Abstract
Dissolved oxygen (DO) in the Lake Biwa hypolimnion reached its lowest level of <1 mg kg−1 in 2007. In this paper, we report the variations in the total dissolvable (TD), dissolved (D), and labile particulate (LP) fractions of Al, Si, P, V, Cr, Mn, Fe, Ni, Cu, Zn, As, Mo, W, and U in Lake Biwa 2007 and 2009. Al and Fe species were predominantly in the form of LP-Al and LP-Fe and were strongly correlated with one another (r = 0.99), suggesting that the weathering of aluminous minerals and the supply of clay mineral particles are the main factors that influence the distributions of Al and Fe. Although D-Al increased in the summer epilimnion, D-Fe was relatively low, probably as a result of uptake by plants. Reductive release of Fe from the bottom was not seen. Mn was also dominated by LP-Mn, but this fraction showed a different distribution to those of LP-Al and LP-Fe. The D-Mn and LP-Mn concentrations varied by factors of 700–1000 and showed marked increases in the bottom water during stratification in 2007. We believe that Mn2+ was released from the sediments and oxidized by DO in the bottom water. Ni, Cu, Zn, and Cr, which exist as cationic species, had LP/TD ratios of 0.1–0.7 and relatively uniform distributions. Si, P, V, As, Mo, W, and U, which form oxoacid species, had LP/TD ratios of 0–0.8. Si, P, and As were characterized by nutrient-like profiles, V, W, and U showed summer maxima in the epilimnion, and Mo had a uniform distribution. TD-Mo increased in the bottom water along with TD-Mn, while TD-V and TD-W showed significant decreases. These results are likely attributable to differences in the adsorption of these elements onto manganese oxides and iron hydroxides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
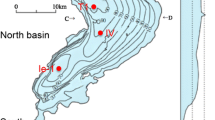
Lake Biwa, located to the east of Kyoto, is the largest freshwater lake in Japan, with a surface area of 674 km2 and a volume of 27.3 km3 (Li et al. 2007, 2011 and references therein; Fig. 1). The main north basin has an average depth of 48 m and a maximum depth of 104 m; it is a monomictic lake with a water residence time of 5.3 years. Lake Biwa has existed at its present location for 430,000 years (Meyers et al. 1993), nurturing unique ecosystems, including around 50 endemic species. It is an important source of freshwater to people in Shiga, Kyoto, Osaka, and Hyogo. The environment of Lake Biwa has changed dramatically since the 1940s due to regional development and global climate change (Nakanishi and Sekino 1996), and the south basin is now fully eutrophic while the north basin is mesotrophic. The dissolved oxygen (DO) concentration in the hypolimnion of the north basin has shown a decreasing trend over the past 50 years (Sohrin et al. 2016). Snowfall has been rare in the catchment area, and lake water convection became weak in the winter of 2006, resulting in the lowest recorded DO level (<1 mg kg−1) in the autumn of 2007. Mass mortality of the endemic goby fish (Gymnogobius isaza) and the lake prawn (Palaemon paucidens), which were highly contaminated with Mn and As, occurred on the lake bottom in 2007 (Hirata et al. 2011; Itai et al. 2012).
We are interested in understanding the changing geochemical cycles of trace elements in Lake Biwa. Reliable data on trace elements in the lake water was first made available in the 1980s, following the development of clean analytical techniques. Kawashima et al. (1988) reported that the total manganese (Mn) concentration increased substantially in the bottom water of the north basin during the stratification period. Sohrin et al. (1997) studied the speciation and variation in the level of arsenic (As) in both the north and south basins. Multielemental analysis is a valuable tool for understanding the geochemical cycles of trace elements. The multielemental determination of dissolved trace elements in Lake Biwa has been made possible by the development of suitable analytical instruments (Haraguchi et al. 1998; Itoh and Haraguchi 1997; Sugiyama et al. 1986, 1992; Zhu et al. 2005). Mito et al. (2004) observed variations in the levels of dissolved elements (Mg, Al, Ca, V, Cr, Mn, Fe, Ni, Zn, As, Sr, Y, W, and U) in the local lake, rivers, and rain water while assessing the elemental budget of Lake Biwa. Harita et al. (2005) observed the vertical distributions of vanadium (V), molybdenum (Mo), and tungsten (W) in Lake Biwa, Lake Ikeda, and Unagi-ike. Sugiyama et al. (2005) reported the seasonal vertical profiles of dissolved elements (Na, Mg, Si, K, Ca, V, Mn, Fe, Ni, Cu, Zn, Sr, Mo, and Ba) and particulate elements (Mg, Al, Ca, Mn, Fe, Ni, Cu, Zn, Sr, and Ba) in Lake Biwa; thus far, this is only study that has considered both the dissolved and particulate species of various trace elements.
Takamatsu et al. (1985) determined the concentrations of 36 elements in the sediments of Lake Biwa and found that the uppermost oxidized layer, where Mn and As were highly concentrated, was only around 2 mm deep. Miyajima (1994) studied the precise behavior of nitrogen (N) and Mn at the oxic/anoxic interface, and Kojima et al. (2003) measured the concentrations of Na, Sc, Mn, Fe, Co, As, Sb, and Sm in pore water of the sediments. Recently, Li et al. (2007, 2011) compiled and discussed the composition of water and sediments in Lake Biwa from a thermodynamic viewpoint.
