Abstract
Plasticity in the larval life history of the Ezo salamander Hynobius retardatus has been reported. In the present study, we monitored larvae of this salamander species in a fragmented forest in the environs of Sapporo, Japan. Overwintering larvae were detected in one of the two ponds examined, i.e., in the pond that was permanent, with water supplied from several spring-fed points. Four seasonal transition phases in water temperature were observed because of the abundant spring-fed groundwater supply and canopy cover from May to October. These phases included a warming period, a constant-high period, a cooling period, and a constant-low period. During the constant-low period, premetamorphic larvae that had already emerged during the cooling period were continuously detected; however, the composition of the developmental stages remained unchanged, with larval growth progressing slowly. There is apparently a critical temperature that represents the threshold for metamorphosis initiation. The critical temperature is expected to be slightly higher than the groundwater temperature at the spring-fed points. Emigration of overwintered larvae resumed during the warming period and continued during the constant-high period in the year following hatching. In the nearby temporary pond, the one-year-old cohort completed metamorphosis during the summer of the hatch year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amphibians have biphasic life cycles, characterized by drastic metamorphosis of aquatic larvae into terrestrial adults. Many amphibians can modify their developmental sequence by extending or reducing their larval period. Phenotypic plasticity in the timing of metamorphosis has been previously reported, and the effects of various abiotic and biotic environmental factors have been studied in anurans and urodeles. Abiotic factors include water temperature (Alvarez and Nicieza 2002; Bizer 1978; Smith-Gill and Berven 1979; Voss 1993), hydroperiod (Laurila and Kujasalo 1999; Phillips et al. 2002; Rowe and Dunson 1995; Wilbur 1987), water level (Beachy 1995), and photoperiod (Kukita et al. 2015). Biotic factors include population density (Newman 1998; Scott 1990), food availability (Krause et al. 2011), and competition and predation (Boone et al. 2002; Orizaola et al. 2013; Segev and Blaustein 2007; Van Buskirk and Yurewicz 1998). Genetic factors have also been suggested (Merila et al. 2000a, b; Nishikawa and Matsui 2008). However, differences in the timing of metamorphosis cannot be interpreted using only one factor; multiple factors are important.
In urodeles, variations in larval life histories have been observed, even in conspecific populations. For example, the banded newt, Triturus vittatus, and the fire salamander, Salamandra salamandra (Segev and Blaustein 2007), display interspecific variations in their life histories. Furthermore, considerable intraspecific variation has been observed in emigration timing between ponds for three salamander species: the spotted salamander (Ambystoma maculatum), marbled salamander (A. opacum), and Eastern newt (Notophthalmus viridescens) (Timm et al. 2007). Populations of tiger salamander (A. tigrinum) in various ponds exhibited several life histories: standard, neotenic, and pedogenic (Bizer 1978). In addition, Bruce (1982, 1985) reported that larvae of the northern two-lined salamander, Eurycea bislineata, exhibit various life histories in different habitats. In anurans, several populations of the North American bullfrog, Rana catesbeiana, overwintered for more than 1 year before metamorphosis (Collins 1979).
In Japan, several studies have shown that certain salamanders, e.g., the Odaigahara salamander (Hynobius boulengeri), Hida salamander (H. kimurae), and clouded salamander (H. nebulosus tokyoensis), within conspecific populations exhibit variations in their larval life histories; i.e., metamorphosis occurs in the same year as hatching (the first year) or in the second or third year after hatching (Kusano 1981; Misawa and Matsui 1997; Nishikawa and Matsui 2008). The Ezo salamander, H. retardatus, is widespread and endemic to the subarctic Hokkaido Island, northern Japan. This salamander normally spawns and hatches in early spring, and metamorphoses during the hatch year. However, previous studies have shown that some larvae overwinter several times in permanent ponds in high-altitude habitats, and consequently metamorphose at 1 year of age or more (Iwasaki and Wakahara 1999; Michimae 2011).
Smith-Gill and Berven (1979) reported a differential sensitivity of growth and differentiation to temperature in amphibians, and that larvae are affected by hormonal systems. Only a few studies have been conducted on the internal mechanisms of metamorphosis in urodeles, whereas many studies have been conducted on anurans (cf. Rose 2005). Morphological features that can serve as indices of metamorphosis in urodeles are not as apparent as those in anurans (Gosner 1960; Taylor and Kollros 1946). Several experiments on the influence of low temperature on the timing of metamorphosis have been conducted for H. retardatus under laboratory conditions. Moriya (1983a, b) demonstrated that thyroid hormone is ineffective at extremely low temperatures, e.g., 4 °C, whereas prolactin (a growth-promoting hormone) stimulated growth at 4 °C.
Fragmented forest occurs at low altitudes in the environs of Sapporo. The Ezo salamander H. retardatus, as well as the Ezo brown frog, R. pirica, spawn during spring in ponds and pools in this region. In one pond, numerous overwintered H. retardatus larvae have been observed in March. This pond is not ephemeral. Pond drying may be an extreme disturbance for larval amphibians, because larvae may not survive if the pond dries before metamorphosis occurs (Semlitsch et al. 1996). On the other hand, pond permanency affects larval metamorphosis by allowing predators such as fishes to persist (Holbrook and Dorn 2016; Semlitsch et al. 1996). Larvae of H. retardatus are strictly carnivorous, and usually prey on the tadpoles of R. pirica that coexist during the breeding season. Intraspecific interactions in the salamander larvae populations (Nishihara-Takahashi 1999) and interspecific interactions between the salamander larvae and the frog tadpoles (Kishida et al. 2014; Michimae and Wakahara 2002; Ohdachi 1994) have been observed. The presence of numerous large H. retardatus larvae in early spring in the permanent pond in Sapporo may be primarily caused by the lack of desiccation and freezing of the pond, and the comparatively weak pressure of intraspecific or interspecific interactions. Since 2013, the authors of the present study have monitored the life histories of salamander larvae in this region. The water temperature has been recorded in the permanent pond as well as in a nearby temporary pond for comparison purposes.
