Abstract
In this contribution, we offer new information about the advertisement call of Peltophryne cataulaciceps, an endemic toad species from Cuba and the smallest bufonid from the West Indies. We measured seven acoustic properties from 17 males and analyzed the variability at the within-individual and between-individual levels, using coefficients of variation, type II ANOVAs, and multivariate analysis. Dominant frequency was distinctly less variable within individuals than the rest of the acoustic properties; call rise time showed the highest variability. Variability between individuals was higher for pulse rate, call duration, and dominant frequency, and the CVb/CVw ratios showed that these acoustic properties are more reliable for individual distinctiveness. Discriminant function analyses assigned 54.1% of the calls to the correct individual, and this classification success increased when smaller groups of individuals were considered in the analysis. Results are compared with studies addressing individual acoustic distinctiveness in anurans. We support that the patterns of advertisement call variation within and among co-occurring males differ among explosive and prolonged breeding species/populations, but additional case studies including other explosive breeding species are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual reproduction creates an intraspecific environment of conflict and competition among individuals where each strives to maximize its genetic contribution to subsequent generations (Alcock 2005). Variation in the spatial and temporal distribution of breeding resources and availability of receptive females determine the dynamic nature of mating system evolution (Shuster 2009). In most anurans, the timing and duration of reproductive activity in response to abiotic conditions can lead to variation in population densities at breeding sites, the relative numbers of males and females, and the strength of male-male competition (Wells 2007). The diversity of anuran temporal breeding patterns has thus been described as a continuum between two extremes: explosive breeders (characterized by the synchronous arrival of females to ephemeral breeding sites) and prolonged breeders (characterized by the asynchronous arrival of females over a prolonged breeding season) (Wells 1977; Wells 2007).
Acoustic communication plays a key role in mate recognition in anurans, and usually, females respond phonotactically to the vocalizations of conspecific males (Gerhardt 1988, 1994; Ryan 1988). Some features of anuran calls are highly stereotyped, with little variation within or among individuals (Gerhardt 1991), while others are more labile and can be strongly affected by social interactions among males in a chorus (Wells 1988). The analysis of the variation in acoustic properties in a behavioral context can provide hints about mechanisms of sound production and their potential for encoding biologically important information (Gerhardt and Huber 2002). In the context of male-male competition, vocal signals can provide important information about the signaler—body size, fighting ability, physiological condition, individual identity—that can be used by male receivers to determine the most appropriate behavioral response (Gerhardt and Bee 2007).
Because assemblages of explosive breeders usually last few days and attain high densities, females of those species are constrained in their ability to compare and select a mate within this scramble competition mating system. Conversely, prolonged breeders have relatively longer breeding periods and usually exhibit lek polygyny or resource defense mating systems where gravid females may arrive at the breeding area over an extended time period and have the potential to evaluate and compare attributes (advertisement call features, visual displays, defended territory, etc.) of different males (Wells 1977; Bee et al. 2013). Logically, the patterns of advertisement call variation within and among co-occurring males should differ among explosive and prolonged breeding species/populations each subjected to a different mating system. In this comparison between the two ends of the breeding phenology spectrum, it is expected that calls of explosive breeding species should be less variable among neighboring calling males as female mate choice and individual recognition should play a lesser role in these scramble competition systems.
Most of the studies on the variability of acoustic signals and its role in the social behavior in anurans has typically involved prolonged breeding species (Sullivan and Leek 1987; Arak 1988; Ryan and Sullivan 1989; Sullivan 1992; Wagner and Sullivan 1995; Castellano and Giacoma 1998; Doglio et al. 2001; Castellano et al. 2003). Comparatively less information exists on the variability in the advertisement calls of explosive breeding anurans (but see Davies and Halliday 1978; Howard and Palmer 1995; Howard and Young 1998; Bee 2007). The analysis of the patterns of variability in advertisement call features, female choice, and social interactions of frogs and toads will benefit from a broader taxonomic scope, necessary to encompass the extraordinary diversity of reproductive modes and mating systems exhibited by anurans (Bee et al. 2013).