We determined the dissolved (D), the total dissolvable (TD), and the labile particulate (LP) concentrations of trace elements (Al, Si, P, V, Cr, Mn, Fe, Ni, Cu, Zn, As, Mo, W, and U) in the water samples collected from Lake Biwa using a clean sampling system in 2007 and 2009. In this paper, we discuss the seasonal variations in the distribution and speciation of the trace elements.
Methods
All materials, such as bottles and micropipette chips, were successively precleaned with a detergent, acids, and water deionized with a Milli-Q system (Millipore). Sampling was carried out on board the training investigation ship Hassaka, which belonged to the University of Shiga Prefecture. The sampling location was offshore near Takashima (35°22′00″N, 136°06′00″E, 91 m depth; T1 in Fig. 1), which is a few kilometers southwest of the deepest part of the basin. A conductivity temperature depth (CTD) system (DataSonde5, Environmental System) was used to measure temperature, DO, pH, and chlorophyll a (Chl a). A 5-L Niskin sampling bottle was prepared by coating the inner surface with Teflon, replacing the spring with silicon tubing to close the caps, and thoroughly cleaning the inside. The Niskin bottle was then deployed with Kevlar wire, the length of which determined the sampling depth. Lake water samples were transferred to Nalgene low-density polyethylene bottles while onboard the vessel, with special attention paid to avoiding contamination by dust. Within a few days, a portion of each sample was filtered through a Nuclepore filter (Whatman) with a pore size of 0.2 µm using a closed filtration system, the solution was driven through the filter using the pressure of N2 gas. High-purity HNO3 (Ultrapur-100, Kanto Chemical) was added to both the filtered and unfiltered samples to achieve a final concentration of 0.1 M, and the resulting solutions were stored at room temperature until analysis. In this study, 0.1 M HNO3 was used, since this matrix is suitable for direct sample introduction onto analytical instruments and the acid concentration does not cause significant contamination of analytes in lake water. The filtered and unfiltered samples were used for the determination of dissolved and total dissolvable fractions, respectively. The unfiltered samples were filtered through a Millex filter (Millipore) with a pore size of 0.45 µm just before analysis. The concentration of silicon (Si) was determined with an inductively coupled plasma-atomic emission spectrometer (ICP-AES; Optima 2000DV, PerkinElmer), while those of the other 13 elements (Al, P, V, Cr, Mn, Fe, Ni, Cu, Zn, As, Mo, W, and U) were determined with a high-resolution inductively coupled plasma mass spectrometer (HR-ICP-MS; Element 2, Thermo Fisher) using the calibration curve method. Detection limits were sufficiently low to permit the study of the distributions of total dissolvable and dissolved fractions for all elements, and the relative standard deviations were ~5 %. The concentration of the labile particulate fraction was calculated as the difference between the total dissolvable and dissolved concentrations. When the concentration of the dissolved fraction was higher than that of the total dissolvable fraction for a particular element, the concentration of the labile particulate fraction was assumed to be zero. Additional details about the analytical method are given in the Electronic supplementary material (ESM).
Results
The results of our study are summarized in Table S3 of the ESM. The vertical profiles of temperature and DO are shown in Fig. 2, and those of Chl a and pH are shown in Fig. S1 of the ESM. In 2007, the DO concentration in the bottom layer decreased during stratification, resulting in the lowest value observed in this study, 1.0 mg kg−1, on November 8. The surface water temperature decreased below 15 °C on November 29, leading to initial destratification. While the DO concentration slightly increased in the bottom water, it showed further decreases at depths of 40–75 m. In 2009, the lowest measured DO concentration was 3.1 mg kg−1. According to observations made by the Lake Biwa Environmental Research Institute in the deepest part of the basin (at a depth of around 100 m), the lowest DO concentration in the bottom water reached 0.3 mg kg−1 in 2007, 0.5 mg kg−1 in 2008, and 1.0 mg kg−1 in 2009 (Jiao et al. 2011).
The vertical profiles of total dissolvable, dissolved, and labile particulate trace elements are presented in Figs. S2, S3, and S4 in the ESM, respectively. Figure 3 shows the vertical profiles of 12 elements on November 29, 2007, which represent characteristic profiles of the trace elements. Statistical data for the total dissolvable, dissolved, and labile particulate concentrations are summarized in Table 1. TD-Mo and D-Mo showed the smallest deviations, with relative standard deviations of around 11 % (n = 130). In contrast, TD-Mn, D-Mn, and LP-Mn varied by factors of 340–1020.
Discussion
Dissolved, total dissolvable, and labile particulate fractions of trace elements
The ranges of dissolved concentrations (Table 1) found in our study are generally consistent with those reported in the literature (Mito et al. 2004; Sugiyama et al. 2005), which supports the accuracy of our results. It has been proposed, on the basis of results derived via an ultrafiltration technique, that many of the trace metals present in Lake Biwa form complexes with dissolved high-molecular-weight organic matter (Zhu et al. 2005). In another previous study, dissolved organic ligands for copper (Cu) were concentrated using immobilized metal ion affinity chromatography and characterized (Wu and Tanoue 2001). In our study, such organic complexes were probably dissociated due to the storage of samples at around pH 1. Further, it has been reported that some D-Fe is present as Fe(II) in the surface lake water (Maruo et al. 2007), but in our study Fe(II) should have been oxidized to Fe(III) during storage. Thus, we do not consider it likely that complexation and redox reactions will have had a significant effect on the determination of dissolved species.