In the present study, we present our findings from the Sapporo permanent and temporary ponds, especially the temperature transitions we discovered, and the relationships between water temperature and H. retardatus larval growth and development.
Methods
Study area
We documented the larval life histories of H. retardatus in two small ponds near a residential area (altitude 60 m) of Sapporo, Hokkaido, Japan. Both ponds were located approximately 200 m from small neighboring valleys of a belt-like forest. This forest was separated from the surrounding forests because of urban construction following the 1970s. Of the two ponds in the forest, the salamander larvae overwintered in one but not the other. The former was a permanent pond with abundant groundwater supplied from several spring-fed points (hereafter referred to as “the permanent pond”). The latter pond was not permanent and partially dried in summer (hereafter referred to as “the temporary pond”).
Both of the ponds were surrounded by broadleaf deciduous trees (e.g., Japanese oak, Quercus crispula, and full moon maple, Acer japonicum). Direct sunlight reached the ponds following leaf fall, but sunlight was intercepted from mid-May to mid-October because of the canopy cover. The surrounding air temperature increased to 30 °C in summer and decreased to −15 °C in winter. In the present study, we used air temperature data from the weather station at the campus of the Rakuno Gakuen University in Ebetsu, which is situated approximately 8 km from the ponds.
Environmental conditions in the permanent pond
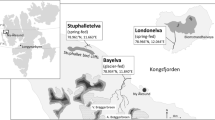
This pond was an impounded springlet at the headwater of a short valley. The spatial structure and thermal distribution of the permanent pond were measured twice: once on March 1, 2014 during winter and again on July 5, 2014 during summer. The depth contours and the longitudinal water temperature gradient of the pond are shown in Fig. 1. The maximum depth of the pond was approximately 1.9 m. The pond had abundant groundwater supplied throughout the year from spring-fed points at the bottom and edge of the pond (Fig. 1a). This continuous discharge of groundwater meant that there was no formation of an obvious vertical thermocline (Fig. 1b, c). The maximum water temperature in winter (March, 9.1 °C) and the minimum water temperature in summer (July, 9.3 °C) were measured near one of the spring-fed points (Fig. 1b, c). The differences between the maximum and minimum water temperatures were 4.7 °C on March 1 and 2.5 °C on July 5. The pond overflows from one outlet, and the water level was stable even after rainfall events or when snowmelt flowed into the pond. Although the surface layer of the lower area of the pond was slightly frozen during midwinter, the upper area was never completely frozen. A thermosensor was placed approximately about 0.5 m below the water surface (Fig. 1a–c) and hourly water temperatures were logged to the nearest 0.1 °C (RTR-52A, T&D Co.).
The spatial structure of the study ponds. a Plan view of the permanent pond. Depth contour lines are shown. The solid diamond shows the position of the serial recording thermosensor. Thin arrows indicate spring-fed locations, and the open arrow indicates the pond outlet. b Longitudinal section view of the permanent pond. The water temperature gradient in winter, March 1, 2014, is shown. c The water temperature gradient in summer, July 5, 2015. d Plan view of the temporary pond
Environmental conditions in the temporary pond
This pond was an enclosed shallow pool intersecting the brook with no spring-fed points in or near the edge of the pond. A bulky litter layer was located at the bottom of the pond. The water depth above the litter layer was measured. The maximum depth of the pond was approximately 0.25 m. The spatial structure and depth contours of the temporary pond are shown in Fig. 1d. The water level rose during snowmelt and following rainy weather, and fell during dry weather. The water surface of the shallow upper area dried and the litter layer was exposed in summer. The entire surface of this pond was covered with snow during winter. A thermosensor was placed on the litter layer approximately 0.15 m below the water surface, and hourly water temperatures were logged to the nearest 0.1 °C (RTR-52A, T&D Co.).
Field survey
Larvae capture and measurements
In the permanent pond, larvae were collected with a dip net every week or twice per month from late February to late November 2014–2015. During the breeding season from March to May, we recorded the number of egg sacs before capturing the larvae. The embryos per egg sac were counted on April 4 and April 11, 2015. Several hatchling larvae were observed near the egg sacs, but they were very small and difficult to capture. To capture larvae that had just emigrated to land, 10 pitfall traps were set around the pond. We checked the traps once or twice per week, and we also captured larvae that had emigrated to land by hand. In the temporary pond, we counted the number of egg sacs from April to May and caught larvae every week or twice per month from early May to early September, 2015. Using digital calipers (Mitutoyo, CD-15PSX), we measured snout–vent length (SVL: from the tip of the snout to the anterior margin of the cloaca) to the nearest 0.01 mm. To obtain these measurements, the larvae were anesthetized by immersion in 0.05 % MS222 (ethyl 3-aminobenzoate methane sulfonate, Sigma–Aldrich). We determined whether the larvae were tail-defective. Collected larvae were released after the effects of the anesthetic had subsided.