The Cuban pineland toad, Peltophryne cataulaciceps Schwartz 1959, is the smallest known species of bufonid from the West Indies (Schwartz and Henderson 1991). This toad inhabits open pinewoods and sandy savannas with palms and herbaceous vegetation, from the extreme southwest of Pinar del Río province, Cuba, and the northern two thirds of Isla de la Juventud (Schwartz 1959; Díaz and Cádiz 2008; Henderson and Powell 2009). Males call in the afternoon, especially after heavy rains, hidden below bushes and grasses in flooded and open areas (Alonso et al. 2007). P. cataulaciceps has been considered as an explosive breeder during the reproductive season; it is uncommon or rare out of this period, and its larvae complete the metamorphosis in 15–18 days (Díaz and Cádiz 2008). We herein characterize the advertisement call of P. cataulaciceps using a sample of males from a breeding aggregation in Isla de la Juventud and examine the patterns of variability of the temporal features and dominant frequency in two levels of analysis: within and between individuals in this single population. We additionally explored the extent to which acoustic variation could allow for individual discrimination in a noisy breeding chorus of this explosive breeding species and which acoustic properties play the leading role.
Materials and methods
Recordings and call analysis
Recordings of the males of P. cataulaciceps were obtained by the authors on August 6, 2006, at the Ecological Reserve “Los Indios,” Isla de Pinos, Cuba (21° 41′ 27.5″ N, 82° 59′ 35.7″ W), using Marantz PMD-430 and Sony WMD6 cassette tape recorders and Sennheiser ME 80 or Realistic Highball microphones. The equipment employed exhibits a flat frequency response (±3 dB) in the 50–15,000 Hz range. To avoid animal perturbation and to improve recording quality (best signal-to-noise ratio), the microphone was placed approximately 50 cm in front of the caller. All recordings were carried out between 15:30 and 18:30 h in an active chorus that started after a very heavy rain. As the calls of P. cataulaciceps are emitted in groups (see results), we recorded the calling activity of each male for at least 2 min; in total, 19 males were recorded, and for 17 of them, three consecutive call groups were available for analysis. Immediately after each recording session, air temperature (AT) was measured with a Miller and Weber quick-reading thermometer (error 0.2 °C). Fifteen calling vouchers were captured and their snout-vent length (SVL) measured to the nearest 0.01 mm with a caliper.
Tape recordings were digitized onto a Pentium computer fitted with a Sound Blaster Creative card at 44.1-kHz sampling frequency and 16-bit resolution, using the software Cool Edit Pro 1.2 (Syntrillium Software Corporation). Sound analysis was performed with the aid of Raven 1.3 software (Bioacoustics Research Program, Cornell Lab of Ornithology), and the following acoustic properties were measured: Dominant frequency (DF) was measured to the nearest 0.02 kHz (FFT 2048 points) in the power spectrum of a 16-pulse segment from the middle of each call. Since no frequency modulation was present in the advertisement calls, this provides a good estimate of call DF. The following temporal properties were measured to the nearest 0.01 s on the oscillograms: call duration (CD), call rise time (CRT) (time span from call onset to the point of maximum amplitude), call-group duration (CGD, defined as a continuous group of calls delimited by intervals of silence at least twice as long as the maximum inter-call interval in the group), and call rate (CR, calculated as the number of calls divided by the duration of the entire call group). Additionally, the pulse rate (PR) was calculated as the ratio between the number of pulses per call and CD. To evaluate within-individual variation, we used the recordings containing three consecutive call groups (17 individuals) and measured call properties in ten calls from the first, second, and last call group; totaling 30 measurements per male.
Statistical analysis
Temperature and body size can affect both spectral and temporal acoustic properties of anuran calls (Ryan and Wilczynski 1991; Gerhardt 1994; Castellano et al. 2002). Regression analysis of each acoustic property on body size (SVL) and AT was used to assess the temperature and size effects on call properties. For this purpose, we used averaged measurements of each call property for every call group and then averaged these values to obtain a male’s mean.
Variation in acoustical properties of the advertisement calls of a chorus of frogs can be structured in several levels. Of these, the variation observed between call groups of a given male and the variation existing between males are very important for individual distinctiveness. To examine these sources of variation in our data, we used model II ANOVAs (Sokal and Rohlf 2000) specifying call group (three levels: first, second, and third) and individuals (17 levels) as random factors and then examined the variance components of these two main factors. As an indicator of the relative importance of variation observed between individuals, we report the partial effect size (η 2) of the factor “individual” included in each model. We checked model assumptions by visual inspections of plots of residuals against fitted values. Regressions and ANOVAs were carried on using Statistica 8.0 software (StatSoft Inc. 2007).