As(III), methylated As, and some unknown organometallic As species are formed in the epilimnion as a result of biological activity, although As(V) is thermodynamically stable under oxic conditions (Hasegawa 1997; Hasegawa et al. 1999, 2001; Sohrin et al. 1997). Most of the As species would be determined by ICP-MS, because the As–C bonds in the organometallic compounds would be dissociated in the argon plasma. However, the efficiency of sample introduction into ICP-MS may differ for the inorganic and organometallic species, causing some errors which are dependent on the As speciation. Dissolved phosphorus (D-P) will consist of phosphate and dissolved organic phosphorus (DOP). Figures S5a–d in the ESM show that the profiles of D-P and D-Si obtained in this study are generally consistent with those of soluble reactive phosphorus (SRP) and silicate, as determined by the Lake Biwa Environmental Research Institute.
The analytical procedure for the total dissolvable fraction is unique to this work. Figures S5e and f show, however, that the profile of TD-P is generally consistent with that of total P determined by the Lake Biwa Environmental Research Institute.
The labile particulate species represent the fraction that is dissociated from particles during storage at a pH of around 1. This fraction will include (1) metal oxides and hydroxides, (2) metal ions adsorbed onto mineral surfaces, and (3) metal ions within particulate organic matter. In previous studies in Lake Biwa, particulate concentrations have been determined after complete acid digestion (Sugiyama et al. 2005), or in situ by X-ray fluorescence spectrometry (Mito et al. 2002). The data from these studies are compared with the labile particulate concentrations obtained in our study in Table S4 in the ESM. For Al and Si, the concentration ranges of the labile particulate species are significantly lower than those of the particulate species. This implies that some of the aluminosilicates and silica are not included in the measured labile particulate fraction. For the other elements, the concentration ranges of the labile particulate and particulate species are similar to one another, suggesting that the labile particulate species represent a major fraction of the particulate species. The LP-Si/LP-P ratio was 8.1 ± 1.2 (mean ± SD, n = 130). For comparison, an average Si/P ratio of 120 has been proposed for diatoms that were naturally growing in Lake Biwa (Nagai et al. 2001). In addition, although the concentrations of suspended particulates of diatom origin increased in the summer (Nagai et al. 2001), the LP-Si/LP-P ratio was relatively high in the winter. This can be explained by the different solubilities of Si and P from particulate matter at around pH 1. Table S5 in the ESM shows that the M/Al ratios of the labile particulate species are generally higher than those of the particulate species determined in previous studies (Mito et al. 2002; Sugiyama et al. 2005) as well as in the crust (Rudnick and Gao 2005).
Correlation coefficients among the total dissolvable, dissolved, and labile particulate fractions are listed in Tables S2–S4 of the ESM. The LP/TD ratio for each element is plotted in order of decreasing average LP/TD in Fig. 4. It should be noted that elements with high correlations also have similar LP/TD ratios. In particular, the correlation coefficient between LP-Al and LP-Fe is 0.99 (p < 0.001, n = 130), and both of these elements have the highest LP/TD ratio (>0.9). Based on these results, the trace elements can be divided into five groups that are discussed separately below: (1) Al and Fe; (2) Mn; (3) Cr, Ni, Cu, and Zn; (4) Si, P, and As; and (5) V, Mo, W, and U.
Al and Fe
Most of the Al and Fe existed as labile particulate species (Figs. 3 and 4). LP-Al and LP-Fe showed a strong linear relationship with one another (Fig. 5a; Eq. 1), suggesting that these elemental concentrations are controlled by aluminosilicate minerals:
A sharp bottom maximum was observed in LP-Al, LP-Fe, and other labile particulate elements on September 5, 2007 (Fig. S4 in the ESM). There were heavy rains during August 29–31 in Hikone, a city to the east of the north basin. It is likely that these rains caused an abrupt intrusion of turbid water to the lake bottom, resulting in these high measured values.
