Abstract
Background
Evidence on the cost effectiveness of deprescribing in multimorbidity is limited.
Objective
To investigate the cost effectiveness of a general practitioner (GP) delivered, individualised medication review to reduce polypharmacy and potentially inappropriate prescribing in older patients with multimorbidity in Irish primary care.
Methods
Within trial economic evaluation, from a healthcare perspective and based on a cluster randomised controlled trial with a 6 month follow up and 403 patients (208 Intervention and 195 Control) recruited between April 2017 and December 2019. Intervention GPs used the SPPiRE website which contained educational materials and a template to support a web-based individualised medication review. Control GPs delivered usual care. Incremental costs, quality adjusted life years (QALYs) generated using the EQ-5D-5L instrument, and expected cost effectiveness were estimated using multilevel modelling and multiple imputation techniques. Uncertainty was explored using parametric, deterministic and probabilistic methods.
Results
On average, the SPPiRE intervention was dominant over usual care, with non-statistically significant mean cost savings of €410 (95% confidence interval (CI): − 2211, 1409) and mean health gains of 0.014 QALYs (95% CI − 0.011, 0.039). At cost effectiveness threshold values of €20,000 and €45,000 per QALY, the probability of SPPiRE being cost effective was 0.993 and 0.988. Results were sensitive to missing data and data collection period.
Conclusions
The study observed a pattern towards dominance for the SPPiRE intervention, with high expected cost effectiveness. Notably, observed differences in costs and outcomes were consistent with chance, and missing data and related uncertainty was non trivial. The cost effectiveness evidence may be considered promising but equivocal.
Trial registration
ISRCTN: 12752680, 20th October 2016.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multimorbidity is associated with adverse outcomes including increased mortality and reduced quality of life, and increased healthcare utilisation and costs [1,2,3]. Unplanned hospital admissions are a key driver of excess healthcare costs related to multimorbidity [4], and these in turn, are frequently the result of adverse drug reactions [5]. Indeed, prescribing for patients with multimorbidity is particularly complex due to related polypharmacy, which is associated with potentially inappropriate prescribing and adverse drug related events [6,7,8]. This polypharmacy is often necessary and appropriate in the context of managing multiple chronic conditions and complex needs. However, higher levels of polypharmacy in multimorbidity have been shown to be associated with higher levels of adverse outcomes, hospital admissions, and related healthcare costs [4, 5, 9]. In this context, healthcare providers caring for patients with multimorbidity are increasingly engaged in medications management and deprescribing practices, which involve the ongoing assessment of both the effectiveness and risks of treatments, and incorporation of their patients’ preferences [10]. To this end, an individualised approach to deprescribing in multimorbidity has been proposed in published multimorbidity and polypharmacy guidelines [11,12,13,14], which highlight that a single disease focus may not be optimal for patients with multimorbidity [15].
Evidence from randomised controlled trials is limited generally on the clinical and cost effectiveness of interventions targeting patients with multimorbidity [16], and interventions targeting patients with polypharmacy [17], with respective 2021 and 2018 Cochrane reviews reporting sparse and mixed results for health benefits and value for money. Evidence of cost effectiveness of medications management and deprescribing interventions for multimorbid patients is even more limited, and reviews have highlighted the need for further research in this area [16,17,18,19]. In the Irish context, the Supporting Prescribing in Older Adults with Multimorbidity in Irish Primary Care (SPPiRE) study reported the clinical effectiveness of a general practitioner (GP) delivered, individualised medication review intervention, that was developed incorporating the concepts of treatment burden and deprescribing and which focused on higher levels of polypharmacy for patients with multimorbidity [20]. The intervention resulted in a small but statistically significant effect in reducing the number of medicines (IRR 0.95, 95% CI 0.899–0.999, p = 0.045) and a weakly significant effect on potentially inappropriate prescriptions (PIP) (OR 0.39, 95% CI 0.140–1.064, p = 0.066). In addition to clinical effectiveness, any decision regarding the adoption of an intervention in clinical practice will depend upon its expected cost effectiveness [21]. The technique of health economic evaluation is concerned with the exploration of cost effectiveness by relating the mean difference in cost between alternative treatment options to their mean difference in effectiveness, and by quantifying the related uncertainty [21]. This paper reports the cost effectiveness results from the health economic evaluation conducted alongside the SPPiRE cluster RCT to assess an intervention targeting reductions in polypharmacy and potentially inappropriate prescribing among older patients with multimorbidity in Irish primary care.
This study adds to the limited evidence base on the cost effectiveness of interventions targeting medications management and desprescribing in patients with multimorbidity. Laberge et al. [18] conducted a systematic review of the economic impact of interventions intended at optimizing medication use in older adults with multimorbidity and polypharmacy. The review included 11 studies and reported that interventions to optimize medication use may provide benefits that outweigh their implementation costs, but the evidence remains limited [18]. In terms of the related and relevant cost effectiveness studies, two recent papers based on randomised controlled trials examined the cost effectiveness of interventions targeting medications management and desprescribing in patients with multimorbidity, one in primary care [22] and one in hospital care [23]. Thorn et al. [22] conducted a health economic evaluation of the 3D randomised control trial study, and found that the evidence for the cost effectiveness of the 3D intervention was equivocal; the results suggesting that there was just over a 50% chance of cost effectiveness at the established UK threshold of £20,000 per QALY from the healthcare perspective [22]. More recently, Salari et al. [23] reported the cost effectiveness findings alongside the OPERAM cluster-randomized trial aimed at testing the effect of a structured pharmacotherapy optimization intervention on preventable drug-related hospital admissions in adults with multimorbidity and polypharmacy aged 70 years or older. The authors reported that the results were not definitive, but were indicative of a pattern towards dominance, potentially resulting from an accumulation of multiple, small, positive intervention effects [23]. This study also adds to the evidence base for the cost effectiveness of interventions targeting multimorbidity and polypharmacy more generally [16,17,18,19]. While comparison of international studies is complicated by the variety of definitions used for multimorbidity, and by the heterogeneity in study designs, further studies are required to explore the health and economic implications of interventions targeting the multimorbid patient population.