We also analyzed both within- and between-male variability of call properties by calculating coefficients of variation (CV = (SD/mean)*100). Within-male variability was evaluated using all the values of call properties for a given male (30 calls from three consecutive call groups). Between-male variability was calculated using the average and standard deviation values of all males recorded. As a measure of the individual distinctiveness of acoustic properties, we calculated the ratio of between-male to within-male (within a single call sequence) coefficients of variation (CVb/CVw), following Bee et al. (2001).
Discriminant function analysis (DFA) has been commonly used to assess the potential for acoustic distinctiveness of individuals in intraspecific choruses (Bee et al. 2001; Fischer et al. 2002). However, these multivariate analyses of a large number of individuals can exaggerate the acoustic discrimination task faced by anuran males or females, which is probably restricted to a small number of adjacent callers (Pettitt et al. 2013; Bee et al. 2016). For these reasons, we implemented a randomized subsample procedure in R to evaluate the efficiency of classification by DFA models when applied to biologically relevant group sizes. We used the data of 30 calls from 17 individuals (510 calls in total) and designed an R-script so that 1000 replicated DFA were carried out including combinations of two to 16 individuals selected randomly without replacement. For each replicate, classification success was estimated by a leave-one-out procedure (a DFA model is iteratively fitted with all but one of the observations, which is reserved for model testing). At the end of each run, an average classification success for a given group size was calculated from the values of the 1000 replicates. We compared the results of these subsampled DFA to those obtained using the full dataset, predicting that classification success would be higher with smaller group sizes. Correlations among predictors can bias the results of DFA; we followed the recommendations of Dormann et al. (2013) and examined the correlation among acoustic variables before its inclusion in DFA using a 0.7 Pearson correlation threshold. No evidence of multicollinearity was found among the five acoustic variables (−0.52 < r < 0.48), and all were included in the DFA.
Results
Advertisement call design
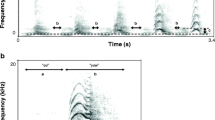
The call is a loud and heavily pulsed trill emitted in groups. Calls have a rather noisy frequency spectrum in which the fundamental frequency is the dominant frequency (Fig. 1a, b). The inner structure of a single call reveals a pattern of perfectly distinguishable pulses with no gaps between them. Pulses have a very short rise time and a longer fall time (Fig. 1c, d, e). Table 1 summarizes the values obtained for the call parameters measured in the population (N = 19 indivs.).
Characteristics of the advertisement calls of Peltophryne cataulaciceps. a Oscillogram (top) and spectrogram (bottom) of a single call [FFT size 2048, Hanning windows, overlap 50%]. b Three consecutive call groups. c Fragments of five consecutive calls from the third group illustrated in b. d Pulse structure of the last call in c. e Ten pulses from the middle of the call in d
Advertisement call variation
The range in male size of the analyzed sample was 20.4–23.9 mm (mean ± SD = 22.13 ± 0.72, ΔSVL = 3.51 mm, N = 15 indivs.), and the temperature range was also slight, 26.2–29.4 °C (mean ± SD = 28.1 ± 0.7, ΔAT = 3.2 °C, N = 19 indivs.). There was no relationship between any of the call variables and SVL (CGD: R 2 = 0.03, intercept = 58.52, F = 1.52, p > 0.05; CR: R 2 = −0.06, intercept = 129.76, F = 0.22, p > 0.05; CD: R 2 = −0.04, intercept = 0.08, F = 0.39, p > 0.05; PR: R 2 = 0.03, intercept = 365.03, F = 1.49, p > 0.05; DF: R 2 = −0.05, intercept = 3.48, F = 0 .39, p > 0.05). We also did not find a relationship between call properties and air temperature (CGD: R 2 = −0.05, intercept = 3.59, F = 0.07, p > 0.05; CR: R 2 = 0.06, intercept = 14.77, F = 2.08, p > 0.05; CD: R 2 = −0.03, intercept = 0.26, F = 0.48, p > 0.05; PR: R 2 = −0.05, intercept = 197.39, F = 0.05, p > 0.05; DF: R 2 = −0.01, intercept = 4.84, F = 0.86, p > 0.05). According to these results, no temperature or size correction was performed, and the original measurements were used in subsequent analysis.