D-Al and D-Fe showed substantial seasonal variations, particularly in the epilimnion, between depths of 0 and 20 m (Figs. S3, S6, and S7 in the ESM). D-Al showed a broad maximum of around 200 nmol kg−1 in August–September. Koshikawa et al. (2002) observed the same trend in 1992–1995 and proposed that it was caused by a decrease in the adsorption of Al onto particulate matter at higher pH levels. However, no clear reverse relationship was found between D-Al and LP-Al in this study. The correlation between D-Al and pH was moderate (r = 0.82, p < 0.001, n = 85), and was as strong as the correlation between D-Al and water temperature (r = 0.84, p < 0.001, n = 116). It is likely that another important controlling factor is enhanced weathering caused by high temperatures and high precipitation in the summer. D-Fe showed a different yearly change to that of D-Al. D-Fe generally remained at less than 30 nmol kg−1, with sporadic spikes in concentration. As a result, the correlation between D-Al and D-Fe was weak:
The D-Fe/D-Al ratio was 0.27 ± 0.18 (n = 65) in the epilimnion and 0.60 ± 0.35 (n = 65) in the hypolimnion. These are significantly lower than all recorded LP-Fe/LP-Al ratios (0.93 ± 0.29; n = 130). The D-Fe/D-Al ratio became particularly low in the summer epilimnion. To put this into context, the budget of D-M in the summer epilimnion is summarized in Table S5 of the ESM. The observed increases at this time were around 200 and 30 nmol kg−1 for D-Al and D-Fe, respectively. Assuming that the increase in D-Al is caused by weathering and that the weathering ratio of Fe/Al is equal to the crustal ratio of 0.3, it can be deduced that 60 nmol kg−1 of D-Fe is supplied by weathering. Thus, there is a missing concentration of 30 nmol kg−1 for D-Fe. The dominant species of D-Fe should be Fe(III), similar to Al(III). Because Fe(II) is observed in surface water of Lake Biwa and its inflowing rivers (Maruo et al. 2007), it is considered very likely that weathering supplies a higher D-Fe/D-Al ratio than the crustal ratio, resulting in a greater missing concentration of D-Fe. In fact, the flux ratio of D-Fe/D-Al in river water was found to be around 0.7 on the basis of river water concentration measurements (Mito et al. 2004). A possible mechanism for the subsequent loss of D-Fe is its uptake by plants, since Fe is an important micronutrient. At present, there are no data on the trace element compositions of phytoplankton in Lake Biwa which could be used to confirm this hypothesis. Given that around 200 nmol kg−1 of LP-P is in the form of P present in suspended organic matter produced by phytoplankton in the offshore epilimnion, and that the Fe/P ratio of phytoplankton is 0.1, according to the compendium (Li 2000), the Fe uptake by phytoplankton is estimated to be only 21 nmol kg−1. Thus, we consider it likely that a significant amount of D-Fe is taken up by land plants, aquatic plants, and algae before reaching the offshore regions.
Fe concentrations did not increase in bottom water at T1, in contrast to those of Mn. This implies that there was no reductive dissolution of Fe from the sediment surface. The Fe concentration was reported to be around 180 µmol kg−1 in pore water at 0–1 cm depth in the sediments (Kojima et al. 2003). Therefore, iron reduction must be occurring within sediments, a few millimeters beneath the sediment surface. However, Fe(II) is oxidized to Fe(III) to form iron hydroxides in the sediment surface layer, meaning that there is no major route for it to the bottom water at present.
Mn
Mn concentrations were also dominated by labile particulate species, but the distribution of Mn was intrinsically different from those of Al and Fe (Figs. 3 and 4, and Figs. S2–S4 in the ESM). Mn is characterized by large variations in concentration, with substantial increases seen in the bottom water during stratification. The TD-Mn concentration reached 9.3 µmol kg−1 on November 29, 2007, which is the highest value that has ever been observed in the north basin. Previous studies in Lake Biwa have reported the Mn concentrations in sediment pore water to be 40–140 µmol kg−1 (Kojima et al. 2003; Miyajima 1994), which, together with our data, imply that manganese reduction is occurring in the sediments. According to a previous study (Miyajima 1994), when Mn(II) in pore water diffuses upward, 26–93 % of the Mn(II) is oxidized to form manganese oxide in the sediment surface layer while the rest diffuses into bottom water. It is likely that the re-oxidation efficiency at the sediment surface decreased with the decrease in DO, resulting in the higher Mn concentrations seen in the bottom water in our study.
In Fig. 5b, the concentrations of D-Mn and LP-Mn are plotted against DO. It can be seen that even when DO is as low as around 1 mg kg−1, LP-Mn concentrations overwhelm those of D-Mn. This result suggests that the oxidation of Mn(II) in lake water is rapid. This is consistent with the findings of laboratory experiments (Kawashima et al. 1988), which have demonstrated that the oxidation of Mn(II) in the lake water is mediated by bacteria and occurs on a timescale of hours. Although it is possible that some Mn(II) was oxidized during the period from sampling to filtration, this effect would be negligible for the huge concentration of LP-Mn.
Mn is also an essential micronutrient for organisms. Assuming that the Mn/P ratio of phytoplankton is 9 × 10−4, as given in the literature (Li 2000), the biogenic LP-Mn in the suspended particles will be only around 0.2 nmol kg−1 (Table S5 in the ESM). Thus, uptake by phytoplankton will not have a significant effect on the distribution or concentration of Mn.
Cr, Ni, Cu, and Zn
Cr, Ni, Cu, and Zn were characterized by LP/TD ratios in the range 0.1–0.7 and distributions that were relatively uniform both vertically and seasonally (Figs. 3, 4, and Figs. S2–S4 in the ESM). Ni, Cu, and Zn are dominated by divalent cations, which form complexes with inorganic and organic ligands (Haraguchi et al. 1998; Itoh and Haraguchi 1997; Wu et al. 2001; Zhu et al. 2005). These elements are also essential micronutrients for aquatic organisms. In the ocean, these metals mostly exist as dissolved species that are taken up from surface water by phytoplankton and regenerated from settling particles in deeper waters; they therefore show nutrient-like vertical profiles (Ezoe et al. 2004). The different behaviors of these elements in Lake Biwa compared to the ocean are probably due to the greater supply of these elements and the higher concentrations of aluminosilicate and metal oxide particles in the lake. The M/Al ratios of the labile particulate species were 20–66 times higher than those in the crust (Table S5 in the ESM), suggesting that these metals are actively scavenged by the particles in Lake Biwa. The M/P ratio of phytoplankton is ~5 × 10−4 for both Ni and Cu (Li 2000), so the LP-Ni and LP-Cu produced by phytoplankton will be ~0.1 nmol kg−1 in Lake Biwa (Table 5 in the ESM). Thus, uptake by phytoplankton can be considered to have undetectable effects on the distributions of Ni and Cu. In contrast, the Zn/P ratio of phytoplankton is 7 × 10−3 (Li 2000), leading to a biogenic LP-Zn concentration of 1.4 nmol kg−1 (Table 5 in the ESM). This suggests that biological uptake may result in significant variations in D-Zn and LP-Zn in the epilimnion. In line with this, our data showed small decreases in D-Zn and small increases in LP-Zn during the summer months.