Methods
Overview
The economic evaluation was conducted insofar as possible, in accordance to the guidelines for the conduct of health economic evaluation in Ireland [24], and the findings are reported in line with the best-practice CHEERS checklist [25]. The perspective of the healthcare system (that is, the Irish health service executive (HSE)) was adopted with respect to costing and health outcomes were expressed in terms of quality adjusted life years (QALYs) gained. The mode of analysis consisted of a trial-based evaluation with a time horizon of 6 months, the trial follow-up period. Given the length of follow up, neither costs nor outcomes were discounted. Data on resource use were collected directly from general practice records, while health outcome data was collected via patient questionnaires at baseline and follow up. The statistical analysis was conducted on an intention to treat basis, and in accordance with guidelines for cluster RCTs [26,27,28,29,30]. Results are presented from complete case and multiple imputation [31] analyses, which was conducted following guidance for hierarchical datasets [30]. Uncertainty was addressed using statistical inference methods, in the form of 95% confidence intervals and hypothesis tests, probabilistic sensitivity analysis, reported in the form of estimated probabilities of the intervention being cost effective at a range of potential threshold values (λ) that the health system may be willing to pay per additional QALY gained [21], and deterministic sensitivity analysis. All analyses were undertaken using Stata 15 and Microsoft Excel statistical software packages.
Randomised controlled trial (RCT)
The methods for the SPPiRE RCT (ISRCTN: 12752680) have been described elsewhere [32]. In brief, SPPiRE was a pragmatic two arm cluster RCT, which was conducted in line with the CONSORT guidelines for cluster RCTs [26]. Ethical approval was granted by the Irish College of General Practitioners Research Ethics Committee. Information about the trial was publicised through a variety of research, teaching and training networks throughout Ireland. Eligible practices that expressed an interest in taking part were formally invited. Practices were eligible to participate if they had at least 300 patients aged ≥ 65 years on their patient panel and they used either one of the two Irish practice management systems in use in over 80% of practices nationally. Practices were excluded if they were currently involved in a medication management or prescribing trial or if they were unable to recruit at least five participants. Eligible patients were aged ≥ 65 years and prescribed ≥ 15 repeat medicines. Patients were excluded if they had been recruited into a practice that was unable to recruit at least four other participants, they were unable to give informed consent, as judged by their GP, or they were unable to attend the practice for a face to face medication review. Recruited GPs ran a patient finder tool embedded in their practice management systems and screened the generated list to ensure only eligible patients were invited. All recruited practice and patients gave full informed consent and baseline data was collected prior to treatment arm allocation.
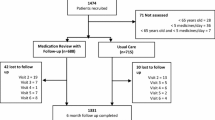
Between April 2017 and December 2019, 139 practices and 1626 patients were invited to take part. A total of 51 practices were recruited giving an overall practice enrolment rate of 36.7%.
Of the patients invited, 403 were recruited into the trial giving an enrolment rate of 24.8%. Recruited practices were randomly allocated using minimisation to the SPPiRE intervention (n = 26) or the usual care control (n = 25) by the independent trial statistician, resulting in 208 patients being randomised to the SPPiRE intervention arm and 195 to the usual care arm. Considering the nature of the intervention, it was not possible to blind GPs or patients to the intervention. Descriptive statistics for the baseline characteristics of the practices and patients for each treatment arm are presented in Table 1. Recruited patients had a mean age of 76.5 years (SD 6.83), a mean number of medicines of 17.37 (SD 3.50), and a mean number of PIPs per person of 2.52 (SD 1.48), identified from a list of 34 pre-specified indicators (see Appendix Table 5). Practices and patients in each group had similar characteristics at baseline.
The SPPiRE intervention, in terms of its implementation strategies and intervention components, are described in detail elsewhere [20] and in the accompanying appendix. In brief, intervention GPs received unique login details to the SPPiRE website where they had access to five training videos and a template for performing the SPPiRE medication review (Appendix Fig. 1). The training videos provided background information on multimorbidity and polypharmacy, PIP, eliciting patient treatment priorities and conducting a brown bag medication review, in which the GP and patient jointly reviewed each medication. In terms of the key intervention components, GPs were instructed to book a double appointment and to ask their patients to bring all their medicines in to the medication review visit with them. The SPPiRE medication review process had two elements; gather and record information and then to discuss and agree changes with their patient based on the recorded information, with a focus on deprescribing medicines that were inappropriate. GPs initially screened the prescription for PIP and then discussed patient treatment priorities and performed a brown bag review where each medicine was discussed in turn with the patient, and issues such as effectiveness, adverse effects and actual drug utilisation were discussed. The website provided suggested treatment alternatives for identified PIP but all treatment decisions were ultimately at the discretion of the individual GP, based on their clinical judgement and their patients’ individual priorities.
Control GPs delivered usual care in Irish general practice. At the time of intervention delivery there was no structured chronic disease management programme in Irish primary care and many patients with multimorbidity attended multiple hospital specialists. All people aged ≥ 70 years of age have access to a medical card which grants free primary care, whereas access in the 65–69 year old age category is means tested on the basis of income. Access to specialists and diagnostics in secondary care is free for the entire population, but shorter waiting lists exist in the private care pathway and for those with private health insurance.
