Abstract
The environmental DNA (eDNA) method is potentially useful to detect the diurnal activity of aquatic organisms. Seasonal changes in the diurnal activities of the endangered semiaquatic water shrew, Chimarrogale platycephalus, were investigated to evaluate the efficiency of the eDNA method in their tracking. We conducted hourly field surveys for a period of 25 consecutive hours in two streams quarterly, using a species-specific primer and camera trap observations. Using qPCR, we compared the frequency and concentration of eDNA detected between day and night, seasons, and streams. In both streams, eDNA was detected consistently with temporal fluctuations during all seasons for nighttime. However, during daytime, eDNA was detected in all seasons except autumn, in which it was detected only in one stream. This suggests that species activity occurs throughout both daytime and nighttime in winter, spring, and summer, and potentially less during the daytime in autumn, probably due to the lack of competition and energy constraints during that season, as most individuals were non-breeding in autumn and avoided the habitation of areas with high density after the dispersal of their offspring. The high eDNA concentration in summer may be attributable to the higher density of non-breeding individuals after the offspring left the nest and/or to the increased activity owing to the competition for food or space. The diurnal activities of species detected using eDNA sampling allow us to obtain detailed ecological information, which is beneficial for managing conservation in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terrestrial and aquatic ecosystems are adjacent to each other and are closely linked through the food web, especially in the upper reaches of streams (Nakano and Murakami 2001). Resource compensation between both of these ecosystems is known to occur in reciprocal directions, that is, land to water (Nakano and Murakami 2001) and water to land (Baxter et al. 2005). As top predators utilize resources from both ecosystems by linking the food webs of both, they consequently occupy an important role in directly or indirectly connecting the food webs in the upper reaches of streams (Baxter et al. 2005). An analysis of the ecological characteristics of top predators, such as seasonal changes in behavioral activities, can lead to a better understanding of food web structures in the upper reaches of streams; however, in this context, few studies have been conducted on semiaquatic mammals, possibly because of direct observational difficulties (Shiozuka et al. 2023), compared to those on birds (e.g., Nakano and Murakami 2001) and large amphibians (e.g., Roon et al. 2022).
Among many endangered small mammals, semiaquatic mammals are among the top predators in the upper reaches of streams (Andermann et al. 2020). Population shrinkage of many species in the semiaquatic mammals has been reported (Hood 2020); however, the behavioral ecology of only a few species have been studied, e.g., the European water shrew Neomys fodiens (Haberl 2002) and the long-tailed otter Lontra longicaudis (Vezzosi et al. 2014). For the conservation management of endangered mammals, knowledge of their diurnal and seasonal behavior patterns is important because it helps to reveal ecological information regarding individuals and populations, such as predation, competition, and habitat use (Kotler et al. 2007; Morris et al. 2009). In this context, the knowledge of semiaquatic mammals should also lead to more effective conservation measures (Sinclair and Byrom 2006). Mammalian diurnal activity is mainly determined by circadian rhythm (Bennie et al. 2014), and that varies considerably seasonally depending on the life history stage, environmental factors such as light and temperature conditions, and biological interactions (Beier and McCullough 1990; Bennie et al. 2014). Few studies have observed diurnal activity or its seasonal variations in endangered small mammal species (Cserkész et al. 2023), possibly owing to observational difficulties. Direct observations, radiotelemetry tracking, and camera-trap capture are used for observing large mammals (Meek and Fleming 2014); however, such traditional methods are difficult to implement for small mammals because many species are nocturnal and tend to move quickly, making photography and observation infeasible (Buchler 1976; Nakazono and Iwasa 2015).
Chimarrogale platycephalus (Temminck 1842), Soricidae; platycephala in the previous species name, Yato et al. (2022) is a Japanese endemic semiaquatic water shrew categorized as an endangered species in Japan (Wildlife Research and EnVision 2022), and conservation of its populations is urgently needed. The species inhabits the upper reaches of mountain streams, forages fish and aquatic insects while swimming in water, and rests behind rocks (Churchfield et al. 2000; Kitagaki 2016). During the life cycle of this species, most of the adults breed in midwinter, and the females give birth in early spring and raise their offspring until they leave the nest in early summer; only a small proportion of the females breed during the fall season and their offspring leave the nest in midwinter (Motoki 2000; Yokohata et al. 2008). Previous studies on the diurnal activity of C. platycephalus have demonstrated a weak nocturnal rhythm: the species was shown to be active throughout both day and night, but more active at night based on direct observations of indoor captive individuals (Motoki 2000) and wild individuals (Fujimoto et al. 2011; Saito et al. 2019; Saitoh et al. 2013; Yokohata et al. 2008). However, the seasonal changes in diurnal activity and/or the changes in life history stages remain unknown because C. platycephalus, like many other small mammals, is difficult to observe in the field.