All advertisement call properties examined in this study exhibited greater variability among individual than within-individual, with the exception of call rise time and call rate, which showed the highest average within-individual coefficients of variation (Table 1). Dominant frequency and pulse rate had the lowest within-individual coefficients of variation. Between males, the dominant frequency was distinctly less variable than the rest of the acoustic properties while call rise time and call duration were the more variable features (Table 1).
Differences between individuals explained an important part of the variation present in each call property in model II ANOVAs, and the effect of call group was much smaller (Fig. 2a). Significant differences were observed between individuals in all models (Table 1). The largest effects of individual variability were observed in call dominant frequency, call duration, and pulse rate, whereas smaller effect sizes were observed in the models derived for call rise time and call rate (Table 1).
Variability observed in five acoustic properties of 17 males of Peltophryne cataulaciceps. a Variance components estimated by model II ANOVAs considering call sequence (white) and individual (black) as random factors; the residual variance of each model is depicted in gray. b Ratio of between male to within male coefficient of variation (CVb/CVw ratios). Names of the acoustical variables, abbreviated as in Table 1
Individual distinctiveness
The analysis of the CVb/CVw ratios showed that pulse rate, call duration, dominant frequency, and call group duration are the acoustic properties most reliable for individual distinctiveness (CVb/CVw > 1). Call rate and call rise time are more variable within than between individuals (CVb/CVw < 1) and thus carry less information for that goal (Fig. 2b).
The DFA generated of the full dataset classified 54.1% of the calls to the correct individual (Fig. 3a). This success rate is significantly greater than the 5.9% value that can be expected under a random classification (chi square = 260.31, df = 1, p < 0.001). The first three roots of the canonical functions explained 95% of the variation in the dataset and were largely determined by DF, PR, and CD (Table 2). Statistical discrimination functions derived from subsampled datasets achieved better classification success. The maximum average classification success (94.4%) was observed when the discernment task was reduced to only two individuals. As the number of individuals included in the analysis increases, the classification success decreased (Fig. 3b).
Acoustic distinctiveness between individual males of Peltophryne cataulaciceps. a Canonical scores of the first two functions from a discriminant function analysis using the full dataset (510 calls from 17 individuals); ellipses indicate the 95% confidence intervals of each individual’s scores, and the contributions of acoustic properties are indicated in the axes. b Average classification success of discriminant functions derived from smaller datasets including 2 to 16 individuals randomly subsampled from the full dataset (the results of the complete dataset are included as a white dot)
Discussion
Although the acoustic measurements obtained in this work are comparable with homologous measurements reported in previous characterizations of the advertisement calls of P. cataulaciceps (Table 3), in this contribution, we offer a most exhaustive description based on a larger dataset, more individuals, more calls, and more properties analyzed. New efforts should be done in the future to characterize additional populations and obtain estimates of the acoustic variability at the inter-population level. All the acoustic data available for this species come from the same locality in Isla de la Juventud in the same season (July–August). This might be a consequence of the scarcity of the species, which is listed as Endangered (Rivalta-González 2012; IUCN 2016). The correct identification of advertisement calls using its spectral and temporal features can be a useful tool to monitor acoustically the presence of this threatened, rare, and explosive breeding species, improving our ecological understanding about its breeding phenology and relative abundance.
Only two previous studies have provided information on the variability of call properties of Cuban Peltophryne (Alonso and Rodríguez 2005; Hernández et al. 2010). Alonso and Rodríguez (2005), after comparing recordings of individual males obtained in two different nights, reported that pulse rate and dominant frequency were the least variable properties of the advertisement call of Peltophryne fustiger. Hernández et al. (2010) observed a similar pattern of within-individual variation in Peltophryne florentinoi. According to our data, dominant frequency and pulse rate are the least variable properties of the calls of P. cataulaciceps. Both variables could be classified as static properties based on the framework of Gerhardt (1991). Low within-male variability in dominant frequency (highly stereotyped within a single call group or individual) and pulse rate has been reported for other frogs and toads and seems to be a general pattern across anurans (Castellano and Giacoma 1998; Howard and Young 1998; Giacoma and Castellano 2001; Castellano et al. 2002). The considerably less variability in dominant frequency can be understood within the context of morphological constraints on sound production in anurans and could be important for species recognition in the ecological scenario where at least other two sympatric species of toads vocalize synchronously in dense choruses (Peltophryne gundlachi and Peltophryne empusa).