The two primary outcomes in the clinical effectiveness analysis were the number of repeat medicines and the proportion of patients with any PIPs. Data on demographics, socioeconomics, medical history, healthcare utilisation and health outcomes were collected at baseline and at 6 months after intervention delivery. Data for prescribed medicines and healthcare utilisation were collected by participating GPs and submitted to the study manager. Data for patient reported health outcome measures were collected by postal questionnaires. Overall, 35 patients (8.66%) were lost to follow-up, 21 of whom died (12 in intervention and 9 in control) during the study period (Appendix Table 6). In addition, 174 or 43% of participants (90 in intervention and 84 in control) did not complete the patient questionnaire at follow up, and had no available data for patient reported health outcomes. There did not appear to be systematic differences between intervention and control in respect of participants lost to follow up or missing data and as a result, such data was assumed to be missing at random (Appendix Table 7). Finally, the study was ongoing during the onset of the COVID-19 pandemic, and follow up of 106 participants (57 in intervention and 49 in control) was after March 2020 and in the midst of the pandemic.
Cost analysis
Three cost components were included in the analysis, all of which were expressed in Euros (€) in 2022 prices (see Appendix Table 8). Unit cost estimates for each activity were based on national data sources and, where necessary, were transformed to Euros (€) in 2022 prices using appropriate indices [33].
The first cost component related to the resources required to implement and operate the SPPiRE intervention in clinical practice. This included a number of fixed, once-off resource outlays including the establishment of the SPPiRE website, and the related educator and administrator time input. In addition, it included a range of variable operation items relating to healthcare professional time input, educational materials and consumables, post, packaging, telephone and travel expenses. This data was recorded prospectively by the study research team. This cost was allocated to all patients in the intervention arm. The impact of halving and doubling the intervention cost estimate were tested in sensitivity analysis.
Second, the costs of medication prescriptions over the trial follow up period were estimated for both treatment arms. A marginal analysis approach was adopted given the volume of prescriptions (approximately 13,800 in total) involved, in that only the costs of medications that were stopped (that is, present at baseline but not follow up: n = 1398), and the costs of medications started (that is, present at follow up but not baseline: n = 1153), were estimated. This process involved applying medication unit cost data to medication utilisation data, and directly informed by recorded information on the prescribed medication name, strength, dosage and quantity. Data on medication utilisation were captured directly from general practice records and categorised by the study team using the World Health Organisation (WHO) Anatomical Therapeutic Chemical (ATC) classification system. Medications covered by Ireland's state drug schemes, unlicensed prescription-only medicines, and non-prescription medicines which are covered or are therapeutic alternatives to covered medications, were included in the analysis. Other non-prescription medicines, non-drug products, and high-tech drugs were excluded. High-tech drugs are prescribed by hospital consultants and their recording in GP records are inconsistent. Unit costs for each medication (based on the specified brand or the most common brand of the medication in Ireland) were obtained from the Irish Pharmacy Union Product File. Unit cost data were applied manually to the medication utilisation data by one member of the study team and checked by a second member. As per guidance from Irish National Centre for Pharmacoeconomics a pharmacy dispensing fee was added to each medication ingredient cost [34]. For the purposes of the incremental cost analysis, a follow up period of 6 months was assumed for the medication costs, but in a manner that reflected the recorded medication dispensing interval. Alternative follow up period assumptions were tested in sensitivity analysis.
Third, costs relating to the use of primary and secondary healthcare services over the course of the trial follow up period were estimated for both treatment arms. This included the costs of GP consultations, outpatient clinic visits, accident and emergency department visits, and hospital inpatient admissions Resource use was captured directly from general practice records at baseline and follow up and for a period of 6 months. A vector of unit costs was applied to calculate the cost associated with each resource activity. In sensitivity analysis, the impact of inflating and deflating the unit costs by an arbitrary figure of 15% for the primary and secondary care services was examined.
For the purposes of the incremental analysis, a ‘total healthcare cost at 6 months follow up’ variable was constructed by aggregating individual resource costs across the follow up period. This comprised of the sum of the costs of the intervention, medications stopped, medications started, and primary and secondary care. A number of alterative total healthcare cost estimates, based on variations in the estimation approach for the intervention and medication cost inputs as detailed above, were tested in sensitivity analysis.
For the complete case analysis, estimation of incremental cost was undertaken using a generalized estimating equations (GEE) [35] regression model, controlling for treatment arm, baseline total cost and clustering. To account for the hierarchical and distributional nature of the cost data, an exchangeable correlation structure, a Gamma variance function and identity link function, was assumed [36, 37]. In addition to the complete case analysis, a multiple imputation analysis was undertaken using the MI package in Stata 15 to generate missing values for individual resource use costs at each time point, which were then summed to generate the imputed total healthcare cost variable. The imputation models employed predictive mean matching drawing from KNN = 5 closest observations, and were estimated using available data on age, gender, treatment arm, number of baseline medications, private health insurance status, medical card status, and general practice setting [30]. For the multiple imputation analysis, estimation was based on M = 10 completed datasets and Rubin’s rules [31] were employed to combine values and produce the coefficients of interest. Alternative multiple imputation assumptions were tested in sensitivity analysis. The analysis was undertaken using the XTGEE, MI estimate and MI predict commands in Stata 15. The mean cost estimates of interest were obtained from the linear predictions from the regression analysis, using the method of recycled predictions [21].