Determination of larval developmental stages
We determined larval developmental stages according to the reference key for the congeneric salamander H. nigrescens (Iwasawa and Yamashita 1991). We used the following primary morphological features to identify the larval stages: after hatching to evident differentiation of all the fingers of the forelimb (Sts 40–55); elongation of the hindlimb begins to primordium of fifth toe faintly recognizable (Sts 56–59); remarkable elongation of second toe (St. 60); fifth toe recognizable, but not totally formed (St. 62); fifth toe clearly recognizable (St. 63); onset of regression of the dorsal fin (St. 64); regression of the dorsal fin reaching half of the body and onset of external gill reduction (St. 65); gills small (St. 66); remarkable regression of the gills (St. 67); and complete disappearance of the gills (St. 68).
Marking of the larvae
To estimate the hatch year and the timing of emigration for the larvae in the permanent pond, we marked 0+ larvae (i.e., larvae in their first year) using Visible Implant Elastomer (VIE) tags injected into the limbs (Northwest Marine Technology, Inc.). From November 2013 to May 2014, we marked overwintering larvae with red VIE tags until observation of newly hatched larvae. Unmarked larvae captured during the marking period were tagged. Furthermore, we injected blue VIE tags from October 2014 to early May 2015.
Statistical analysis
We compared the distribution of SVL for each metamorphic stage, Sts 65–68, using the nonparametric Kruskal–Wallis test. To determine whether larval metamorphosis progressed simultaneously with growth, we analyzed the SVL of larvae captured in the pond and on land surrounding the pond using the Mann–Whitney U test.
Moreover, to determine whether larvae grew and developed during the wintering period, we analyzed larval SVL distributions and the composition of developmental stages from early autumn 2014 to early spring 2015 using the Kruskal–Wallis test. We conducted additional analyses using the Steel–Dwass nonparametric multiple comparisons test.
Results
Water temperature in the ponds
The successive changes in water temperature during 2013–2015 in both ponds are shown in Fig. 2. In the permanent pond, we observed four seasonal transition phases in water temperature: a warming period during spring, a constant-high period from summer to early autumn, a cooling period from mid-autumn to early winter, and a constant-low period during winter. The length of each period differed slightly depending on the year considered. However, the average length of each period was as follows: the constant-high period lasted from June 10 to September 20 (2013: mean temperature, in °C, ± SD = 10.77 ± 0.19, 2014: 10.78 ± 0.25, and 2015: 10.68 ± 0.27), and the constant-low period from December 10 to February 28 (2013–2014: 4.78 ± 0.50, 2014–2015: 5.65 ± 0.39). The mean temperature for the constant high period in 2014 was approximately 1.5 °C higher than that of the water temperature near the spring-fed point in the permanent pond on July 5, 2014 (Fig. 1c). The water temperature was lower than the air temperature during the constant-high period, and it was significantly higher than the air temperature during the constant-low period. During the warming and cooling periods, an inversion occurred between the air and water temperatures. The end of the warming period roughly coincided with the beginning of canopy closure above the pond. Furthermore, the cooling period began when the daily minimum air temperature was lower than the water temperature, and occurred simultaneously with leaf fall.
Successive changes in water temperature in the permanent and temporary ponds from April 2013 to November 2015. The temperature in the permanent pond is shown in red, the temperature in the temporary pond is shown in blue, and the air temperature is shown in gray. Note the four water temperature periods per year in the permanent pond: warming, constant-high, cooling, and constant-low periods. The water temperature data in the temporary pond from September 2014 to April 2015 were lost because of technical problems with the logger. Data for air temperature were obtained from a weather monitoring station at Rakuno Gakuen University, approximately 8 km from the ponds
In the temporary pond, fluctuations in water temperature were large, and the timing of the seasonal transitions differed depending on the year. We recognized three seasonal transition phases in water temperature: a warming period from spring to summer, a cooling period from autumn to winter, and a constant-low period during winter. Snow and ice covered the surface of the pond and ice almost reached the bottom litter layer; accordingly, the water temperature dropped to 1.0 °C in winter (Fig. 2). The great majority of the benthos and aquatic insects overwintered in the litter layer. The water temperature data collected from September 2014 to April 2015 were lost because of technical problems with the logger. Mean water temperature (°C) (±SD) from June 10 to September 20 in 2013 and 2014 was 15.79 ± 1.60 and 16.68 ± 1.88, respectively. Furthermore, the maximum water temperature was 19.6 °C in early August 2013, 21.5 °C in late June 2014, and 22.7 °C in early August 2015.
Spawning and hatching
In the permanent pond, we began spawning surveys on March 15, 2014, but we did not observe egg sacs of H. retardatus. We found two fresh egg sacs on March 22, and 88 egg sacs from March 22 to April 26. The first hatch day was observed on April 26, with the time to hatching being approximately 30 days in 2014. The first observed egg sacs on February 28, 2015, were fresh, and we found 107 egg sacs from February 28 to April 25, 2015. Hatching was observed from April 4 to May 30, 2015, and the time to hatching was the same as in 2014 (approximately 30 days). We also found eight egg masses of the sympatric amphibian R. pirica between April 5 and April 19, 2014, and 11 egg masses between March 24 and March 31, 2015. The R. pirica embryos in the egg jellies disappeared before the salamander hatching, and newly hatched tadpoles were not captured in the permanent pond.
In the temporary pond, 38 egg sacs of H. retardatus were observed from April 19 to May 3, 2014, and 75 egg sacs from April 4 to May 9, 2015. Egg masses of R. pirica were also found in this pond. A total of 38 egg masses were discovered from April 12 to 19, 2014, and 26 egg masses from April 4 to 25, 2015. Hatching of R. pirica occurred during late April and hatching of H. retardatus during early May.