Recently, non-invasive and simple methods for studying semiaquatic species have been developed instead of invasive methods, which often should be avoided when studying endangered species (Shiozuka et al. 2023); a promising approach is the environmental DNA (eDNA) method. For aquatic species that continuously inhabit underwater environments, the eDNA method is a useful tool for determining their presence/absence, abundance, and biomass (Rees et al. 2014). It is further useful for semiaquatic species; for example, Shiozuka et al. (2023) developed the eDNA method for C. platycephalus in Japanese streams by sampling multiple streams during different seasons to test the performance by comparing with camera-trap detections. Using eDNA, it is possible to determine the activity and abundance of semiaquatic species under water because the eDNA content of aquatic organisms changes depending on their activity and life history stage (Inui et al. 2021; Tsuji and Shibata 2021; Wacker et al. 2019).
The objective of this study was to determine the seasonal changes in diurnal activity of the water shrew in the field. We conducted hourly censuses during 25-h field surveys seasonally (spring, summer, autumn, and winter) using a species-specific primer developed by Shiozuka et al. (2023). We compared the frequency and concentration of C. platycephalus eDNA detected at day/night between seasons and streams. In parallel, direct observation by camera-trap capture was performed, and all results were compared to assess or evaluate the utility of the eDNA method for the estimation of seasonal changes of the diurnal activity of semiaquatic species.
Materials and methods
Study areas
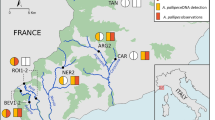
Field surveys were performed at two stations along two typical mountain streams in the northern Kii Peninsula, Japan; one in a tributary flowing into the Takami River (Kinokawa River system, Nara Prefecture), and another in a tributary flowing into the Nabari River (Yodogawa River system, Mie Prefecture; Fig. 1). For the protection of this species, the latitude and longitude of the locations will not be disclosed. Inhabitation by C. platycephalus at the two stations had been previously confirmed by fecal surveys (Shiozuka et al. 2023). The stations were, respectively, located in the middle-streams, within watershed areas of 3.5 and 8.5 km2. The average stream width was approximately 2 m, and the canopy coverage at each station was approximately 60% according to measurements by ruler and imagery taken by iPhone with a fisheye lens camera (Bostionye, Jian, China) on the day of the survey.
Field survey for camera-trap photography and water sampling
We conducted the field survey quarterly with camera-trap photography (Trophy Cam 24MP, Bushnell, Overland Park, KS, USA) and sampled water at each station from autumn 2020 to summer 2021. In the tributaries along the Takami River (hereafter Stream A) and Nabari River (Stream B), we conducted the surveys during October 20–21 and November 24–25, 2020 (autumn, non-breeding or occasional second breeding season, including pregnant period of C. platycephalus), February 4–5 and 10–11, 2021 (winter, the principal and first breeding season, including mating and pregnant periods), May 10–11 and 14–15, 2021 (spring, the nesting period in the first breeding season), and August 4–5 and 10–11, 2021 (summer, the non-breeding season), respectively. Throughout the period of water sampling, camera-trap photography equipment (Trophy cam 24MP, Bushnell, Overland Park, KS, USA) was continuously placed near rocks where feces of C. platycephalus were observed. The camera trap was strapped to a riparian tree at the streamside to acquire photography within a 5 m reach of the stream. The shutter was released when a moving object entered the field of view, and the camera collected imagery 0.3 s after moving detection within 24 m from the camera (detection range 38º forward) using LED lights with wavelengths not visible to animals.
Each water sampling was conducted for a duration of 25 h, described as follows. At both stations, a 1-L water sample was collected directly from both (right- and left-hand) sides of the stream center, at a location 10 m downstream of the rock on which the camera captured defecating by subjects, using a DNA-free polypropylene bottle. Two 1-L water samples were taken hourly from 12:00 to 13:00 on the following day. A 0.1% volume of benzalkonium chloride solution was added to the water samples, which were stirred immediately, to prevent DNA degradation (Yamanaka et al. 2017). The water samples were stored in a cooler box containing 2 L of DNA-free distilled water (DW) as a cooler blank. The water temperature of the stream surface during sampling was measured using a digital stick thermometer (TP15JP, ThermoPro, Tront, Canada).