Effectiveness analysis
Health outcomes were expressed in terms of QALYs gained at 6 months, calculated based on participant responses to the EuroQol [36] EQ-5D-5L instrument, collected via questionnaire at baseline and follow up. The EQ-5D-5L consists of five dimensions: mobility, self-care, usual activities, pain or discomfort and anxiety or depression; and each dimension has five levels of severity: no problems, slight problems, moderate problems, severe problems, or unable/extreme problems. In completing the EQ-5D-5L, an individual is located in one of 3125 health state, each of which may be transformed into a health state index score or ‘utility’ using values elicited from the relevant general population. The index score ranges from 1, which is equivalent to perfect health, to 0, which is equivalent to death, and below 0, with negative scores for those health states that are valued as worse than death. The EQ-5D-5L value set scoring algorithm for Ireland, which was generated using a hybrid time trade-off and discrete choice experiment approach, produces health utility index scores ranging from − 0.974 to 1 [39]. For economic evaluation, QALYs gained over a period of time are calculated using the area under the curve method, which involves weighting the time spent in EQ-5D-5L health states by their relevant index scores [40]. For the purposes of the incremental analysis, a ‘QALYs gained at 6 months variable’ was constructed using the EQ-5D-5L index scores at baseline and 6 months follow up. The statistical and multiple imputation analysis techniques adopted were similar to those for the cost analysis described above; adopting a GEE regression model estimated controlling for treatment arm, baseline EQ-5D-5L score and clustering, assuming an exchangeable correlation structure, a Gaussian variance function and an identity link function [36].
Cost effectiveness analysis
The net benefit framework, which allows for costs and effects, and their correlation, to be combined into a single variable, enables the identification of the cost effectiveness of a treatment relative to a comparator [21, 41]. In this case, net benefit (nb) is defined as follows:
where eijk is the health outcome for the ith person in the jth cluster in treatment arm k, λ is the cost effectiveness threshold value, and cijk is the cost. Applying this framework, a treatment is defined to be cost effective, at a given threshold value, λ, if its corresponding net benefit is greater than that of its comparator: that is, if the incremental net benefit is greater than zero. Point estimates for the mean differences in cost and effectiveness between the alternatives must be estimated, and an explicit examination of the uncertainty surrounding these point estimates conducted. The probabilistic analysis of uncertainty incorporates both the sampling uncertainty around the mean cost effectiveness estimates and the uncertainty around the true cost effectiveness threshold value [42]. In the Irish context, cost effectiveness thresholds in the range of €20,000 to €45,000 per QALY are generally recommended for decision-making [24], although these are not universally employed. Further, when undertaking analysis using data from cluster RCTs, techniques which recognise the correlation and clustering in the cost and effect data are recommended [27,28,29].
Given the divergence in missing data patterns between cost and QALY variables, and the need to account for the correlation between both variables, the incremental cost effectiveness findings, both in the form of the net benefits point estimates and the expected cost effectiveness probabilities, are presented solely for the multiple imputation analysis. Net benefit statistics at thresholds of €20,000 and €45,000 were generated, and incremental net benefits were estimated using a GEE regression model, controlling for treatment arm and clustering, assuming an exchangeable correlation structure, a Gaussian variance function and an identity link function. The probabilistic analysis was conducted using a two-stage, non-parametric bootstrapping technique [43], which was based on 2000 bootstrap replications of the linear predictions for total healthcare cost and QALYs. The analysis was undertaken using the XTGEE, MI estimate, MI predict and TSB commands in Stata 15.
Deterministic sensitivity analysis
An extensive range of deterministic sensitivity analysis was conducted to test the robustness of the base-case results to variations in the methods and assumptions employed. The results are presented for comparison purposes in terms of incremental costs, QALYs and cost effectiveness probabilities at the €20,000 and €45,000 per QALY thresholds. First, alternative multiple imputation approaches, employing different variables and assumptions were employed. Second, alternative regression model specifications were tested. Third, a number of assumptions relating to the costing methods were varied. Fourth, an analysis was conducted which explicitly accounted for the impact of the COVID-19 pandemic on the study and for the 106 individuals (intervention = 57 and control = 49) whose data were followed up after March 2020. Fifth, alternative probabilistic methods for the generation of the cost effectiveness probabilities were tested.
Results
Descriptive statistics for health outcomes, resource use and costs at baseline and follow up are summarised in Table 2. The total cost of implementing the intervention was €53,492, resulting in a mean cost per participant estimate of €257. In terms of total healthcare cost over the 6 month follow up period, the mean cost per patient in the SPPiRE intervention arm was €3745 (standard deviation (SD): 6441) and €4115 (SD: 10,319) in the usual care control arm. In terms of health outcomes, mean QALYs gained per patient at 6 months was 0.261 (SD: 0.171) in the SPPiRE intervention arm and 0.235 (SD: 0.167) in the usual care arm. Descriptive statistics for EQ-5D-5L responses are presented in Appendix Table 9. Missing data for the intervention and control arms at baseline and follow up are presented alongside Table 2.
The results from the incremental cost, QALY and cost effectiveness analyses are presented in Table 3 for the complete case analysis and for the multiple imputation analysis. On average, the SPPiRE intervention was the dominant strategy over usual care. In the complete case analysis, the intervention was associated with a non-statistically significant mean cost saving of €615 (95% CI − 2566, 1336) and a non-statistically significant mean gain of 0.005 QALYs (95% CI − 0.011, 0.020) relative to the control. In the multiple imputation analysis, the intervention was associated with a mean cost saving of €401 (95% CI (− 2211, 1409) and a mean gain of 0.014 QALYs (95% CI − 0.011, 0.039). Univariate analysis comparing the means for individual resource costs and total costs, and EQ-5D-5L and QALYs gained scores are presented in Appendix Table 10. Moving to the incremental cost effectiveness results, the incremental net monetary benefit statistics at the €20,000 and €45,000 thresholds were at estimated €985 (95% CI 217.09, 1752.57) and €1647 (95% CI 184.59, 3108.62) respectively. In terms of expected cost effectiveness based on the available and imputed data, the probability that the SPPiRE intervention is cost effective was 0.993 and 0.988 at threshold values of €20,000 and €45,000 per QALY respectively.