Growth and development in the permanent pond
Numerous aquatic larvae were observed during all seasons in the permanent pond. Several larvae with reducing gills (Sts 65–67) were found on land, as well as those which had completely lost their gills (St. 68) (Fig. 3). These larvae were classified as larvae that had newly emigrated from water to land, not as juveniles. We captured 139 and 147 tail-defective larvae in 2014 and 2015, respectively. Cannibalistic behavior of larvae was occasionally observed. Dragonfly naiads and Japanese crayfish Cambaroides japonicus, which are potential predators of salamander larvae, were occasionally captured. We frequently captured numerous Gammaridea, and some benthos, including Tubificidae and nymphs of Chironomidae, which are prey of salamander larvae.
Cumulative size–frequency histograms with composition of the developmental stages of larvae Hynobius retardatus captured in the permanent pond and on surrounding land between April and October a 2014 and b 2015. Larvae captured in the pond are shown below the x-axis; larvae with shortened gills and juveniles on land are above the x-axis. Green bars indicate the St. 65 larval developmental stage, orange St. 66, blue St. 67, and red St. 68
Size–frequency histograms of larval SVL with corresponding developmental stages from April 5, 2014 to October 10, 2015 are shown in Figs. 4 and 5, respectively. Only one group of larvae observed in early to mid-April 2014 (Fig. 4a) and 2015 (Fig. 4n, o) consisted of overwintered individuals, most of which were at Sts 60–64, with a few at St. 65. After new larvae hatched, a smaller group of larvae emerged in late April, with two separated peaks in the histogram from May to July 2014 (Fig. 4b–e) and 2015 (Figs. 4p, 5a–d). In mid-June, the developmental stage of the smaller group of larvae remained below St. 56 (Figs. 4d, 5c). In early July 2014 (Fig. 4e) and 2015 (Fig. 5d), the latest developmental stage of larvae in the smaller group was St. 60, whereas in the larger group, the latest stage was St. 68 and the earliest-stage larvae were at St. 63. Several of the larvae in the smaller group reached the same size as the slow-growing larvae in the larger group by mid-July, and the two groups joined (Fig. 4f). The number of larger individuals decreased and, as a result, the bimodal pattern changed to unimodal in early August 2014 (Fig. 4g) and late July 2015 (Fig. 5e). After the larval population exhibited the unimodal histogram, it was difficult to distinguish overwintered larvae by body size.
Size–frequency histograms with composition of the developmental stages of larvae Hynobius retardatus in the permanent pond from April 2014 to April 2015. a Apr 5, 2014; b May 10, 2014; c May 24, 2014; d Jun 14, 2014; e Jul 5, 2014; f Jul 19, 2014; g Aug 2, 2014; h Aug 30, 2014; i Sep 14, 2014; j Oct 11, 2014; k Oct 25, 2014; l Nov 22, 2014; m Feb 28, 2015; n Apr 4, 2015; o Apr 11, 2015; p Apr 25, 2015
Comparison of the size–frequency histograms with composition of the developmental stages of larvae Hynobius retardatus in the permanent and temporary pond in 2015. a May 9; b June 6; c Jun 20; d Jul 4; e Jul 25; f Aug 1; g Aug 25; h Oct 10 in the permanent pond. i May 9; j Jun 6; k Jun 20; l Jul 4; m Jul 25; n Aug 1; o Aug 18 in the temporary pond
Aquatic larvae at Sts 65–67 were caught during April and early October in the pond. Emigrated larvae, whose developmental stages were Sts 65–68, were captured from May 10 to August 30, 2014 (150 individuals) and from May 2 to September 1, 2015 (101 individuals). Between late October 2014 and early April 2015, the observed developmental stages of the larvae were Sts 60–64, whereas larvae at St. 65 were not observed (Fig. 4k–n). The overwintered larvae resumed their development and larvae at St. 65 re-emerged during mid-April 2015 (Fig. 4o).
We marked 418 larvae with red VIE tags from November 2013 to May 2014, and 58 larvae with red tags were re-captured by July 26, 2014. Moreover, we marked 765 larvae with blue VIE tags from October 2014 to April 2015, and re-captured 175 larvae with blue tags by August 11, 2015. No larvae with red tags were found after August 2014 (Fig. 6).
Time sequence in snout–vent length (SVL) distributions and recapture of marked Hynobius retardatus larvae from October 2013 to May 2015. Marking of 0+ larvae with VIE tags was done several times (indicated by colored arrows) from November to April; red tags for the wintering cohort in 2013, blue tags for 2014. Recaptured larvae with red or blue marks and larvae that emigrated without marks are shown as a green cross
The overlap in the size distribution of the 2014 cohort with that of the previous year occurred between July 19 and August 30, 2014 (Fig. 4f–h), and the overlap of the 2015 cohort with the 2014 cohort occurred between July 25 and August 25, 2015 (Fig. 5e–g). In contrast, the composition of the developmental stages in each cohort was separated by a gap. The gap between the latest stage of the new cohort and the earliest stage of the previous cohort was at St. 64 on July 19 and St. 65 on August 2, 2014, and at St. 63 on August 1 and Sts 64–67 on August 25, 2015. Consequently, cohorts of larvae were distinguished by their developmental stages. The growth in SVL in the 2014 cohort from just after hatching in late spring until the next summer is shown in Fig. 7a, and that of the 2015 cohort is shown in Fig. 7b. In the 2014 cohort, the size distributions between August 17 and March 31 of the next year were significantly different (Kruskal–Wallis, p < 0.05). The size distribution on November 22 was significantly different from that of February 28 and March 31 in the next year (Steel–Dwass, p < 0.05), and those of August 17 and October 25 did not differ (p > 0.05). In the 2015 cohort, the size distributions of August 11 and December 12 were significantly different (Kruskal–Wallis, p < 0.05). The size distribution on October 10 was significantly different from that of November 7 and December 12 (Steel–Dwass, p < 0.05), but did not differ from those of August 25 and September 26.