Water filtration and DNA extraction
In the laboratory, the two collected water samples were vacuum-filtered through a 47-mm GF/F glass filter (pore size: 0.7 μm, Cytiva, Marlborough, Massachusetts, USA). We incorporated an equipment blank as a negative control; 1 L of DNA-free DW was filtered after filtering the samples on each sampling day. A cooler blank was filtered during the filtering of samples on each sampling day. DNA from the negative controls was tested along with the samples to check contamination during sample preparation/transportation in the field and/or filtration in the laboratory. The filter was wrapped in a commercial aluminum foil and stored at − 20 °C until DNA extraction.
DNA was extracted from the filters as described by Uchii et al. (2016) and Minamoto et al. (2021). Each filter was incubated at 56 °C for 30 min in a mixed buffer of 400 μL of Buffer AL (Qiagen, Hilden, Germany) and 40 μL of Proteinase K (Qiagen, Hilden, Germany) using a Salivette tube (Sarstedt, Nümbrecht, Germany). The Salivette tube with the filter was centrifuged at 3500×g for 5 min. After adding 220 μL of Tris-ethylenediaminetetraacetic acid (EDTA) buffer (10 mM Tris–HCl and 1 mM EDTA at pH 8.0), the filter was centrifuged at 5000×g for 5 min. The DNA in the eluted solution was purified using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The DNA was eluted in 200 μL of Buffer AE (Qiagen, Gilden, Germany) and stored at − 20 °C until real-time quantitative polymerase chain reaction (qPCR) analysis.
Real-time qPCR
For qPCR, we used a species-specific primer–probe set to amplify an 81-bp fragment of the 16S ribosomal gene of the mitochondrial DNA of C. platycephalus (Shiozuka et al. 2023). The eDNA concentration was measured using a StepOne qPCR system (Applied Biosystems, Waltham, Massachusetts, USA). Each PCR mixture contained 900 nM of each primer (F, R), 125 nM probe in 1 × PCR master mix (TaqPath CG, Life Technologies, Carlsbad, CA, USA), and 2 μL of DNA solution. The total volume of each reaction mixture was 20 μL. The PCR conditions were 2 min at 95 °C and 55 cycles of 15 s at 95 °C and 60 s at 60 °C. Each sample was evaluated in triplicate; a positive value for any of the replicates was considered to indicate the presence of C. platycephalus DNA, considering that the limit of detection of qPCR for the three replicates was one copy per reaction (Shiozuka et al. 2023). We performed qPCR measurement according to the “Minimum Information for Publication of Quantitative qPCR Experiments (MIQE)” checklist (Bustin et al. 2009).
A standard curve for the target gene was constructed using dilution series of 10,000, 1000, 100, and 10 copies per PCR template. For the standard curve, we used standard DNA isolated from plasmid cloning; we used the standard DNA isolated from plasmid cloning linearized with restriction endonuclease. The qPCR results were analyzed using StepOne software ver. 2.3 (Applied Biosystems, Waltham, Massachusetts). The R2 values of the standard curves ranged from 0.978–0.998, and the PCR efficiencies ranged from 87.18–113.88%. The DNA concentration in the collected water (DNA copies L−1) was calculated from the volume of the filtered water (2 L). Mean DNA copy number in triplicates was calculated from each DNA copy numbers, including a negative detection as zero. The limit of quantification of qPCR for the three replicates was three copies per reaction (Shiozuka et al. 2023). The PCR setup and qPCR were performed in two separate rooms to avoid contamination.
Statistical analysis
All statistical analyses were performed using R ver. 4.1.1 (R Core Team 2022). We set the criterion of significance at 0.05 (α = 0.05). Time-series data of the eDNA concentration in each stream and season (N = 25 for each) were tested by the Ljung–Box test to evaluate the significance of the autocorrelation using the “Box.test” function. Then we confirmed that all time-series data were not significantly auto-correlated with χ2 < 2.30, p > 0.130. To compare the day/night eDNA detection frequency in each stream and season, we tested the effects of streams and seasons on the binary frequency of eDNA detections (0, 1) in the day/night using the generalized linear model (GLM, error distribution: binomial) with the “glm” function. To evaluate the significance of the factors (i.e., streams and seasons), we performed the likelihood ratio test with χ2 using the “ANOVA” function of the “car” package. We also performed three-way analysis of variance (ANOVA) to analyze eDNA concentrations for three factors: season, stream, and day/night using the “aov” function. For the ANOVA, we used only positive values because we aimed to compare the eDNA concentration when eDNA was detected. Deleting zero values induces the unbalance of sample size and consequently decreases homogeneity of variance among the treatments; therefore, we performed Levene’s test for homogeneity of variance on the data used for ANOVA and confirmed the homogeneity of variance (Levene’s statistics = 1.586 p = 0.2113). Tukey’s multiple comparisons test was performed to assess the differences in seasons using the “TukeyHSD” function.