The results from the sensitivity analysis are presented in Table 4 and in Appendix Tables 13 and 14. The results generally confirmed the robustness of the findings from the base case analyses. The results from the analysis for the post COVID-19 data collection cohort revealed stronger evidence in favour of the SPPiRE intervention. In the complete case analysis, the intervention was associated with a statistically significant cost saving of €6084 (95% confidence interval (CI): − 11268, − 901) and a non-statistically significant gain of 0.019 QALYs (95% CI: − 0.017; 0.055) per patient relative to the control (see Appendix Table 13). The cost savings were driven by statistically significant reductions in hospitalisation and emergency department costs in the intervention arm within this subgroup (see Appendix Table 14). Finally, employing the Monte Carlo simulation method for the probabilistic analysis resulted in lower probability estimates of 0.770 and 0.841 at threshold values of €20,000 and €45,000 respectively.
Discussion
This paper reports the findings from a within trial economic evaluation which observed a pattern towards dominance for the SPPiRE intervention over usual care for patients with multimorbidity in Irish general practice. This potentially resulted from an accumulation of multiple, small, positive, albeit statistically insignificant intervention impacts. That is, cost savings, arising from reduced hospital services utilisation which went to offset the intervention implementation costs, and health gains, contributed to the dominant cost effectiveness point estimates. Notably, uncertainty in the analysis, and particularly the issue relating to postal questionnaire non-response and resulting missing data at follow up, were non trivial factors, and should be carefully considered when interpreting our findings.
The incremental costs and QALYs estimates were not individually statistically significant in the complete case or multiple imputation analysis, and were therefore consistent with chance findings. That said, the SPPiRE RCT was not powered to specifically detect differences in costs or QALYs. Indeed, trial-based economic evaluation is often faced with inappropriate sample size constraints [44]; thereby raising the possibility that important differences between treatment arms cannot be detected at conventional levels of power and significance. To address this concern, it is recommended that the evidence should be presented in the form of expected cost effectiveness probabilities, rather than by relying solely on showing significance at conventional levels [44]. In this case, we report the estimated probabilities for the SPPiRE intervention and find them to be in the region of 90% across a range of potential cost effectiveness threshold values for Ireland, and this remained consistent in a series of sensitivity analysis.
Importantly however, given the extent of the missing data on health outcomes, the expected cost effectiveness results were based on data generated from the multiple imputation analysis. While missing data did not appear to be systematically different in nature across treatment arms, it was substantial in totality, with only 57% of postal questionnaire data available for analysis. Further, the observed pattern of results for the patient cohort with data collection occurring post the onset of COVID-19 poses additional questions that require further scrutiny and analysis. While this may be a chance finding, it raises legitimate concerns regarding the interpretation of our expected cost effectiveness results, and whether or not they should be used for healthcare resource allocation decisions.
Taken all of the above together, we conclude that the evidence for the cost effectiveness of the SPPiRE intervention should be considered promising but equivocal. That said, it is ultimately the remit of decision makers to determine whether the level of evidence presented is sufficient to justify the adoption of the SPPiRE intervention in clinical practice. These findings supplement those from the parallel clinical effectiveness study which found that the SPPiRE intervention resulted in a statistically significant, but small reduction in the number of medicines and in a weakly significant effect on PIP [20]. Our findings also reflect those from the existing evidence base for the cost effectiveness of interventions targeting multimorbidity and polypharmacy, and support calls for further studies to explore the health and economic implications of interventions targeting the multimorbid patient population [16,17,18,19].
Strengths and limitations
This study had a number of strengths and limitations. The trial recruited to target a vulnerable group of patients with substantial disease and treatment burden and a high baseline prevalence of potentially inappropriate prescribing, and the SPPiRE intervention was both safe and feasible [24]. There were a number of limitations relating to the conduct of the RCT, as outlined in the main trial publication, which also apply to the economic evaluation. For example, only a quarter of invited patients agreed to participate, although no other intervention study with this degree of polypharmacy as an inclusion criterion could be identified for comparison. Further, a chance imbalance in the number of days from baseline to follow-up between groups was identified. However, a sensitivity analysis including the number of days to follow-up as a covariate revealed that there was no significant effect on the results.
The sample size of the trial was based on the clinical primary endpoint and may have been insufficient to detect statistically significant changes in the health economic outcomes. Further, outcome measures were assessed at just one-time point, 6 months after intervention completion. There is a possibility that the full effect of the intervention may not have been captured by assessing outcomes at just one point in time. Significantly, only 57% of patients reported patient-reported outcome measures at follow-up, which the QALYs gained variable was based upon. Missing data was therefore an important and significant consideration and details on missing data at each data collection point are presented. After consideration of missing data patterns, we proceeded with the assumption that the data were missing at random and multiple imputation was undertaken to impute missing values using the MI command in Stata 15. The variables included in the imputation models were pragmatically chosen by the study team. This approach may be criticised on the basis that values for resource cost and EQ-5D-5L scores were imputed independently. Furthermore, general practice surgery was included as a fixed effect in the imputation model, reflecting recent guidance that the imputation model should be compatible with the analysis model: that is, both should reflect the multilevel nature of the data [30]. The approach of including the cluster variable as a fixed effect in the imputation model may be problematic in some cases [45]; however, we deemed it to be appropriate. Finally, given the concerns raised above, it may be argued that the multiple imputation methods adopted have produced artificially low estimates of uncertainty in this case.
In terms of the health economic evaluation, the time horizon of the economic evaluation was limited to the trial follow-up period of 6 months; thereby excluding costs and benefits that arise beyond 6 months and over the remainder of the patients’ lifetime. This is likely be particularly relevant in the context of chronic disease, for which short term interventions may have long term implications. However, the concept of modelling long term health outcomes and costs for multimorbidity is an important area of future study.