Time series boxplots of larval snout–vent length (SVL). a 2014 hatched cohort in the permanent pond; b 2015 cohort in the permanent pond; c 2015 cohort in the temporary pond. Box indicates the interquartile range, the central horizontal bar in a box indicates the median, and whiskers indicate the data range
Size–frequency histograms of emigrated larvae at Sts 65–68 during April and October in 2014 and 2015 are shown in Fig. 5, with those of larvae at Sts 65–67 in the pond shown for comparison. Larvae at St. 65 were rarely found on land. The SVL distributions of each larval stage, Sts 65–68, did not differ during 2014, although they were different in 2015 (Kruskal–Wallis, p < 0.05). The size of St. 65 was somewhat larger than that of St. 68 in 2015 (Steel–Dwass, p < 0.05). The size distributions of metamorphosing larvae, Sts 65–68, captured in the pond were not different from those captured on land in 2014, although the larvae captured in the pond were larger than those captured on land in 2015 (Mann–Whitney U, p < 0.05). The SVLs of the smallest larvae that had emigrated to the land were 27.79 mm at St. 67 on June 28, 2014 and 27.58 mm at St. 68 on June 6, 2015.
Growth and development in the temporary pond
Size–frequency histograms of larval SVL with the corresponding developmental stages from May 9 to August 18, 2015 are shown in Fig. 5i–o. Newly hatched larvae grew and developed rapidly. The latest-stage larvae reached St. 63 on June 20 (Fig. 5k) and St. 64 on June 24 (not shown in Fig. 5). The metamorphosing larvae at Sts 65–67 were first observed on July 4, with the smallest observed larva (27.11 mm in size) at St. 67 on August 1 (Fig. 5l–o). Only five emigrated larvae, all at St. 68, were captured from July 11 to August 11. The smallest emigrated larva was 30.28 mm on August 11 (not shown in Fig. 5). No larvae were found after late August in the pond.
The SVL growth of the 2015 cohort larvae in the temporary pond is shown in Fig. 7c. The larvae grew continuously after hatching until early July. The size distributions between July 4 and August 18, when emigrating larvae were observed, rose and fell continuously and were significantly different (Kruskal–Wallis, p < 0.05).
We obtained 44 tail-defective larvae out of 274 larvae that were captured from late May to mid-August. Cannibalistic behavior of larvae was occasionally observed. Dragonfly naiads, which are predators of the salamander larvae, were usually captured in the pond. Japanese crayfish, C. japonicus, were not captured. Tadpoles of the brown frog, R. pirica, developed concurrently with larvae of H. retardatus. Metamorphosed larvae at St. 68 were captured on land during early July and early August in 2015. Neither tadpoles in the water nor juveniles on land were observed after mid-August. Pleuroceridae, Gammaridea, Tubificidae, and nymphs of Chironomidae, which are all prey items of the salamander larvae, were also captured.
Discussion
Variation in larval life histories
In the temporary pond, larvae at St. 65 were found from early July to mid-August in 2015, and emigrated larvae were also captured during this period. The range of SVL fluctuated as soon as the metamorphosing larvae emerged. The disappearance of larger-sized larvae was probably caused by the departure of new juveniles to terrestrial life, although smaller specimens continued to grow. These results clearly indicate that the larvae of one cohort grow and develop to complete metamorphosis in the year of hatching.
In the permanent pond, contrary to the temporary pond, the salamander larvae included 0+, 1+ (hatched in the previous year), or 2+ (hatched the year before last). We marked wintering larvae in the permanent pond that hatched in 2013 with red VIE tags, and captured several of them from April to July 2014. However, we did not capture any red-tagged larvae after August 2014. These results indicate that no larvae overwintered twice in the permanent pond, which is different from previous reports that some larvae of H. retardatus in high-altitude habitats overwinter several times (Iwasaki and Wakahara 1999; Michimae 2011).
Larvae initiating metamorphosis were first captured on April 5, 2014, and larvae emigrating from the permanent pond were first captured on May 10, 2014. The emigrated larvae included Sts 68–66, and St. 65 was also observed until July 19. Larvae at St. 65 were not obtained either in the pond or on land, but larvae at Sts 66–68 were captured between August 2 and August 30. Larvae at St. 65 were observed again between September 14 and October 11, but were never captured after October 25. During the period from late August to late November, the growth in SVL in the 2014 cohort larvae appeared to halt, but smaller specimens continued to grow. These results suggest that 0+ larvae initiating metamorphosis emigrated from the pond before late autumn. Thus, it is presumed that both 1+ larvae and 0+ larvae emigrated to land from the pond, suggesting that two larval life histories were present in the permanent pond.
In 2015, larvae at St. 65 were captured in the permanent pond between April 11 and July 25. Larvae that emigrated from May 2 to September 1 were 1+ larvae, similar to the 2014 cohort. Larvae older than St. 65, however, were never observed after mid-September. The SVL in the 2015 cohort larvae was not different from late August to mid-October, but smaller specimens continued to grow until December. Accordingly, some of the 0+ larvae metamorphosed and emigrated from the pond.