Results
The sunset/sunrise times, water temperature, number of captures by the camera trap, and frequency of eDNA detection are listed in Table 1. In Stream A, C. platycephalus individuals were captured by camera traps twice (19:06 and 1:33) in winter and four times (19:49, 20:24, 22:22, and 23:03) in summer (Table 1 and Fig. 2); however, no individuals were captured in Stream B throughout all four seasons (Table 1). In both streams, eDNA of C. platycephalus was detected in every season (Fig. 3 and Table 1). In all seasons, eDNA was detected consistently during nighttime, but there were temporal fluctuations; and during daytime, eDNA was also detected in all seasons except for Stream A in autumn (Fig. 3). For Stream A, the frequency of eDNA detection (0 or 1) in the daytime was significantly lower than that in the nighttime in autumn (GLM likelihood ratio test, p = 0.044, Table 2); however, no significant difference between day and night was observed in any seasons other than autumn in Stream A and all seasons in Stream B (GLM likelihood ratio test, p > 0.05, Table 2). eDNA was detected across all seasons in Stream A between 21:00 and 22:00; however, this diurnal pattern was not observed in Stream B. Seasonal changes in the eDNA concentrations are shown in Fig. 4. The three-way ANOVA to test diurnal, seasonal, and between-stream differences in the eDNA concentrations showed that only the seasonal difference was significant (F = 26.11, p < 0.001), with the interaction between seasons and streams (seasons × streams, F = 7.37, p < 0.001, Table 3). Tukey’s multiple comparison tests revealed that eDNA concentrations were significantly higher during summer in both streams (p < 0.05, Fig. 4). Each qPCR assay included three no-template controls, which showed no amplification.
Discussion
We found the activity of C. platycephalus in both streams in all seasons during nighttime using the eDNA detection method, even in the absence of observations by camera trap. However, eDNA detections during daytime depended on the season and the stream. In winter, spring, and summer, eDNA was detected during daytime without any differences from nighttime; however, during daytime in autumn, eDNA concentration was low in one stream and not detected in the other. This study is the first to quantitatively demonstrate that C. platycephalus activity in the field occurred throughout the entire day in three seasons (winter, spring, and summer) and its nocturnal-like habit occurred only in autumn.
Several previous empirical studies have suggested that this species is more active during nighttime than during daytime but forages throughout both day and night (Fukumoto et al. 2015; Motoki 2000; Saitoh et al. 2013; Saito et al. 2019; Yokohata et al. 2008). With the exception of autumn, the result on eDNA detection in this study is consistent with that observed in previous studies. Because the species forages in the water but defecates, breeds, and rests on land (Ohdachi et al. 2015), it is likely that the frequency or concentration of eDNA detected in stream water indicates foraging activities of the species. Thus, the frequency of eDNA detection should correspond to times of active foraging. The continuous eDNA detection observed throughout both day and night suggests that the species has a fast metabolic rate (Kitagaki 2016), similar to that of other semiaquatic shrews (Nagorsen 1996; Nowak and Walker 1999; Rychlik and Jancewicz 2002), and that the species needs to forage during both day and night to maintain its body temperature and activity. This is possibility supported by the result that the species is more active during both daytime and nighttime during cooler winter than autumn, and during this season, most adults are mating and/or pregnant and need to increase their energy intake.
In contrast, during autumn, when most individuals except for a few pregnant females are non-breeding (Motoki 2000; Yokohata et al. 2008), the species could be less active during the daytime and biased toward nocturnal behavior. These results may suggest that the species is less active during the daytime when there are no constraints of energy or competition. Although the species primarily feeds on aquatic insects (Shiozuka et al. 2022), most aquatic insects actively crawl on the surface of the streambed and drift in the water column at nighttime (Katano et al. 2005; Kohler 1985; Waters 1972), and hence the nocturnal foraging by the water shrew is expected to provide sufficient food.
eDNA concentrations were significantly higher during summer, which corresponds to the non-breeding season after the offspring leave the nest. The increasing population density could be a principal reason for the higher eDNA concentrations, as eDNA concentration increases with an increase in population density (e.g., Doi et al. 2017; Tillotson et al. 2018). Another possible reason for the elevated eDNA concentrations during summer could be an increase in foraging activity, which has been previously demonstrated in the species (Yonezawa et al. 2020). There is also the possibility of increasing activity in response to the low availability of aquatic insects because the density and abundance of aquatic insects are lowest during the summer (Nakano and Murakami 2001). Although this study could not distinguish between the possible explanations (high density and high activity) for the higher eDNA concentration during summer, either or both of the possibilities could have affected eDNA concentrations throughout daytime and nighttime during the summer.
