordanella floridae. Anim Behav 69:661–668. https://doi.org/10.1016/j.anbehav.2004.05.019
Kodric-Brown A (1977) Reproductive success and the evolution of breeding territories in pupfish (Cyprinodon). Evolution 1977:750–766. https://doi.org/10.2307/2407437
Kodric-Brown A (1978) Establishment and defence of breeding territories in a pupfish (Cyprinodontidae: Cyprinodon). Anim Behav 26:818–834. https://doi.org/10.1016/0003-3472(78)90147-1
Kodric-Brown A (1986) Satellites and sneakers: opportunistic male breeding tactics in pupfish (Cyprinodon pecosensis). Behav Ecol Sociobiol 19:425–432. https://doi.org/10.1007/bf00300545
Kodric-Brown A, Mazzolini P (1992) The breeding system of pupfish, Cyprinodon pecosensis: effects of density and interspecific interactions with the killifish, Fundulus zebrinus. Environ Biol Fish 35:169–176. https://doi.org/10.1007/BF00002191
Kodric-Brown A, Strecker U (2001) Responses of Cyprinodon maya and C. labiosus females to visual and olfactory cues of conspecific and heterospecific males. Biol J Linn Soc 74:541–548. https://doi.org/10.1006/bijl.2001.0598
Leiser JK (2003) When are neighbours ‘dear enemies’ and when are they not? The responses of territorial male variegated pupfish, Cyprinodon variegatus, to neighbours, strangers and heterospecifics. Anim Behav 65:453–462. https://doi.org/10.1006/anbe.2003.2087
Leiser JK, Itzkowitz M (2002) The relative costs and benefits of territorial defense and the two conditional male mating tactics in the Comanche Springs pupfish (Cyprinodon elegans). Acta Ethol 5:65–72. https://doi.org/10.1007/s10211-002-0066-1
Leiser J, Itzkowitz M (2003) The costs and benefits of territorial neighbours in a Texas pupfish (Cyprinodon bovinus). Behaviour 140:97–112. https://doi.org/10.1163/156853903763999926
Leiser JK, Itzkowitz M (2004) Changing tactics: dominance, territoriality, and the responses of “primary” males to competition from conditional breeders in the variegated pupfish (Cyprinodon variegatus). Behav Process 66:119–130. https://doi.org/10.1016/j.beproc.2004.01.008
Leiser JK, Bryan CM, Itzkowitz M (2006) Disruption of dear enemy recognition among neighboring males by female Leon Springs pupfish, Cyprinodon bovinus. Ethology 112:417–423. https://doi.org/10.1111/j.1439-0310.2006.01166.x
Leiser JK, Gagliardi‐Seeley JL, Wisenden BD, Itzkowitz M (2015) Mating patterns of female Leon Springs pupfish Cyprinodon bovinus. J Fish Biol 87:604–615 https://doi.org/10.1111/jfb.12738
Liu RK (1969) The comparative behavior of allopatric species (Teleostei-Cyprinodontidae: Cyprinodon). Dissertation, University of California, Los Angeles
Loiselle PV (1981) Garmanella pulchra: a sparkling killifish from the Yucatan. Freshwater Marine Aquarium Magazine 4(44–46):76–77
Loiselle PV (1983) Filial cannibalism and egg recognition by males of the primitively custodial teleost Cyprinodon macularius californiensis Girard (Atherinomorpha: Cyprinodontidae). Ethol Sociobiol 4:1–9. https://doi.org/10.1016/0162-3095(83)90002-X
Maher CR, Lott FD (2000) A review of ecological determinants of territoriality within vertebrate species. Am Midl Nat 14:1–29. https://doi.org/10.1674/0003-0031(2000)143[0001:aroedo]2.0.co;2
Martin P, Bateson P (1986) Measuring behaviour: an introductory guide. Cambridge University Press, Cambridge
Martin CH, Wainwright PC (2013) Multiple fitness peaks on the adaptive landscape drive adaptive radiation in the wild. Science 339:208–211. https://doi.org/10.1126/science.1227710
Mertz JC, Barlow GW (1966) On the reproductive behavior of Jordanella floridae (Pisces: Cyprinodontidae) with special reference to a quantitative analysis of parental fanning. Zeitschrift Tierpsych 23:537–554. https://doi.org/10.1111/j.1439-0310.1966.tb01611.x
Munthali SM (1996) Territoriality and nutritional condition in Cynotilapia afra (Gunther) and Pseudotropheus zebra (Boulenger), cichlidae in Lake Malawi National Park, Malawi. J Appl Ichthyol 12:131–134. https://doi.org/10.1111/j.1439-0426.1996.tb00076.x