Timing of metamorphosis
In the permanent pond, spawning and hatching occurred during the warming period, and the season of spawning and hatching was earlier than that of the temporary pond. Larval development progressed gradually during the constant-high period. The mean water temperature in the permanent pond during the constant-high period was 10.8 °C, which is considerably lower than that of the temporary pond, which was 16.2 °C. During cooling period in the permanent pond, all of the larvae were earlier than St. 64, and they suspended their development during the constant-low period. On the other hand, larval growth continued, and a larger proportion of the larvae were greater than the smallest size of the metamorphosed larvae (e.g., 27.58 mm in 2015).
Cessation of metamorphosis in the salamander H. retardatus under certain laboratory conditions has been reported in several studies (Arai and Wakahara 1993; Iwasaki and Wakahara 1999; Moriya 1983a). After the larvae were transferred from 22 to 10 °C water, they completed metamorphosis. However, they did not undergo metamorphosis when transferred from 22 to 4 °C, even when they were transferred just prior to transformation (Moriya 1983a), suggesting there are temperature-sensitive mechanisms for metamorphosis. However, the endocrinological mechanism whereby larval metamorphosis progresses and the developmental stages in which the mechanism is active has not been fully investigated in salamanders. In bullfrogs, cessation of the metamorphic response because of low temperatures in the peripheral tissues and cells has been demonstrated (Frieden et al. 1965). Molecular biological studies in bullfrogs have shown several temperature-sensitive processes in the thyroid hormone-signaling pathway and gene expression (Mochizuki et al. 2012; Murata and Yamauchi 2005).
We found many larvae at Sts 65–67 that had already emigrated from the permanent pond, even though they still had exposed gills. They were assumed to be in the preliminary stage of complete metamorphosis (St. 68). In 2014, several 0+ larvae that had reached St. 65 by mid-October disappeared for a brief period. These larvae might have progressed to metamorphosis and emigrated to the terrestrial environment. However, other larvae, earlier than St. 64, ceased to continue their development in low temperatures from November 2014 to early April 2015, thereby overwintering without metamorphosis.
The SVLs of the larvae that emigrated from the pond at Sts 65–68 were almost the same as the SVLs of the larvae at Sts 65–67 that remained in the pond. The minimum size of the larvae that emigrated from the pond was identical in 2014 and 2015, and the available size to progress to metamorphosis was estimated to be approximately 28 mm. These results suggest that when larvae initiate metamorphosis they suspend growth and devote their energy to metamorphosis.
From our field study results, we refer to the lowest temperature at which preparation for metamorphosis was completed as a hypothetical critical temperature (HCT). We suggest that the HCT acts as a threshold for initiating metamorphosis between St. 64 and 65. Larvae at earlier stages than St. 64 did not progress their development when the water temperature fell below the HCT, and these larvae consequently overwintered in the permanent pond. Therefore, HCT is required to generate plasticity in the metamorphic timing of this salamander species. Furthermore, we suggested that the HCT is nearly the same but slightly higher than the water temperature at the spring-fed points during winter (9.1 °C). If larvae moved closer to a spring-fed point, where the water temperature was highest in winter, larval developmental stages would have progressed to metamorphosis. Emigrating to a snowy terrestrial environment in winter may increase mortality risk.
Intra- and interspecific interactions
In the temporary pond, tail-defective larvae were often captured during the survey. Salamander larvae suffered injuries via predation from dragonfly larvae that were usually captured at the same time as the salamander larvae, and from intraspecific interactions, including cannibalism in H. retardatus (Michimae and Wakahara 2002; Nishihara-Takahashi 1999; Ohdachi 1994). In the temporary pond, abiotic factors, such as water temperature and water level (Beachy 1995), and biotic factors, such as predation and competition (Boone et al. 2002; Orizaola et al. 2013; Segev and Blaustein 2007; Van Buskirk and Yurewicz 1998), could hasten larval development to complete metamorphosis in the year of hatching.
Tail-defective larvae were also captured in the permanent pond. Dragonfly larvae were scarce in this pond, implying that the salamander larvae suffered injuries from intraspecific interactions rather than from predation. The preying upon larvae of a smaller cohort (i.e., 0+ larvae) by larvae in a larger cohort (i.e., 1+ larvae) affects the survival and activity rates of 0+ larvae (Boone et al. 2002; Segev and Blaustein 2007; Wissinger et al. 2010). In H. retardatus, larvae that fed on conspecifics reportedly metamorphosed faster than larvae that fed on benthos (Michimae 2011; Michimae and Emura 2012). Thus, 1+ larvae preying on 0+ larvae might affect the development of 1+ larvae. In addition, the number of embryos of R. pirica decreased following hatching, and almost all tadpoles quickly disappeared after hatching. In a sympatric breeding environment, tadpoles can lessen their risk of predation or mutilation by hatching earlier than the salamanders (Ohdachi 1994), but the predation on embryos and tadpoles of the native frog by the large 1+ salamander larvae may have occurred in the permanent pond. Overall, 1+ larvae can prey on the next cohort larvae of H. retardatus, as well as on the tadpoles of R. pirica.
Features of the permanent pond that caused larval development to be slower than that in the temporary pond were its lower water temperature during summer, the depth of the pond with a stable water level, and its moderate number of predators and competitors.