The eDNA concentrations were relatively low during the winter, which is the breeding season. Aquatic organisms that externally fertilize, such as bivalves (Wacker et al. 2019), fish (Erickson et al. 2016; Inui et al. 2021; Tsuji and Shibata 2021), and amphibians (Buxton et al. 2017), release gametes into the water when spawning, and aquatic reptiles (De Souza et al. 2016) are internally fertilized but lay eggs in water; therefore, for many of these organisms, eDNA concentrations and detection frequencies are expected to increase during the breeding season. However, because the water shrew utilizes internal fertilization and its reproductive behavior occurs on land (Ohdachi et al. 2015), no increase in eDNA was observed during the breeding season. Unfortunately, in this case, eDNA may not be useful for estimating the breeding season of semiaquatic mammals.
No difference in diurnal activity patterns was observed between winter and spring, i.e., between the main breeding and post-breeding seasons. It has been reported that other closely related species of shrew (Sorex and Crocidura) do not change their behavior from the pre- to the post-breeding season, irrespective of sex (von Merten et al. 2020). Similarly, C. platycephalus also did not change its behavior pattern between the breeding and non-breeding seasons. The fact that the eDNA concentration increased from winter and spring to summer without any changes in the diurnal pattern could indicate an increase in the population density, amount of activity, or both, of the newly nestling offspring. Moreover, the decrease in the frequency of eDNA detections during the daytime in autumn suggests the possibility that the diurnal pattern could change to be closer to nocturnal behavior during autumn, when most individuals do not breed, which may be due to offspring dispersal and/or a decrease in competition.
Although eDNA is considered to be an effective tool for evaluating the total amount of activity and life history stages of aquatic organisms (e.g., Inui et al. 2021), this study quantitatively demonstrates that eDNA is an effective tool for evaluating activities of semiaquatic organisms in water. Based on previous studies on eDNA distribution in streams (e.g., Nevers et al. 2020; Wilcox et al. 2016; Wood et al. 2020, 2021, cage experiments in the streams), it is assumed that eDNA likely originates from a range of 0–5 km. Therefore, it is possible that a population within that range is being detected. Although small semiaquatic organisms are often missed by camera traps during both day and night (Buchler 1976; Shiozuka et al. 2023), in this case, the eDNA survey was able to detect their underwater behaviors and the distribution in the broader ranges, including upper reaches, even when camera traps failed to detect them, demonstrating that eDNA surveys can provide more precise information on diurnal activity changes of wild animal populations. At least for the Japanese water shrew, eDNA could directly represent foraging activities. Moreover, even for smaller organisms making temporary use of the water, it should be possible to capture diurnal activity changes in the field using the eDNA method with precise timing and local area settings. In this study, the range of eDNA detected was not estimated.
This study has two main limitations. First, we conducted only a 25-h sampling duration in each season; thus, the results are somewhat dependent on the field conditions of the sampling days, e.g., stream flow and weather. Second, although we monitored behaviors using eDNA and camera traps at two specific mountain stream sites in the northern Kii Peninsula, the species is actually distributed across the Japanese Archipelago, with its various climates, habitat conditions, and genomes. Hence, the various environmental factors (e.g., watershed area, elevation, stream width, and current speed) and population factors (e.g., population size and genotypes) may influence eDNA and camera-trap detection. Therefore, to understand the behaviors of the species and its eDNA dynamics in depth, future studies need to measure eDNA on multiple days, across various habitats, and in different seasons.