The possible valence states of Cr are +3 and +6, with Cr(VI) forming an oxyanion and Cr(III) forming complex species. Thermodynamically, Cr(VI) will be dominant in oxic water while Cr(III) dominates under conditions of manganese reduction (Turner et al. 1981). Thus far, there have been no studies documenting the valence speciation for Cr in Lake Biwa. However, our data indicate that Cr has a similar LP/TD ratio and distribution to those of Ni, Cu, and Zn, implying that the Cr is predominantly in the +3 valence state in Lake Biwa.
Sporadic maxima of Cr, Ni, Cu, and Zn were seen in surface water. These peaks are not considered to be related to weathering because there were no concurrent maxima in Al and Fe and because the supply of D-M by weathering is estimated to be low for Cr, Ni, Cu, and Zn (Table S5 in the ESM). It is possible that some of these metals were derived from atmospheric deposition (Lantzy and Mackenzie 1979). In support of this, the dissolved concentrations of these metals were found to be considerable in rain water collected near Lake Biwa: 0.32–11.8 nmol kg−1 for Cr, 0.60–61.7 nmol kg−1 for Ni, and 34–3023 nmol kg−1 for Zn (no data for Cu; Mito et al. 2004).
In bottom water, TD-Cr and TD-Zn showed slight decreases during stratification, while D-Cu decreased and LP-Cu increased. These changes are only small and relatively insignificant, so we do not discuss them further here.
Si, P, and As
The LP/TD ratio was 0.3–0.8 for P and 0–0.1 for Si and As (Fig. 4). The concentrations of their dissolved species were low in surface water and high in deeper waters during stratification (Fig. 3 and Fig. S3 in the ESM). These profiles can be explained by biological uptake in surface water, followed by remineralization of dissolved species from settling biogenic particles in deep water. The bottom water concentrations were higher in 2009 than in 2007. The profiles became uniform during the winter as a result of vertical mixing of water. The dissolved species of Si is Si(OH)4, which is essential for diatoms to form silica frustules. This biogenic silica would undergo very little dissolution during the storage of unfiltered lake water samples, so it cannot be quantified through the measurement of LP-Si.
The major dissolved species of P is phosphate, which is essential for all organisms. Other dissolved species of P include natural and anthropogenic dissolved organic matter that incorporates phosphate through a phosphate bond. DOP becomes dominant when phosphate is depleted in surface water (Kim et al. 2006). In inflowing and outflowing rivers of Lake Biwa, the ratios of phosphate to SRP ranged from 0.06 to 0.79 (Maruo et al. 2016). P can also exist as phosphate in particulate matter. The phosphate bond is easily dissociated through hydrolysis, so LP-P is expected to represent most of the total particulate P. In 2007 and 2009, LP-P showed increases of around 200 nmol kg−1 in the summer epilimnion, which would be a good measure of the P present in suspended particles produced by phytoplankton.
Although both arsenite and organometallic As species, such as dimethylarsinate, occur in the stratified epilimnion, arsenate is the major species of As in Lake Biwa (Sohrin et al. 1997). Arsenate has a similar molecular structure to phosphate and forms an unstable arsenate ester with ADP instead of the phosphate ester of ATP, leading to the breakdown of energy metabolism (Andreae 1986). Figure 5c illustrates the linear relationship between D-As and D-P:
This linearity suggests that a proportion of the As content circulates with P within the lake. However, the high intercept suggests that some As also remains in the lake water. Such behavior seems consistent with biochemical mechanisms that address the problem of arsenic toxicity by both depleting As relative to P during uptake and transforming As to harmless organometallic compounds (Andreae 1986). Additionally, the D-As concentration became significantly higher than the regression line in the bottom water in 2009. This is attributed to the fact that As can have a higher flux from sediments than P under suboxic conditions. The D-As/D-P ratio of around 0.05 observed in the bottom water is close to the As/P ratio of ferromanganese concretions collected from the bottom of the north basin (Takamatsu et al. 1993).
V, Mo, W, and U
The LP/TD ratios for V, Mo, W, and U were in the range 0–0.3 (Fig. 4). The dissolved concentrations of V, W, and U increased in the epilimnion during stratification (Fig. 3 and Fig. S3 in the ESM), whereas in suboxic bottom water, TD-V and TD-W substantially decreased while TD-Mo increased (Fig. S2 in the ESM). The respective valence states are +5 for V and +6 for Mo, W, and U, and these elements form oxoacid species in oxic lakes and sea water. However, the vertical profiles observed in Lake Biwa are generally different from the uniform and conservative profiles of these elements in the ocean.