For the purpose of this study, the lack of a temperature or size effect on the call properties is a desirable condition which allows for straightforward comparisons among males. It is likely, however, that male size and temperature do have a statistical effect on the call features of P. cataulaciceps, but more variation in these predictors is necessary to detect it.
In “explosive breeders” (Wells 1977), reproduction occurs over a short period (e.g., <24 h) and commonly takes place in ephemeral or otherwise spatially and temporally unpredictable water bodies. This reproductive dynamic is characteristic of many anurans, mainly from the temperate region: Bufonidae, Ranidae, Pelobatidae (Van Gelder and Hoedemaekers 1971; Oldham 1974; Wells 1977; Gittins et al. 1980; Elmberg 1990; Kusano and Fukuyama 1989; Hartel et al. 2007; Wells 2007). Schwartz (1959) collected only two P. cataulaciceps specimens not associated with choruses. Our field observations suggest that this species is an explosive breeder with its mating activity limited to a few days each season. In the same study site, we have conducted fieldwork for several days in the rainy seasons of 1996, 1999, and 2006, and during the first two visits, only very sparse vocal activity was heard. It was only after a heavy rain in 2006 that a breeding aggregation was observed in a shallow and ephemeral rain puddle. As in other explosive breeding species, the sound of the chorus of P. cataulaciceps males could serve as an acoustic beacon that facilitates gravid female orientation toward the breeding aggregation (Bogert 1960; Oldham 1966, 1967; Wells 1977; Gerhardt and Klump 1988; Bee 2007). It has been demonstrated that some species of frogs learn to recognize the calls of their familiar neighbors based on the individually distinctive calls (Bee and Gerhardt 2001a, b). Based on our observations, P. cataulaciceps males are not territorial and change their calling sites frequently. It can thus be speculated that the “dear enemy” effect is not present in this species and males probably do not learn the calls of familiar neighbors. Although this hypothesis will require field observations of a larger number of individuals and experimental testing, this contribution could be a useful initial point for future comparative analysis.
Although other studies have been carried out across multiple nights, the values of the CVb/CVw ratio obtained for the acoustic properties of P. cataulaciceps calls (minimum–maximum 0.8–1.7) are lower than those reported for other species of anurans: Rana catesbeiana (1.1–4.7) (Bee and Gerhardt 2001a), Rana clamitans (1.2–2.5) (Bee et al. 2001), Pseudacris maculata (0.6–4.8) (Bee et al. 2010), Anomaloglossus beebei (0.8–3.2) (Pettit et al. 2013), and Hyla versicolor (0.7–3.1) (Reichert 2013). Contrary to P. cataulaciceps, all these species have a relatively longer breeding period, and it might be hypothesized that individual distinctiveness is a less advantageous feature in explosive breeding species where the high spatial density of extremely excited males might result in quick female interception with little room for the females to pick and choose a mate. However, the values of the CVb/CVw ratio reported for three other species with prolonged breeding seasons, and tropical distributions, are comparable to those obtained in this study: Odorrana tormota (0.58–1.0) (Feng et al. 2009), Allobates femoralis (0.68–1.85) (Gasser et al. 2009), and Oophaga pumilio (0.75–1.76) (Meuche et al. 2013). In the case of O. tormota, the authors hypothesized that the relatively low acoustic differentiation between individuals results from the evolutionary pressure resulting from ultrasonic emissions. The other two examples of low CVb/CVw ratio (A. femoralis and O. pumilio) are terrestrial species with a relatively long breeding season during which males actively defend calling territories (Gasser et al. 2009; Meuche et al. 2013). Future advances with this and other explosive breeder species could be useful to corroborate a possible hypothesis relating reproductive dynamic and call variability (ecological patterns and potential individual discrimination).
Wells (1988) suggested that in many anurans, the females probably enter a chorus and move to an area where several males are calling, but mates probably are chosen from a small subset of males in the chorus. The inverse relationship between the predicted acoustic distinctiveness and the number of individuals included in the comparison is likely an inherent property of DFA, and future studies should attempt to compare this relationship across several species or use null model simulations in order to obtain a better estimate on the group size effect in DFA. However, our results are in line with others that reported an increase in classification success when smaller groups of individuals are considered (Bee and Gerhardt 2001a; Bee et al. 2001).