References
Andermann T, Faurby S, Turvey ST, Antonelli A, Silvestro D (2020) The past and future human impact on mammalian diversity. Sci Adv 6:eabb2313. https://doi.org/10.1126/sciadv.abb2313
Baxter CV, Fausch KD, Carl Saunders W (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol 50:201–220. https://doi.org/10.1111/j.1365-2427.2004.01328.x
Beier P, McCullough DR (1990) Factors influencing white-tailed deer activity patterns and habitat use. Wildl Monogr 109:3–51
Bennie JJ, Duffy JP, Inger R, Gaston KJ (2014) Biogeography of time partitioning in mammals. Proc Natl Acad Sci USA 111:13727–13732. https://doi.org/10.1073/pnas.1216063110
Buchler ER (1976) A chemiluminescent tag for tracking bats and other small nocturnal animals. J Mammal 57:173–176. https://doi.org/10.2307/1379522
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
Buxton AS, Groombridge JJ, Zakaria NB, Griffiths RA (2017) Seasonal variation in environmental DNA in relation to population size and environmental factors. Sci Rep 7:46294. https://doi.org/10.1038/srep46294
Churchfield S, Barber J, Quinn C (2000) A new survey method for water shrews (Neomys fodiens) using baited tubes. Mamm Rev 30:249–254. https://doi.org/10.1046/j.1365-2907.2000.00074.x
Cserkész T, Kiss C, Sramkó G (2023) Seasonal and diel activity patterns of small mammal guilds on the Pannonian Steppe: a step towards a better understanding of the ecology of the endangered Hungarian birch mouse (Sicista trizona) (Sminthidae, Rodentia). Mamm Res 68:13–25. https://doi.org/10.1007/s13364-022-00656-0
Doi H, Inui R, Akamatsu Y, Kanno K, Yamanaka H, Takahara T, Minamoto T (2017) Environmental DNA analysis for estimating the abundance and biomass of stream fish. Freshw Biol 62:30–39. https://doi.org/10.1111/fwb.12846
Erickson RA, Rees CB, Coulter AA, Merkes CM, McCalla SG, Touzinsky KF, Walleser L, Goforth RR, Amberg JJ (2016) Detecting the movement and spawning activity of bigheaded carps with environmental DNA. Mol Ecol Resour 16:957–965. https://doi.org/10.1111/1755-0998.12533
Fujimoto R, Ando M, Ogawa H (2011) Efficient capture methods for the Japanese water shrew Chimarrogale platycephala (in Japanese). J Agric Sci Tokyo Univ Agric 55:290–296
Fukumoto S, Ushimaru A, Minamoto T (2015) A basin-scale application of environmental DNA assessment for rare endemic species and closely related exotic species in rivers: a case study of giant salamanders in Japan. J Appl Ecol 52:358–365. https://doi.org/10.1111/1365-2664.12392
Haberl W (2002) Food storage, prey remains and notes on occasional vertebrates in the diet of the Eurasian water shrew, Neomys fodiens. Folia Zool 51:93–102
Hood GA (2020) Semi-aquatic mammals: ecology and biology. Johns Hopkins University Press, Baltimore, Maryland. https://doi.org/10.1353/book.78278
Inui R, Akamatsu Y, Kono T, Saito M, Miyazono S, Nakao R (2021) Spatiotemporal changes of the environmental DNA concentrations of amphidromous fish Plecoglossus altivelis in the spawning grounds in the Takatsu River, western Japan. Front Ecol Evol 9:622149. https://doi.org/10.3389/fevo.2021.622149
Katano I, Isobe Y, Oishi T (2005) Diel periodicity in the drift of the fourth instar Micrasema quadriloba (Trichoptera: Brachycentridae) larvae in relation to body size. Korean J Ecol Environ Issue SPC 38:17–21
Kitagaki K (2016) Development of a device for outdoor recording of hunting behavior of Japanese water shrew, Chimarrogale platycephala (in Japanese). Nat Environ Sci Res 29:25–30. https://doi.org/10.32280/nesr.29.0_25
Kohler SL (1985) Identification of stream drift mechanisms: an experimental and observational approach. Ecology 66:1749–1761. https://doi.org/10.2307/2937371
Kotler BP, Morris DW, Brown JS (2007) Behavioral indicators and conservation: wielding “the biologist’s tricorder.” Isr J Ecol Evol 53:237–244. https://doi.org/10.1560/IJEE.53.3.237
Meek P, Fleming P (2014) Camera trapping: wildlife management and research. CSIRO Publishing, Collingwood. https://doi.org/10.1071/9781486300402