The increases in D-V and D-W in the stratified epilimnion have been observed in previous studies (Harita et al. 2005; Sugiyama 1989). These authors have proposed that the increases are due to the release of the respective elements from sediments and suspended particles following an increase in pH. Figure 5d illustrates that D-U increases when the pH increases from 6.5 to 8.0. Similar relationships were observed for D-V versus pH and D-W versus pH. Thus, this pH-dependent release seems to be an important mechanism for the increases in the concentrations of these metals. However, the dissolved concentrations are not a simple function of pH, and the maximum concentrations varied depending on the sampling date. Another influential factor may therefore be a variable weathering flux from rivers, as supported by the fact that D-V, D-W, and D-U show significant correlations with D-Al (Table S3 in the ESM; p < 0.001). The estimated weathering supply of D-M will be low for V, W, and U, assuming that it is proportional to the crustal M/Al ratios (Table S5 in the ESM). However, since the oxoacid species are highly soluble, it is likely that the D-M/D-Al ratio increases significantly during weathering, which will have a considerable impact on the distribution of V, W, and U in Lake Biwa. Based on river water concentrations, the flux ratios of D-M/D-Al from the rivers feeding Lake Biwa were found to be 2 × 10−2 for V and 3 × 10−4 for W and U (Mito et al. 2004). This enhancing effect is likely to be negligible for Mo, since the estimated weathering supply of around 5 × 10−4 nmol kg−1 is far lower than the D-Mo concentration of around 4 nmol kg−1 in Lake Biwa. It is also considered that uptake by phytoplankton will have a negligible effect on the distributions of these elements (Table S5 in the ESM).
In suboxic bottom water, a decrease in concentration was observed for TD-V, D-V, TD-W, D-W, D-Mo, and D-U, while an increase was observed for TD-Mo, LP-Mo, and LP-U. Figure 5e illustrates the relationship between D-U and LP-Mn, in which the D-U concentration varies greatly when LP-Mn is less than 200 nmol kg−1, and the D-U concentration becomes constant when LP-Mn is higher than 1000 nmol kg−1. Similar relationships were observed between the LP-Mn concentration and those of D-V and D-W. These results suggest that the concentrations of D-V, D-W, and D-U are controlled by adsorption on manganese oxides when the concentration of LP-Mn is high. These elements seem to be strongly influenced by the manganese oxide content, because they usually have a low LP/TD ratio (Fig. 3), and thus were probably scavenged from suboxic bottom water by manganese oxide particles. Beneath the Mn redox boundary in sediments, the manganese oxides were reduced and dissolved, resulting in the release of the adsorbed elements. V and W were then adsorbed onto hydrous ferric oxides in the sediments, leading to the depletion of TD-V and TD-W in bottom water (Fig. 6a). Due to the fact that the adsorption of U onto hydrous ferric oxides is weaker than that of V and W at a neutral pH (Fujinaga et al. 2005), a portion of the U returned to the bottom water, resulting in slightly higher values (Fig. 6b). In the case of Mo, D-Mo showed a small decrease, while LP-Mo showed a large increase in bottom water. A linear relationship was observed between LP-Mo and LP-Mn (Fig. 5f; p < 0.001). Mo is adsorbed onto both manganese and iron oxides, but this adsorption is weaker than for the other elements (Fujinaga et al. 2005). As a result, Mo was not as effectively scavenged by manganese oxides and was also not trapped by iron oxides at the sediment surface, resulting in the bottom water maximum of LP-Mo.
Schematic models of the reactions of trace elements in bottom water and sediments. The yellow layer represents the oxidized surface layer of sediments. a A trace element X, such as V and W, is effectively adsorbed onto ferric hydroxides, resulting in negligible release of dissolved species from sediments. b Another trace element X, such as Mo and U, is not effectively adsorbed onto ferric hydroxides, resulting in substantial release of dissolved species from sediments
Conclusions
Seasonal changes in trace elements (Al, Si, P, V, Cr, Mn, Fe, Ni, Cu, Zn, As, Mo, W, and U) in the waters of Lake Biwa in Japan were studied in the context of total dissolvable, dissolved, and labile particulate concentrations in 2007 and 2009. Weathering supply was considered the major factor controlling the distributions of Al and Fe. Biological uptake had a significant impact on the distributions of Si, P, Fe, and As. Sporadic surface maxima occurred for Cr, Ni, Cu, and Zn. High M/Al ratios for the labile particulate species suggested an abundance of Mn and Fe oxide particles, resulting in unique speciations and distributions of each trace element. V, W, and U increased in the summer epilimnion, probably due to an increase in desorption from Mn–Fe oxides with the increase in pH and because of the increase in weathering supply in the summer. During stratification, LP-Mn showed large increases in suboxic bottom water, suggesting that Mn2+ was supplied from sediments and was rapidly oxidized in the bottom water. Although DO decreased to less than 1 mg kg−1, Fe did not increase in bottom water, suggesting that iron reduction has not yet occurred at the sediment surface. Under these conditions, each trace element showed characteristic behaviors, such as the decreases in TD-V and TD-W and the increase of TD-Mo in the bottom water.