Relationships between larval life histories and forest environments
Thus, overwintering by larvae in the permanent pond might be controlled by retardation of larval development and delayed metamorphosing. Determining the occurrence and ratio of overwintering larvae is crucial to understanding the causes and effects of environmental changes and larval life histories. For example, it is likely that the majority of the larvae will metamorphose without wintering in the permanent pond if the water temperature rises several degrees. Monitoring groundwater is important for predicting changes in larval life histories and for recognizing undetected disturbances in this area.
In the present study, HCT was almost the same as the water temperature at the spring-fed point. However, it is unclear whether HCT varies between populations depending on the geographical conditions of the habitat or whether it is a physiologically fixed determinant of the species. Groundwater temperature is dependent on the annual mean air temperature in the Japanese archipelago (Arai 2009), and the local groundwater temperature was roughly estimated from the altitude and latitude of the site (Nakano et al. 1996). Thus, it is necessary to conduct additional surveys in other populations at higher altitudes and/or latitudes where overwintering larvae are usually observed (cf. Iwasaki and Wakahara 1999). In addition, laboratory experiments should be conducted to verify whether there is a difference in temperature sensitivity between larvae from various populations. Moreover, to determine the absolute numerical value of HCT, additional laboratory experiments performed under standardized thermal regimes are needed.
The water temperature of the pond might be influenced by several physical factors, such as light conditions, heat exchange at the surface layer, and inflow discharge. Light conditions changed dramatically with canopy growth of the surrounding deciduous broadleaf trees. Skelly et al. (2002) reported that canopy cover may be of great importance to resident amphibians; they discovered that conditions in enclosures with closed canopy ponds were associated with reduced performance of amphibians, and the average water temperature was 5 °C warmer in waters under an open canopy than under a closed canopy. In the present study, the increase in water temperature in the permanent pond during the warming period was stalled by leaf emergence around the pond. Moreover, water temperature remained constant at 10.8 °C when the canopy was closed during summer, even though the air temperature increased to 30 °C. In contrast, the water temperature in the temporary pond increased after the surrounding tree canopy closed. This might have occurred because of the lack of spring-fed points in this pond and its shallowness, which led to an influence of the air temperature at the surface and the inflow water from the upper stream. Therefore, abundant spring water regulated the progress of larval development and metamorphosis in the permanent pond. The pond water temperature would increase if the surrounding trees were removed or the groundwater vein was damaged from extensive urban development. As a result, the growth and development of salamander larvae would accelerate, and many larvae would metamorphose during the hatching year.
References
Alvarez D, Nicieza AG (2002) Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct Ecol 16:640–648
Arai T (2009) Climate change and variations in the water temperature and ice cover of inland waters. Jpn J Limnol 70:99–116
Arai T, Wakahara M (1993) Hemoglobin transition from larval to adult types in normally metamorphosing, metamorphosed and metamorphosis-arrested Hynobius retardatus. Zool Sci 10:637–644
Beachy CK (1995) Effects of larval growth history on metamorphosis in a stream-dwelling salamander (Desmognathus ochrophaeus). J Herpetol 29:375–382
Bizer JR (1978) Growth rates and size at metamorphosis of high elevation populations of Ambystoma tigrinum. Oecologia 34:175–184
Boone MD, Scott DE, Niewiarowski PH (2002) Effects of hatching time for larval ambystomatid salamanders. Copeia 2002:511–517
Bruce RC (1982) Larval periods and metamorphosis in two species of salamanders of the genus Eurycea. Copeia 117–127
Bruce RC (1985) Larval period and metamorphosis in the salamander Eurycea bislineata. Herpetologica 41:19–28
Collins JP (1979) Intrepopulation variation in the body size at metamorphosis and timing of metamorphosis in the bullfrog, Rana catesbeiana. Ecolgy 60:738–749
Frieden E, Wahlborg A, Howard E (1965) Temperature control of the response of tadpoles to triiodothyronine. Nature 205:1173–1176
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Holbrook JD, Dorn NJ (2016) Fish reduce anuran abundance and decrease herpetofaunal species richness in wetlands. Freshw Biol 61:100–109
Iwasaki F, Wakahara M (1999) Adaptable larval life histories in different populations of the salamander, Hynobius retardatus, living in various habitats. Zool Sci 16:667–674
Iwasawa H, Yamashita K (1991) Normal stages of development of a hynobiid salamander Hynobius nigrescens Stejneger. Jpn J Herpetol 14:39–62
Kishida O, Costa Z, Tezuka A, Michimae H (2014) Inducible offences affect predator–prey interactions and life-history plasticity in both predators and prey. J Anim Ecol 83:899–906
Krause ET, Steinfartz S, Caspers BA (2011) Poor nutritional conditions during the early larval stage reduce risk-taking activities of fire salamander larvae (Salamandra salamandra). Ethology 117:416–421
Kukita S, Gouda M, Ikeda S, Ishibashi S, Furuya T, Nakamura K (2015) Effects of photoperiod and temperature on growth and development in clouded salamander (Hynobius nebulosus) larvae. Zool Sci 32:266–271