Very little is known about the mating system of P. cataulaciceps. From our observations, two alternatives are at least possible: the first is that females quickly select the preferred male from a small subset of males in the chorus, using acoustic features to discriminate among them. The other possibility would be that the females do not discriminate the subtle acoustic differences among neighboring males and mate with the one that remains in amplectant position while resisting the clasping attempts of other competitors. This pattern has been observed in other explosive anuran species (Wilbur 1978; Kruse 1981; Woodward 1982). In addition to the acoustic features measured in this study, individual distinctiveness could also benefit from differences in the intensity of the calls, as have been described for other anurans (Castellano et al. 2000; Bush et al. 2001; Gerhardt 2005), but then the relative position of the callers could easily misinform the females approaching the chorus. In general, additional field observations and experimental studies are needed to improve our knowledge of the mating system of P. cataulaciceps.
References
Alcock J (2005) Animal behaviour—an evolutionary approach. 8th ed. Sinauer Associates, Sunderland
Alonso R, Rodríguez A (2003) Advertisement calls of Cuban toads of the genus Bufo (Anura: Bufonidae). Phyllomedusa 2:75–82
Alonso R, Rodríguez A (2005) Intra-population variation in the advertisement calls of the Cuban giant toad Bufo fustiger (Anura: Bufonidae). Rev Biol 10:89–93
Alonso R, Rodríguez A, Márquez R (2007) Guía sonora de los anfibios de Cuba. Sound guide of the amphibians of Cuba. Audio CD + booklet. Alosa sons de la natura, Barcelona, Spain
Arak A (1988) Female mate selection in the natterjack toad: active choice or passive attraction? Behav Ecol Sociobiol 22:317–327
Bee MA (2007) Selective phonotaxis by male wood frogs (Rana sylvatica) to the sound of a chorus. Behav Ecol Sociobiol 61:955–966
Bee MA, Gerhardt HC (2001a) Neighbour-stranger discrimination by territorial male bullfrogs (Rana catesbeiana): I. Acoustic basis. Anim Behav 62:1129–1140
Bee MA, Gerhardt HC (2001b) Neighbour-stranger discrimination by territorial male bullfrogs (Rana catesbeiana): II. Perceptual basis. Anim Behav 62:1141–1150
Bee MA, Reichert MS, Tumulty J (2016) Assessment and recognition of rivals in anuran contests. In: Mitani JC, Simmons LW, Barrett L, Healy S, Zuk M, Naguib M. (eds) Advances in the study of behavior. Academic Press Elsevier, Cambridge, pp 161–249
Bee MA, Schwartz JJ, Summers K (2013) All’s well that begins wells: celebrating 60 years of animal behaviour and 36 years of research on anuran social behaviour. Anim Behav 85:5–18
Bee MA, Kozich CE, Blackwell KJ, Gerhardt HC (2001) Individual variation in advertisement calls of territorial male green frogs, Rana clamitans: implications for individual discrimination. Ethology 107:65–84
Bee MA, Cook JM, Love EK, O’Bryan LR, Pettitt BA, Schroder K, Vélez A (2010) Assessing acoustic signal variability and the potential for sexual selection and social recognition in boreal chorus frogs (Pseudacris maculata). Ethology 116:564–576
Bogert CM (1960) The influence of sounds on the behavior of amphibians and reptiles. In: Lanyon WE, Tavolga WN (eds) Animal sounds and communication. American Institute of Biological Sciences, Washington, DC, pp 137–320
Bush SL, Gerhardt HC, Schul J (2001) Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim Behav 63:7–14
Castellano S, Giacoma C (1998) Stabilizing and directional female choice for male calls in the European green toads. Anim Behav 56:275–287
Castellano S, Giacoma C, Ryan MJ (2003) Call degradation in diploid and tetraploid green toads. Biol J Linn Soc 78:11–26
Castellano S, Rosso A, Laoretti F, Doglio S, Giacoma C (2000) Call intensity and female preferences in the European green toad. Ethology 106:1129–1141
Castellano S, Cuatto B, Rinella R, Rosso A, Giacoma C (2002) The advertisement call of the European treefrogs (Hyla arborea): a multilevel study of variation. Ethology 108:75–89
Davies NB, Halliday TR (1978) Deep croaks and fighting assessment in toads Bufo bufo. Nature 274:683–685
Díaz LM, Cádiz A (2008) Guía taxonómica de los anfibios de Cuba. AbcTaxa 4:1–294