References
Andreae MO (1986) Organoarsenic compounds in the environment. In: Craig PJ (ed) Organometallic compounds in the environment. Wiley, New York, pp 198–228
Ezoe M, Ishita T, Kinugasa M, Lai X, Norisuye K, Sohrin Y (2004) Distributions of dissolved and acid-dissolvable bioactive trace metals in the North Pacific. Geochem J 38(6):535–550. doi:10.2343/geochemj.38.535
Fujinaga T, Sohrin Y, Isshiki K (eds) (2005) Chemistry of the oceans and lakes: studies using trace elements (in Japanese with extensive English and Japanese references). Kyoto University Press, Kyoto
Haraguchi H, Itoh A, Kimata C, Miwa H (1998) Speciation of yttrium and lanthanides in natural water by inductively coupled plasma mass spectrometry after preconcentration by ultrafiltration and with a chelating resin. Analyst 123(5):773–778. doi:10.1039/A708253C
Harita Y, Hori T, Sugiyama M (2005) Release of trace oxyanions from littoral sediments and suspended particles induced by pH increase in the epilimnion of lakes. Limnol Oceanogr 50(2):636–645. doi:10.4319/lo.2005.50.2.0636
Hasegawa H (1997) The behavior of trivalent and pentavalent methylarsenicals in Lake Biwa. Appl Organomet Chem 11(4):305–311. doi:10.1002/(SICI)1099-0739(199704)11:4<305::AID-AOC586>3.0.CO;2-6
Hasegawa H, Matsui M, Okamura S, Hojo M, Iwasaki N, Sohrin Y (1999) Arsenic speciation including ‘hidden’ arsenic in natural waters. Appl Organomet Chem 13(2):113–119. doi:10.1002/(SICI)1099-0739(199902)13:2<113::AID-AOC837>3.0.CO;2-A
Hasegawa H, Sohrin Y, Seki K, Sato M, Norisuye K, Naito K, Matsui M (2001) Biosynthesis and release of methylarsenic compounds during the growth of freshwater algae. Chemosphere 43(3):265–272. doi:10.1016/s0045-6535(00)00137-5
Hirata SH, Hayase D, Eguchi A, Itai T, Nomiyama K, Isobe T, Agusa T, Ishikawa T, Kumagai M, Tanabe S (2011) Arsenic and Mn levels in Isaza (Gymnogobius isaza) during the mass mortality event in Lake Biwa. Japan. Environ Pollut 159(10):2789–2796. doi:10.1016/j.envpol.2011.05.007
Itai T, Hayase D, Hyobu Y, Hirata SH, Kumagai M, Tanabe S (2012) Hypoxia-induced exposure of isaza fish to manganese and arsenic at the bottom of Lake Biwa, Japan: experimental and geochemical verification. Environ Sci Technol 46(11):5789–5797. doi:10.1021/es300376y
Itoh A, Haraguchi H (1997) Dissolved sates of trace metal ions in natural water as elucidated by ultrafiltration/size exclusion chromatography/ICP-MS. Anal Sci 13(Suppl.):393–396. doi:10.2116/analsci.13.Supplement_393
Jiao C, Aoki S, Okumura Y, Minami M, Yada M, Ishikawa K, Nakajima T, Ishikawa T, Tsujimura S (2011) Analytical monitoring of decreasing oxygen and its effects on the north basin ecosystem in Lake Biwa (in Japanese). Res Rep Lake Biwa Env Res Inst 7:150–181
Kawashima M, Takamatsu T, Koyama M (1988) Mechanisms of precipitation of manganese(II) in Lake Biwa, a fresh water lake. Water Res 22(5):613–618. doi:10.1016/0043-1354(88)90062-0
Kim C, Nishimura Y, Nagata T (2006) Role of dissolved organic matter in hypolimnetic mineralization of carbon and nitrogen in a large, monomictic lake. Limnol Oceanogr 51(1):70–78. doi:10.4319/lo.2006.51.1.0070
Kojima S, Saito T, Takada J, Furukawa M, Oda H, Nakamura T, Yokota K (2003) Neutron activation analysis of trace elements at sediment–water interface in the Biwa Lake, Japan. J Radioanal Nucl Chem 255(1):119–123. doi:10.1023/A:1022292100994
Koshikawa MK, Sugiyama M, Hori T (2002) Seasonal variation of dissolved aluminum concentration in harmonic-type Lake Biwa, Japan. Limnology 3(1):1–9. doi:10.1007/s102010200000
Lantzy RJ, Mackenzie FT (1979) Atmospheric trace metals: global cycles and assessment of man’s impact. Geochim Cosmochim Acta 43(4):511–525. doi:10.1016/0016-7037(79)90162-5
Li Y-H (2000) A compendium of geochemistry. Princeton University Press, Princeton
Li Y-H, Takamatsu T, Sohrin Y (2007) Geochemistry of Lake Biwa sediments revisited. Limnology 8(3):321–330. doi:10.1007/s10201-007-0223-3
Li YH, Sohrin Y, Takamatsu T (2011) Lake Biwa and the ocean: geochemical similarity and difference. Limnology 12(1):89–101. doi:10.1007/s10201-010-0326-0
Maruo M, Toda M, Satori T, Obata H (2007) Distribution and speciation of Fe(II) in the waters of Lake Biwa and inflowing rivers (in Japanese with English abstract). Jpn J Limnol 68(3):415–423. doi:10.3739/rikusui.68.415
Maruo M, Ishimaru M, Azumi Y, Kawasumi Y, Nagafuchi O, Obata H (2016) Comparison of soluble reactive phosphorus and orthophosphate concentrations in river waters. Limnology 17:7–12. doi:10.1007/s10201-015-0463-6