While the cost analysis was conducted from the health service perspective and included an extensive range of resource use activities, certain resource items were not captured. For example, other medications costs to those started and stopped, community care costs, private out-of-pocket patient costs such as private health insurance premiums, and broader costs to society such as productivity losses were not captured in the analysis. Nonetheless, there is little evidence to suggest that the inclusion of these resources categories would fundamentally change the results presented. The resource utilisation data was collected directly from practice records and was compiled to a high standard of completeness and precision. Given the volume of prescription data, we assumed that follow up period of 6 months for all medication costings. In addition, the calculation approach of the total health care cost variable is open to criticism and it resulted in 17 participants having negative costs, since the savings from stopped medications outweighed their other cost outlays. The sensitivity analysis indicated that these assumptions had no bearing on the overall results. The process of conducting cost analysis in Ireland is also compromised by the lack of nationally available unit cost data. In estimating unit costs for individual resource activities, we endeavoured at all times to be conservative in any assumptions adopted.
We employed appropriate methods for the statistical analysis of cost and effect data collected alongside cluster RCTs. To account for potential covariate imbalances between treatment arms at baseline [28], we estimated separate multilevel regression models for costs and QALYs, controlling for baseline costs and health outcome covariates. To jointly account for correlation and clustering, we adopted a two-stage non-parametric bootstrapping technique [43]. While the methods adopted were appropriate, arguments could be made for a number of alternative approaches. For comparative purposes, probabilistic results for the complete case analysis from the Monte Carlo simulation approach were presented, and generated lower probability estimates than the nonparametric and parametric methods. Notably, this method does not account for both clustering and correlation as per the recommendations, and may be less precise in quantifying uncertainty in cost effectiveness results [46].
In conclusion, due to ageing population dynamics, the provision of safe, effective, equitable and efficient healthcare services for those with complex multimorbidity will become an ever pressing challenge. Given the equivocal nature of the cost effectiveness results presented, implementation of the SPPiRE intervention cannot be recommended. Further research is needed to support health policy and healthcare decision makers to address the issue of excess polypharmacy in multimorbidity. Future studies should carefully consider the design of data collection methods for patient reported outcomes among patients with multimorbidity.
Data availability
Data will be made available on reasonable request.
References
Fortin, M., Lapointe, L., Hudon, C., Vanasse, A., Ntetu, A.L., Maltais, D.: Multimorbidity and quality of life in primary care: a systematic review. Health Qual. Life Outcomes 2, 51 (2004). https://doi.org/10.1186/1477-7525-2-51PMC526383
Mair, F.S., Gallacher, K.I.: Multimorbidity: what next? Br. J. Gen. Pract. 67(659), 248–249 (2017). https://doi.org/10.3399/bjgp17X690965
Nunes, B.P., Flores, T.R., Mielke, G.I., Thumé, E., Facchini, L.A.: Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch. Gerontol. Geriatr. 67, 130–138 (2016). https://doi.org/10.1016/j.archger.2016.07.008. (Epub 2016/08/09)
Payne, R.A., Abel, G.A., Guthrie, B., Mercer, S.W.: The effect of physical multimorbidity, mental health conditions and socioeconomic deprivation on unplanned admissions to hospital: a retrospective cohort study. CMAJ 185(5), E221–E228 (2013). https://doi.org/10.1503/cmaj.121349. (Epub 2013/02/21)
Kongkaew, C., Noyce, P.R., Ashcroft, D.M.: Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann. Pharmacother. 42(7), 1017–1025 (2008). https://doi.org/10.1345/aph.1L037. (Epub 2008/07/03)
Spinewine, A., Schmader, K.E., Barber, N., Hughes, C., Lapane, K.L., Swine, C., et al.: Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet 370(9582), 173–184 (2007). https://doi.org/10.1016/S0140-6736(07)61091-5. (Epub 2007/07/17)
Wallace, J., Paauw, D.S.: Appropriate prescribing and important drug interactions in older adults. Med. Clin. North Am. 99(2), 295–310 (2015). https://doi.org/10.1016/j.mcna.2014.11.005. (Epub 2015/02/24)
Guthrie, B., McCowan, C., Davey, P., Simpson, C.R., Dreischulte, T., Barnett, K.: High risk prescribing in primary care patients particularly vulnerable to adverse drug events: cross sectional population database analysis in Scottish general practice. BMJ 342, d3514 (2011). https://doi.org/10.1136/bmj.d3514. (Epub 2011/06/23)
Payne, R.A., Abel, G.A., Avery, A.J., Mercer, S.W., Roland, M.O.: Is polypharmacy always hazardous? A retrospectivecohort analysis using linked electronic health records from primary and secondary care. Br. J. Clin. Pharmacol. 77(6), 1073–1082 (2014). https://doi.org/10.1111/bcp.12292
Gnjidic, D., Le Couteur, D.G., Kouladjian, L., Hilmer, S.N.: Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin. Geriatr. Med. 28(2), 237–253 (2012). https://doi.org/10.1016/j.cger.2012.01.006
McCarthy, C., Sheeran-Purcell, P., Fitzgerald, L., Cafferty, O., Sheeran-Purcell, L.: Medication Review: A Guide for GPs: Quick Reference Guide. ICGP Quality in Practice Committee, Dublin (2020)
Mair, F.S., May, C.R.: Thinking about the burden of treatment. BMJ 349, g6680 (2014). https://doi.org/10.1136/bmj.g6680