Doglio SS, Castellano S, Giacoma C (2001) The role of the advertisement call in the competitive interactions among males of the European green toad, Bufo viridis. In: Atti 1° Covegno Societas Herpetologica Italica. Italy
Dormann C, Elith J, Bacher S, Buchmann C, Carl G, Carré G, García-Marquéz JR, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46
Elmberg J (1990) Long-term survival, length of breeding season, and operational sex ratio in a boreal population of common frogs, Rana temporaria L. Can J Zool 68:121–127
Feng AS, Riede T, Arch VS, Yu ZL, Xu ZM, Yu XJ, Shen JX (2009) Diversity of the vocal signals of concave-eared torrent frogs (Odorrana tormota): evidence for individual signatures. Ethology 115:1015–1028
Fischer J, Hammerschmidt K, Cheney DL, Seyfarth RM (2002) Acoustic features of male baboon loud calls: influences of context, age, and individuality. J Acoust Soc Am 1115:1465–1474
Gasser H, Amézquita A, Hödl W (2009) Who is calling? Intraspecific call variation in the aromobatid frog Allobates femoralis. Ethology 115:596–607
Gerhardt HC (1988) Acoustic properties used in call recognition by frogs and toads. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. John Wiley, New York, pp 455–483
Gerhardt HC (1991) Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim Behav 42:615–635
Gerhardt HC (1994) The evolution of vocalizations in frogs and toads. Annu Rev Ecol Syst 25:293–324
Gerhardt HC (2005) Acoustic spectral preferences in two cryptic species of grey treefrogs: implications for mate choice and sensory mechanisms. Anim Behav 70:39–48
Gerhardt HC, Klump GM (1988) Phonotactic responses and selectivity of barking treefrogs (Hyla gratiosa) to chorus sounds. J Comp Physiol A 163:795–802
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press, Chicago
Gerhardt HC, Bee MA (2007) Recognition and localization of acoustic signals. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Hearing and sound communication in amphibians. Springer, New York, USA, pp 113–146
Giacoma C, Castellano S (2001) Advertisement call variation y speciation in the Bufo viridis complex. In: Ryan MJ (ed) Anuran communication. Smithsonian Institution Press, pp 205–209
Gittins SP, Parker AG, Slater FM (1980) Population characteristics of the common toad (Bufo bufo) visiting a breeding site in Mid-Wales. J Anim Ecol 49:161–173
Hartel T, Sas I, Pernetta AP, Geltsch IC (2007) The reproductive dynamics of temperate amphibians: a review. North West J Zool 3:127–145
Hernández M, Alonso R, Rodríguez A (2010) Advertisement call of Peltophryne florentinoi (Anura: Bufonidae), an endemic toad from Zapata Swamp. Cuba Amphibia-Reptilia 31:265–272
Henderson RW, Powell R (2009) Natural history of West Indian reptiles and amphibians. Florida, University Press of Florida, Gainesville
Howard RD, Palmer JG (1995) Female choice in Bufo americanus: effects of dominant frequency and call order. Copeia 1995:212–217
Howard RD, Young JR (1998) Individual variation in male traits and female mating preferences in Bufo americanus. Anim Behav 55:1165–1179
IUCN (2016) Peltophryne cataulaciceps. The IUCN red list of threatened species v. 2016-2. Http://www.iucnredlist.org [accessed 13 October 2016]
Kruse KC (1981) Mating success, fertilization potential, and male body size in the American toad (Bufo maericanus). Herpetologica 37:228–233
Kusano T, Fukuyama K (1989) Breeding activity of a stream-breeding frog (Rana sp.). In: Matsui M et al (eds) Current Herpetology in East Asia. Herpetological Society of Japan, Kyoto, pp 314–322
Meuche I, Brusa O, Linsenmair KE, Keller A, Pröhl H (2013) Only distance matters—non-choosy females in a poison frog population. Front Zool 10:29. doi:10.1186/1742-9994-10-29
Oldham RS (1966) Spring movements in the American toad, Bufo americanus. Can J Zool 44:63–100
Oldham RS (1967) Orienting mechanisms of the green frog, Rana clamitans. Ecology 48:477–491
Oldham RS (1974) Mate attraction by vocalization in members of the Rana pipiens complex. Copeia 1974:982–984
Pettitt BA, Bourne GR, Bee MA (2013) Advertisement call variation in the golden rocket frog (Anomaloglossus beebei): evidence for individual distinctiveness. Ethology 119:244–256