Myrberg AA Jr, Thresher RE (1974) Interspecific aggression and its relevance to the concept of territoriality in reef fishes. Am Zool 14:81–96. https://doi.org/10.1093/icb/14.1.81
Ord TJ (2021) Costs of territoriality: a review of hypotheses, meta-analysis, and field study. Oecologia 197:615–631. https://doi.org/10.1007/s00442-021-05068-6
Paciorek T, Al-Shaer L, Itzkowitz M (2014) How territoriality affects the density of an egg predator: Habitat renovation and reintroduction as a method of conserving two endangered desert spring fish. Curr Zool 60:527–533. https://doi.org/10.1093/czoolo/60.4.527
Parenti LR (1981) A phylogenetic and biogeographic analysis of cyprinodontiform fishes (Teleostei, Atherinomorpha). B Am Mus Nat Hist 168:335–557
Parker A, Kornfield I (1995) Molecular perspective on evolution and zoogeography of cyprinodontid killifishes (Teleostei; Atherinomorpha). Copeia 1995:8–21. https://doi.org/10.2307/1446795
Peiman K, Robinson B (2010) Ecology and evolution of resource-related heterospecific aggression. Q Rev Biol 85:133–158. https://doi.org/10.1086/652374
Plath M, Strecker U (2008) Behavioral diversification in a young species flock of pupfish (Cyprinodon spp.): shoaling and aggressive behavior. Behav Ecol Sociobiol 62:1727–1737
Richter HJ (1975) The snakeskin killie, Garmanella pulchra. Tropical Fish Hobbyist 23:38–40: 42–44, 46–48
Schütz D, Pachler G, Ripmeester E, Goffinet O, Taborsky M (2010) Reproductive investment of giants and dwarfs: specialized tactics in a cichlid fish with alternative male morphs. Funct Ecol 24:131–140. https://doi.org/10.1111/j.1365-2435.2009.01605.x
St Mary CM, Noureddine CG, Lindström K (2001) Environmental effects on male reproductive success and parental care in the Florida flagfish Jordanella floridae. Ethology 107:1035–1052. https://doi.org/10.1046/j.1439-0310.2001.00747.x
Thresher RE (1976) Field analysis of the territoriality of the threespot damselfish, Eupomacentrus planifrons (Pomacentridae). Copeia 1976:266–276. https://www.jstor.org/stable/1443946
Vega-Cendejas ME, de Santillana MH (2004) Fish community structure and dynamics in a coastal hypersaline lagoon: Rio Lagartos, Yucatan, Mexico. Estuar Coast Shelf S 60:285–299. https://doi.org/10.1016/j.ecss.2004.01.005
von Kuerthy C, Tschirren L, Taborsky M (2015) Alternative reproductive tactics in snail shell-brooding cichlids diverge in energy reserve allocation. Ecol Evol 5:2060–2069. https://doi.org/10.1002/ece3.1495
Zacharia PU (2017) Trophic levels and methods for stomach content analysis of fishes. Course Manual Summer School on Advanced Methods for Fish Stock Assessment and Fisheries Management. Lecture Note Series No. 2/2017. Kochi, India: Central Marine Fisheries Research Institute
Acknowledgements
We thank A. F. Echelle for helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization of the project, field observations, and funding (ODC); laboratory work (ODC, TMMC, SVC); data analysis and manuscript preparation (ODC, TMMC, SVC, AAE).
Corresponding author
Ethics declarations
Ethics approval
We follow the current standards for handling and euthanizing animals of the Government of Mexico (NOM-062-ZOO-199); and the guidelines for researchers issued by The International Council for Laboratory Animal Science.
Consent for publication
All authors consent to publish.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Domínguez-Castanedo, O., Muñoz-Campos, T.M., Valdez-Carbajal, S. et al. Behavioral ecology in co-occurring territorial males of the pupfishes, Garmanella pulchra and Cyprinodon artifrons, at reciprocally asymmetric densities in a mangrove floodplain. acta ethol 27, 51–64 (2024). https://doi.org/10.1007/s10211-023-00433-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-023-00433-5