Minamoto T, Miya M, Sado T, Seino S, Doi H, Kondoh M, Nakamura K, Takahara T, Yamamoto S, Yamanaka H, Araki H, Iwasaki W, Kasai A, Masuda R, Uchii K (2021) An illustrated manual for environmental DNA research: water sampling guidelines and experimental protocols. Environ DNA 3:8–13. https://doi.org/10.1002/edn3.121
Morris DW, Kotler BP, Brown JS, Sundararaj V, Ale SB (2009) Behavioral indicators for conserving mammal diversity. Ann N Y Acad Sci 1162:334–356. https://doi.org/10.1111/j.1749-6632.2009.04494.x
Motoki T (2000) New breeding method of the water shrew, Chimarrogale platycephala and the diurnal activity by using the new breeding apparatus (in Japanese). Ann Environ Sci Shinshu University 223:7–40
Nagorsen DW (1996) Opossums, shrews and moles of British Columbia. UBC Press. https://doi.org/10.5962/p.358300
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA 98:166–170. https://doi.org/10.1073/pnas.98.1.166
Nakazono S, Iwasa MA (2015) An evaluation of the use of automatic infrared sensor cameras for ecological research of terrestrial small mammals (in Japanese). Mamm Sci 55:59–65. https://doi.org/10.11238/mammalianscience.55.59
Nevers MB, Przybyla-Kelly K, Shively D, Morris CC, Dickey J, Byappanahalli MN (2020) Influence of sediment and stream transport on detecting a source of environmental DNA. PLoS One 15:e0244086. https://doi.org/10.1371/journal.pone.0244086
Nowak RM, Walker EP (1999) Walker’s mammals of the world, vol 1. JHU Press. https://doi.org/10.56021/9780801857898
Ohdachi SD, Ishibashi Y, Iwasa MA, Fukui D, Saitoh T (2015) The wild mammals of Japan, 2nd edn. Shoukadoh Book Sellers, Kyoto
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org
Rees HC, Maddison BC, Middleditch DJ, Patmore JRM, Gough KC (2014) The detection of aquatic animal species using environmental DNA—a review of eDNA as a survey tool in ecology. J Appl Ecol 51:1450–1459. https://doi.org/10.1111/1365-2664.12306
Roon DA, Dunham JB, Bellmore JR, Olson DH, Harvey BC (2022) Influence of riparian thinning on trophic pathways supporting stream food webs in forested watersheds. Ecosphere 13:e4219. https://doi.org/10.1002/ecs2.4219
Rychlik L, Jancewicz E (2002) Prey size, prey nutrition, and food handling by shrews of different body sizes. Behav Ecol 13:216–223. https://doi.org/10.1093/beheco/13.2.216
Saito H, Hashimoto H, Hino T, Motokawa M (2019) How does the Japanese water shrew Chimarrogale platycephalus cross the concrete walls of check dams? Mamm Study 44:1–11. https://doi.org/10.3106/ms2017-0056
Saitoh H, Kazama K, Hino T (2013) Environmental factors affecting capture rate of the Japanese water shrew, Chimarrogale platycephala. Mamm Sci 53:117–121. https://doi.org/10.11238/mammalianscience.53.117
Shiozuka N, Katano I, Kanzaki T, Kikuchi R, Sato N, Nakashita R, Kudo S, Ikeda H, Azuma N (2022) Isotopic diet analysis of the Japanese water shrew Chimarrogale platycephala to estimate their feeding habits and the usefulness of body hair samples. Mamm Study 48:19–29. https://doi.org/10.3106/ms2022-0021
Shiozuka N, Katano I, Doi H, Nakamura M, Shirako T, Ichiyanagi H (2023) Diurnal detection of environmental DNA of the semi-aquatic water shrew Chimarrogale platycephala using 25-h water sampling in streams. Landscape Ecol Eng 19:69–77. https://doi.org/10.1007/s11355-022-00526-6
Sinclair AR, Byrom AE (2006) Understanding ecosystem dynamics for conservation of biota. J Anim Ecol 75:64–79. https://doi.org/10.1111/j.1365-2656.2006.01036.x
De Souza LS, Godwin JC, Renshaw MA, Larson E (2016) Environmental DNA (eDNA) detection probability is influenced by seasonal activity of organisms. PLoS One 11:e0165273. https://doi.org/10.1371/journal.pone.0165273
Tillotson MD, Kelly RP, Duda JJ, Hoy M, Kralj J, Quinn TP (2018) Concentrations of environmental DNA (eDNA) reflect spawning salmon abundance at fine spatial and temporal scales. Biol Conserv 220:1–11. https://doi.org/10.1016/j.biocon.2018.01.030
Tsuji S, Shibata N (2021) Identifying spawning events in fish by observing a spike in environmental DNA concentration after spawning. Environ DNA 3:190–199. https://doi.org/10.1002/edn3.153
Uchii K, Doi H, Minamoto T (2016) A novel environmental DNA approach to quantify the cryptic invasion of non-native genotypes. Mol Ecol Resour 16:415–422. https://doi.org/10.1111/1755-0998.12460