Kusano T (1981) Growth and survival rate of the larvae of Hynobius nebulosus tokyoensis Tago (Amphibia, Hynobiidae). Res Popul Ecol 23:360–378
Laurila A, Kujasalo J (1999) Habitat duration, predation risk and phenotypic plasticity in common frog (Rana temporaria) tadpoles. J Anim Ecol 68:1123–1132
Merila J, Laurila A, Laugen AT, Rasanen K, Pahkala M (2000a) Plasticity in age and size at metamorphosis in Rana temporaria: comparison of high and low latitude populations. Ecography 23:457–465
Merila J, Laurila A, Pahkala M, Rasanen K, Laugen AT (2000b) Adaptive phenotypic plasticity in timing of metamorphosis in the common frog Rana temporaria. Ecoscience 7:18–24
Michimae H (2011) Plasticity in the timing of a major life-history transition and resulting changes in the age structure of populations of the salamander Hynobius retardatus. Biol J Linn Soc 102:100–114
Michimae H, Emura T (2012) Correlated evolution of phenotypic plasticity in metamorphic timing. J Evol Biol 25:1331–1339
Michimae H, Wakahara M (2002) A tadpole-induced polyphenism in the salamander Hynobius retardatus. Evolution 56:2029–2038
Misawa Y, Matsui M (1997) Larval life history variation in two populations of the Japanese salamander Hynobius kimurae (Amphibia, Urodela). Zool Sci 14:257–262
Mochizuki K, Goda T, Yamauchi K (2012) Gene expression profile in the liver of Rana catesbeiana tadpoles exposed to low temperature in the presence of thyroid hormone. Biochem Biophys Res Commun 420:845–850
Moriya T (1983a) The effect of temperature on the action of thyroid hormone and prolactin in larvae of the salamander Hynobius retardatus. Gen Comp Endocrinol 49:1–7
Moriya T (1983b) Cytological changes induced by low temperature in the thyroid glands of larvae of the salamander Hynobius retardatus. Gen Comp Endocrinol 49:8–14
Murata T, Yamauchi K (2005) Low-temperature arrest of the triiodothyronine-dependent transcription in Rana catesbeiana red blood cells. Endocrinology 146:256–264
Nakano S, Kitano F, Maekawa K (1996) Potential fragmentation and loss of thermal habitats for charrs in the Japanese archipelago due to climatic warming. Freshw Biol 36:711–722
Newman RA (1998) Ecological constraints on amphibian metamorphosis: interactions of temperature and larval density with responses to changing food level. Oecologia 115:9–16
Nishihara-Takahashi A (1999) Faster growth of head size of pre-feeding larvae in a cannibalistic population of the salamander Hynobius retardatus. Zool Sci 16:303–307
Nishikawa K, Matsui M (2008) A comparative study on the larval life history in two populations of Hynobius boulengeri from Kyushu, Japan (Amphibia: Urodela). Curr Herpetol 27:9–22
Ohdachi S (1994) Growth, metamorphosis, and gape-limited cannibalism and predation on tadpoles in larvae of salamanders Hynobius retardatus. Zool Sci 11:127–131
Orizaola G, Dahl E, Nicieza AG, Laurila A (2013) Larval life history and anti-predator strategies are affected by breeding phenology in an amphibian. Oecologia 171:873–881
Phillips CA, Johnson JR, Dreslik MJ, Petzing JE (2002) Effects of hydroperiod on recruitment of mole salamanders (genus Ambystoma) at a temporary pond in Vermilion County, Illinois. Trans Ill Acad Sci 95:131–139
Rose CS (2005) Integrating ecology and developmental biology to explain the timing of frog metamorphosis. Trends Ecol Evol 20:129–135
Rowe CL, Dunson WA (1995) Impacts of hydroperiod on growth and survival of larval amphibians in temporary ponds of central Pennsylvania, USA. Oecologia 102:397–403
Scott DE (1990) Effects of larval density in Ambystoma opacum an experiment in large scale field enclosures. Ecology 71:296–306
Segev O, Blaustein L (2007) Priority effects of the early breeding fire salamander on the late breeding banded newt. Hydrobiologia 583:275–283
Semlitsch RD, Scott DE, Pechmann JHK, Gibbons JW (1996) Structure and dynamics of an amphibian community: evidence from a 16-year study of a natural pond. In: Cody ML, Smallwood JA (eds) Long-term studies of vertebrate communities. Academic, San Diego, pp 217–248
Skelly DK, Freidenburg LK, Kiesecker JM (2002) Forest canopy and the performance of larval amphibians. Ecology 83:983–992
Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113:563–586
Taylor AC, Kollros JJ (1946) Stages in the normal development of Rana pipiens larvae. Anat Rec 94:7–23
Timm BC, McGarigal K, Gamble LR (2007) Emigration timing of juvenile pond-breeding amphibians in western Massachusetts. J Herpetol 41:243–250
Van Buskirk J, Yurewicz KL (1998) Effects of predators on prey growth rate: relative contributions of thinning and reduced activity. Oikos 82:20–28
Voss SR (1993) Relationship between stream order and length of larval period in the salamander Eurycea wilderae. Copeia 1993:736–742
Wilbur HM (1987) Regulation of structure in complex systems experimental temporary pond communities. Ecology 68:1437–1452
Wissinger SA, Whiteman HH, Denoel M, Mumford ML, Aubee CB (2010) Consumptive and nonconsumptive effects of cannibalism in fluctuating age-structured populations. Ecology 91:549–559
Acknowledgments
We would like to thank Dr. Masami Wakahara for helpful discussions. We thank the members of our laboratory for supporting the field survey.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Toshifumi Minamoto.
Rights and permissions
About this article
Cite this article
Midori, T., Kuwahara, T. & Yamashiki, N. Retardation of larval development in the salamander Hynobius retardatus in a permanent pond with abundant spring water. Limnology 18, 287–299 (2017). https://doi.org/10.1007/s10201-016-0506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-016-0506-7