Reichert MS (2013) Sources of variability in advertisement and aggressive calling in competitive interactions in the grey treefrog, Hyla versicolor. Bioacoustics. doi:10.1080/09524622.2013.777942
Rivalta-González V (2012) Hoja de datos del taxon: Peltophryne cataulaciceps. In: González Alonso H, Rodríguez Schettino L, Rodríguez A, Mancina CA, Ramos García I (eds) Libro Rojo de los Vertebrados de Cuba. Editorial Academia, La Habana, pp 88–89
Ryan MJ (1988) Constraints and patterns in the evolution of anuran acoustic communication. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds.). The evolution of the amphibian auditory system. New York, Wiley, pp 637–677
Ryan MJ, Sullivan BK (1989) Transmission effects on temporal structure in the advertisement call of two toads, Bufo woodhousei and Bufo valliceps. Ethology 80:182–189
Ryan MJ, Wilczynski W (1991) Evolution of intraspecific variation in the advertisement call of a cricket frog (Acris crepitans, Hylidae). Biol J Linn Soc 44:249–271
Schwartz A (1959) A new species of toad, Bufo cataulaciceps from Isla de Pinos and Western Cuba. Proc Biol Soc Washington 72:109–120
Schwartz A, Henderson RW (1991) Amphibians and reptiles of the West Indies: descriptions, distributions, and natural history. University of Florida Press, Gainesville
Shuster SM (2009) Sexual selection and mating systems. Proc Natl Acad Sci U S A 106:10009–10016
Sokal RR, Rohlf FJ (2000) Biometry: the principles and practice of statistics in biological research, 3rd edn. W. H, Freeman and Company, New York
StatSoft Inc. (2007) STATISTICA (data analysis software system), version 8.0. www.statsoft.com
Sullivan BK (1992) Sexual selection and calling behaviour in the American toad (Bufo americanus). Copeia 1992:1–7
Sullivan BK, Leek MR (1987) Acoustic communication in Woodhouse’s toad (Bufo woodhousei). II. Response of females to variation in spectral and temporal components of advertisement calls. Behaviour 103:16–36
Van Gelder JJ, Hoedemaekers HCM (1971) Sound activity and migration during the breeding period of Rana temporaria, L., R. arvalis Nilsson, Pelobates fuscus Laur. and Rana esculenta L. J Anim Ecol 40:599–568
Wagner WE, Sullivan BK (1995) Sexual selection in the Gulf Coast toad, Bufo valliceps: female choice based on variable characters. Anim Behav 49:305–319
Wells KD (1977) The social behaviour of anuran amphibians. Anim Behav 25:666–693
Wells KD (1988) The effects of social interactions on anuran vocal behavior. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds.). The evolution of the amphibian auditory system. New York, Wiley, pp 433–454
Wells KD (2007) The ecology and behavior of amphibians. IL. University of Chicago Press. Chicago, Chicago
Wilbur HM (1978) Complex life cycles. Annu Rev Ecol Syst 11:67–93
Woodward BD (1982) Sexual selection and non-random mating pattern in desert anurans (Bufo woodhousei, Scaphiopus couchi, S. multiplicatus and S. bombifrons). Copeia 1982:351–355
Acknowledgements
We thank the local authorities of the Empresa para la Conservación de la Flora y la Fauna and Centro de Gestion y Servicios Ambientales y Tecnológicos (CGSAT) for their logistic support. We also extend our thanks to all the personnel of the Ecological Reserve “Los Indios,” for their hospitality and field assistance. Y. C. Martínez helped in the field during the recording sessions. P. Narins and IdeaWild foundation generously provided part of the equipment employed. This work was partially funded by the project “Creación, conservación y manejo de colecciones zoológicas” of the Institute of Ecology and Systematic, Havana, Cuba. AR’s work was supported by a postdoctoral research fellowship from the Alexander von Humboldt Foundation. Access and collection permits were provided by Centro de Inspección y Control Ambiental (CICA). Two anonymous referees provided helpful insights and comments on the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alonso Bosch, R., Rodríguez, A. & Quinta, M.H. Advertisement call variation and individual acoustic distinctiveness in the explosive breeding toad Peltophryne cataulaciceps (Anura: Bufonidae). acta ethol 20, 197–205 (2017). https://doi.org/10.1007/s10211-017-0261-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-017-0261-8