Vezzosi RI, Eberhardt AT, Raimondi VB, Pautasso AA (2014) Seasonal variation in the diet of Lontra longicaudis in the Paraná River basin, Argentina. Mammalia 78:451–463. https://doi.org/10.1515/mammalia-2013-0053
von Merten S, Dingemanse NJ, Mathias MD, Rychlik L (2020) Individual behavior, behavioral stability, and pace of life within and among five shrew species. Behav Ecol Sociobiol 74:1–14. https://doi.org/10.1007/s00265-019-2793-6
Wacker S, Fossøy F, Larsen BM, Brandsegg H, Sivertsgård R, Karlsson S (2019) Downstream transport and seasonal variation in freshwater pearl mussel (Margaritifera Margaritifera) eDNA concentration. Environ DNA 1:64–73. https://doi.org/10.1002/edn3.10
Waters TF (1972) The drift of stream insects. Annu Rev Entomol 17:253–272. https://doi.org/10.1146/annurev.en.17.010172.001345
Wilcox TM, McKelvey KS, Young MK, Sepulveda AJ, Shepard BB, Jane SF, Whiteley AR, Lowe WH, Schwartz MK (2016) Understanding environmental DNA detection probabilities: a case study using a stream-dwelling char Salvelinus fontinalis. Biol Conserv 194:209–216. https://doi.org/10.1016/j.biocon.2015.12.023
Wildlife Research and EnVision, Ministry of the environment, Government of Japan, the Search System of Japanese Red Data. http://jpnrdb.com. Accessed 13 Jun 2022
Wood ZT, Erdman BF, York G, Trial JG, Kinnison MT (2020) Experimental assessment of optimal lotic eDNA sampling and assay multiplexing for a critically endangered fish. Environ DNA 2:407–417. https://doi.org/10.1002/edn3.64
Wood ZT, Anaïs L-R, LeBlanc F, Trudel M, Kinnison MT, McBrine CG, Pavey SA, Gagne N (2021) Spatial heterogeneity of eDNA transport improves stream assessment of threatened salmon presence, abundance, and location. Front Ecol Evol 9:2021. https://doi.org/10.3389/fevo.2021.650717
Yamanaka H, Minamoto T, Matsuura J, Sakurai S, Tsuji S, Motozawa H, Hongo M, Sogo Y, Kakimi N, Teramura I, Sugita M, Baba N, Kondo A (2017) A simple method for preserving environmental DNA in water samples at ambient temperature by addition of cationic surfactant. Limnology 18:233–241. https://doi.org/10.1007/s10201-016-0508-5
Yato T, Okabe S, Ikeda Y, Motokawa M (2022) Comments on species taxonomy for Japanese mammals in “illustrated checklist of the mammals of the world” (in Japanese). Taxa 53:31–47. https://doi.org/10.19004/taxa.53.0_31
Yokohata Y, Kawada S, Ichiyanagi H (2008) A report on the workshop “Recent advances in the ecology and conservation of the Japanese water shrew, Chimarrogale platycephala” (in Japanese). Mamm Sci 48:175–176. https://doi.org/10.11238/mammalianscience.48.175
Yonezawa S, Ushio M, Yamanaka H, Miya M, Takayanagi A, Isagi Y (2020) Environmental DNA metabarcoding reveals the presence of a small, quick-moving, nocturnal water shrew in a forest stream. Conserv Genet 21:1079–1084. https://doi.org/10.1007/s10592-020-01310-5
Acknowledgements
We thank Dr. Yoichi Yusa and Dr. Hiroaki Sato for their helpful comments in interpreting data. The present study was supported in part by JSPS KAKENHI Grants (15K00596 and 18K11678) to I.K. and by the Sasakawa Kagaku Research Grant (2021-5033) and JST SPRING, Grant No. JPMJSP2115 to N.S. Our investigations in this study complied with the current laws of Japan in which they were performed.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: Izumi Katano, Hideyuki Doi, Masatoshi Nakamura, Tomoyasu Shirako, Hidetaka Ichiyanagi. Data curation and formal analysis: Nao Shiozuka, Shun Nagayama, Izumi Katano, Hideyuki Doi. Writing—original draft: Izumi Katano, Hideyuki Doi. Writing—review and editing: Nao Shiozuka, Izumi Katano, Hideyuki Doi, Masatoshi Nakamura, Tomoyasu Shirako, Shun Nagayama, Hidetaka Ichiyanagi.
Corresponding authors
Additional information
Handling Editor: Noboru Okuda.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shiozuka, N., Katano, I., Doi, H. et al. Seasonal changes in the diurnal behavior of Chimarrogale platycephalus evaluated using environmental DNA. Limnology (2024). https://doi.org/10.1007/s10201-024-00760-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10201-024-00760-x