Meyers PA, Takemura K, Horie S (1993) Reinterpretation of late Quaternary sediment chronology of Lake Biwa, Japan, from correlation with marine glacial-interglacial cycles. Quat Res 39(2):154–162. doi:10.1006/qres.1993.1019
Mito S, Kawashima M, Sohrin Y (2002) Characterization of suspended solids in Lake Biwa by measuring their elemental composition of Al, Si, P, S, K, Ca, Ti, Mn, and Fe. Limnology 3(1):11–19. doi:10.1007/s102010200001
Mito S, Sohrin Y, Norisuye K, Matsui M, Hasegawa H, Maruo M, Tsuchiya M, Kawashima M (2004) The budget of dissolved trace metals in Lake Biwa, Japan. Limnology 5(1):7–16. doi:10.1007/s10201-003-0111-4
Miyajima T (1994) Mud-water fluxes of inorganic nitrogen and manganese in the pelagic region of Lake Biwa: seasonal dynamics and impact on the hypolimnetic metabolism. Arch Hydrobiol 130(3):303–324
Nagai M, Sugiyama M, Hori T (2001) Environmental chemistry of rivers and lakes, part VII. Fractionation by calculation of suspended particulate matter in Lake Biwa into three types of particles of different origins. Limnology 2(3):147–155. doi:10.1007/s10201-001-8030-8
Nakanishi M, Sekino T (1996) Recent drastic changes in Lake Biwa bio-communities, with special attention to exploitation of the littoral zone. GeoJournal 40(1–2):63–67
Rudnick RL, Gao S (2005) Composition of the continental crust. In: Rudnick RL (ed) The crust. Treatise on geochemistry, vol 3. Elsevier–Pergamon, Oxford, pp 1–64
Sohrin Y, Matsui M, Kawashima M, Hojo M, Hasegawa H (1997) Arsenic biogeochemistry affected by eutrophication in Lake Biwa, Japan. Environ Sci Technol 31(10):2712–2720. doi:10.1021/es960846w
Sohrin Y, Nakashima Y, Maruo M (2016) Trace elements influenced by environmental changes in Lake Biwa: (II) Chemical variations in the hypolimnion over the last half-century. Limnology (in press)
Sugiyama M (1989) Seasonal variation of vanadium concentration in Lake Biwa, Japan. Geochem J 23:111–116. doi:10.2343/geochemj.23.111
Sugiyama M, Fujino O, Kihara S, Matsui M (1986) Preconcentration by dithiocarbamate extraction for determination of trace elements in natural waters by inductively-coupled plasma atomic emission spectrometry. Anal Chim Acta 181:159–168. doi:10.1016/S0003-2670(00)85230-7
Sugiyama M, Hori T, Kihara S, Matsui M (1992) A geochemical study on the specific distribution of barium in Lake Biwa, Japan. Geochim Cosmochim Acta 56(2):597–605. doi:10.1016/0016-7037(92)90084-V
Sugiyama M, Hori T, Kihara S, Matsui M (2005) Geochemical behavior of trace elements in Lake Biwa. Limnology 6(2):117–130. doi:10.1007/s10201-005-0144-y
Takamatsu T, Kawashima M, Matsushita R, Koyama M (1985) General distribution profiles of thirty-six elements in sediments and manganese concretions of Lake Biwa. Jpn J Limnol 46(2):115–127. doi:10.3739/rikusui.46.115
Takamatsu T, Kawashima M, Takada J, Matsushita R (1993) Characteristics in elemental composition of ferromanganese concretions from Lake Biwa. Jpn J Limnol 54(4):281–291. doi:10.3739/rikusui.54.281
Turner DR, Whitfield M, Dickson AG (1981) The equilibrium speciation of dissolved components in freshwater and sea water at 25° C and 1 atm pressure. Geochim Cosmochim Acta 45(6):855–881. doi:10.1016/0016-7037(81)90115-0
Wu F, Tanoue E (2001) Molecular mass distribution and fluorescence characteristics of dissolved organic ligands for copper(II) in Lake Biwa, Japan. Org Geochem 32(1):11–20. doi:10.1016/S0146-6380(00)00155-8
Wu F, Midorikawa T, Tanoue E (2001) Fluorescence properties of organic ligands for copper(II) in Lake Biwa and its rivers. Geochem J 35(5):333–346. doi:10.2343/geochemj.35.333
Zhu Y, Hattori R, Rahmi D, Okuda S, Itoh A, Fujimori E, Umemura T, Haraguchi H (2005) Fractional distributions of trace metals in surface water of Lake Biwa as studied by ultrafiltration and ICP-MS. Bull Chem Soc Jpn 78(11):1970–1976. doi:10.1246/bcsj.78.1970
Acknowledgments
This study was partly supported by a fund for the mobile site type research from the Institute of Sustainability Science, Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Ken'ichi Osaka.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakashima, Y., Shimizu, A., Maruo, M. et al. Trace elements influenced by environmental changes in Lake Biwa: (I) Seasonal variations under suboxic hypolimnion conditions during 2007 and 2009. Limnology 17, 151–162 (2016). https://doi.org/10.1007/s10201-015-0474-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-015-0474-3