Reeve, E., Gnjidic, D., Long, J., Hilmer, S.: A systematic review of the emerging definition of “deprescribing” with network analysis: implications for future research and clinical practice. Br. J. Clin. Pharmacol. (2015). https://doi.org/10.1111/bcp.12732
Skou, S.T., Mair, F.S., Fortin, M., Guthrie, B., Nunes, B.P., Miranda, J.J., Boyd, C.M., Pati, S., Mtenga, S., Smith, S.M.: Multimorbidity. Nat. Rev. Dis. Primers. 8(1), 48 (2022). https://doi.org/10.1038/s41572-022-00376-4
Clyne, B., Smith, S.M., Hughes, C.M., Boland, F., Bradley, M.C., Cooper, J.A., et al.: Effectiveness of a multifaceted intervention for potentially inappropriate prescribing in older patients in primary care: a cluster-randomized controlled trial (OPTI-SCRIPT Study). Ann. Fam. Med. 13(6), 545–553 (2015). https://doi.org/10.1370/afm.1838. (Epub 2015/11/11)
Smith, S.M., Wallace, E., Clyne, B., Boland, F., Fortin, M.: Interventions for improving outcomes in patients with multimorbidity in primary care and community setting: a systematic review. Syst. Rev. 10, 271 (2021). https://doi.org/10.1186/s13643-021-01817-z
Rankin, A., Cadogan, C.A., Patterson, S.M., Kerse, N., Cardwell, C.R., Bradley, M.C., et al.: Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst. Rev. (2018). https://doi.org/10.1002/14651858.CD008165.pub4
Laberge, M., Sirois, C., Lunghi, C., Gaudreault, M., Nakamura, Y., Bolduc, C., Laroche, M.L.: Economic evaluations of interventions to optimize medication use in older adults with polypharmacy and multimorbidity: a systematic review. Clin. Interv. Aging 5(16), 767–779 (2021). https://doi.org/10.2147/CIA.S304074
Romano, S., Figueira, D., Teixeira, I., Perelman, J.: Deprescribing interventions among community-dwelling older adults: a systematic review of economic evaluations. Pharmacoeconomics 40(3), 269–295 (2022). https://doi.org/10.1007/s40273-021-01120-8. (Epub 2021 Dec 16)
McCarthy, C., Clyne, B., Boland, F., Moriarty, F., Flood, M., Wallace, E., Smith, S.: GP-delivered medication review of polypharmacy, deprescribing, and patient priorities in older people with multimorbidity in Irish primary care (SPPiRE Study): a cluster randomised controlled trial. PLoS Med. 19(1), e1003862 (2022). https://doi.org/10.1371/journal.pmed.1003862
Drummond, M.F., Sculpher, M.J., Claxton, K., Stoddart, G.L., Torrance, G.W.: Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press (2015)
Thorn, J., Man, M.S., Chaplin, K., Bower, P., Brookes, S., Gaunt, D., Fitzpatrick, B., Gardner, C., Guthrie, B., Hollinghurst, S., Lee, V., Mercer, S.W., Salisbury, C.: Cost-effectiveness of a patient-centred approach to managing multimorbidity in primary care: a pragmatic cluster randomised controlled trial. BMJ Open 10(1), e030110 (2020). https://doi.org/10.1136/bmjopen-2019-030110
Salari, P., O’Mahony, C., Henrard, S., Welsing, P., Bhadhuri, A., Schur, N., Roumet, M., Beglinger, S., Beck, T., Jungo, K.T., Byrne, S., Hossmann, S., Knol, W., O’Mahony, D., Spinewine, A., Rodondi, N., Schwenkglenks, M.: Cost-effectiveness of a structured medication review approach for multimorbid older adults: Within-trial analysis of the OPERAM study. PLoS One 17(4), e0265507 (2022). https://doi.org/10.1371/journal.pone.0265507
Health, Information and Quality Authority (HIQA). Guidelines for the Economic Evaluation of Health Technologies in Ireland. 2020. http://www.hiqa.ie/publication/guidelines-economic-evaluation-health-technologies-ireland
Husereau, D., Drummond, M., Augustovski, F., et al.: Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. 20, 23 (2022). https://doi.org/10.1186/s12916-021-02204-0
Campbell, M.K., Elbourne, D.R., Altman, D.G.: CONSORT statement: extension to cluster randomised trials. BMJ 328, 702–708 (2004)
Gomes, M., Ng, E.S.W., Grieve, R., Nixon, R., Carpenter, J., Thompson, S.G.: Developing appropriate methods for cost-effectiveness analysis of cluster randomized trials. Med. Decis. Making 32(2), 350–361 (2012)
Gomes, M., Grieve, R., Nixon, R., Ng, E.S., Carpenter, J., Thompson, S.G.: Methods for covariate adjustment in cost-effectiveness analysis that use cluster randomised trials. Health Econ. 21(9), 1101–1118 (2012)
Ng, E.S., Diaz-Ordaz, K., Grieve, R., Nixon, R.M., Thompson, S.G., Carpenter, J.R.: Multilevel models for cost-effectiveness analyses that use cluster randomised trial data: An approach to model choice. Stat. Methods Med. Res. (2013). https://doi.org/10.1177/0962280213511719
Gomes, M., Díaz-Ordaz, K., Grieve, R., Kenward, M.: Multiple imputation methods for handling missing data in cost-effectiveness analyses that use data from hierarchical studies an application to cluster randomized trials. Med. Decis. Making 33(8), 1051–1063 (2013)
Rubin, D.: Multiple Imputation for Nonresponse in Surveys. Wiley, Chichester (1987)
McCarthy, C., Clyne, B., Corrigan, D., Boland, F., Wallace, E., Moriarty, F., et al.: Supporting prescribing in older people with multimorbidity and significant polypharmacy in primary care (SPPiRE): a cluster randomized controlled trial protocol and pilot. Implement. Sci. 12(1), 99 (2017). https://doi.org/10.1186/s13012-017-0629-1. (Epub 2017/08/03)
Central Statistics Office. Dublin (www.cso.ie). (Accessed June 2022)
Guidelines for Inclusion of Drug Costs in Pharmacoeconomic Evaluations v3.2
Hardin, J.W., Hilbe, J.M.: Generalised Estimating Equations. Chapman and Hall/CRC Press, London (2003)
Rodríguez, G.: Multilevel generalized linear models. In: de Leeuw, J., Meijer, E. (eds.) Handbook of Multilevel Analysis. Springer, New York (2008). https://doi.org/10.1007/978-0-387-73186-5_9
Thompson, S.G., Nixon, R.M., Grieve, R.: Addressing the issues that arise in analysing multicentre cost data with application to a multinational study. J. Health Econ. 25, 1015–1028 (2006)
EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 16(3):199–208 (1990). https://doi.org/10.1016/0168-8510(90)90421-9 (Epub 1990/11/05)
Hobbins, A., Barry, L., Kelleher, D., Shah, K., Devlin, N., Goni, J.M.R., O’Neill, C.: Utility values for health states in Ireland: a value set for the EQ-5D-5L. Pharmacoeconomics 36(11), 1345–1353 (2018). https://doi.org/10.1007/s40273-018-0690-x
Orenstein, D., Kaplan, R.: Measuring the quality of well-being in cystic fibrosis and lung transplantation: the importance of the area under the curve. Chest 100, 1016–1018 (1991)
Hoch, J., Rock, M., Krahn, A.: Using the net benefit regression framework to construct cost-effectiveness acceptability curves: an example using data from a trial of external loop recorders versus Holter monitoring for ambulatory monitoring of “community acquired” syncope. BMC Health Serv. Res. (2006). https://doi.org/10.1186/1472-6963-6-6
Fenwick, E., O’Brien, B., Briggs, A.: Cost-effectiveness acceptability curves: facts, fallacies and frequently asked questions. Health Econ. 13, 405–415 (2004)
Ng, E.S., Grieve, R., Carpenter, J.: Two-stage non-parametric bootstrap sampling with shrinkage correction for clustered data. Stand. Genomic Sci. 13(1), 141–164 (2013)
Briggs, A.H.: A bayesian approach to stochastic cost-effectiveness analysis: an illustration and application to blood pressure control in type 2 diabetes. Int. J. Technol. Assess. Health Care 17(1), 69–82 (2001)
Diaz-Ordaz, K., Kenward, M.G., Grieve, R.: Handling missing values in cost-effectiveness analyses that use data from cluster randomised trials. 2012. J. R. Stat. Soc. Ser. A. http://araiv.org/1206.6070v1 [stat.ME]
Bachmann, M.O., Fairall, L., Clark, A., Mugford, M.: Methods for analyzing cost effectiveness data from cluster randomized trials. Cost Eff. Resour. Alloc. 6(5), 12 (2007). https://doi.org/10.1186/1478-7547-5-12
Acknowledgements
We thank all the patients and GP practice staff who took part in this research. Membership of the wider SPPiRE Study group who contributed to this research (administration, pilot study, practice recruitment, data collection, data entry, and website development) were Tom Fahey, Derek Corrigan, Bridget Kiely, Aisling Croke, James Larkin, Oscar James, Clare Lambert, and Brenda Quigley. The independent members of the trial steering committee were Patricia Kearney (Chair), Andrew Murphy, Cathal Cadogan, Carmel Hughes, and Brid Nolan (PPI).
Funding
Open Access funding provided by the IReL Consortium. This research is funded by the HRB Primary Care Clinical Trial’s Network, Ireland (Grant CTN-2021-002).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
See Fig. 1.
SPPiRE Intervention—Proctor implementation strategy
1. Specify and operationalize implementation strategies:
-
Training videos were created to explain the importance of the topic and the approach for the medication review. A training manual for GPs provided similar information in written format, including background evidence for the inclusion of the relevant potentially inappropriate prescriptions (PIPs).
2. Tailor strategies to context:
-
The study manager tracked the progress of reviews by checking data on the SPPiRE website. Practices that were behind schedule were contacted to encourage them to perform the reviews and to offer further information or support if needed. Practices had the flexibility to adopt scheduling strategies that suited their specific context. Some practices performed opportunistic reviews instead of scheduled ones, this was not part of the original intervention plan. Modifications included conducting phone reviews in response to COVID-19, although this only affected one practice.
3. Engage stakeholders:
-
The trial management committee (TMC) included GP input, and the study manager was a GP, ensuring awareness of the context. The trial steering committee (TSC) had patient and public involvement (PPI) input to ensure that patient-facing materials were appropriate.
4. Training and education:
-
The training videos and manuals served as the primary educational tools for GPs, covering both the importance of the intervention and the practical steps for conducting medication reviews.
5. Implementation planning:
-
A trial Gantt chart was used for planning, but the intervention period had to be extended due to slow progress. Adjustments were made in consultation with the TMC and TSC.
6. Monitor and evaluate implementation:
-
A parallel mixed methods process evaluation was conducted, including quantitative data from website usage and qualitative data from semi-structured interviews with a purposive sample of intervention GPs and patients.
7. Continuous quality improvement:
-
Although not directly related to implementation strategies, safety data were collected on an ongoing basis. GPs were given a process for reporting any adverse drug withdrawal events (ADEs).
8. Sustainability planning:
-
There were no specific strategies planned or implemented for sustainability beyond the intervention period.
SPPiRE Intervention—TIDieR Checklist for Intervention
1. Brief name:
-
SPPiRE
2. Why:
-
To improve the quality and safety of prescribing and reduce treatment burden for patients with complex multimorbidity (defined as ≥ 15 repeat medicines).
3. What (materials):
-
Web platform: Guided GPs through the medication review process.
-
Training materials: Videos and manuals provided to GPs for preparation.
-
Patient materials: Instructions for patients to bring all their medications ("brown bag").
4. What (procedures):
-
Session length: 30 min, conducted once.
-
Components of the review:
-
1.
Check for PIP: The GP identified any potentially inappropriate prescriptions.
-
2.
Brown bag review: The GP and patient reviewed each medication to check for actual drug utilisation, side effects, and effectiveness.
-
3.
Discuss treatment priorities: The GP asked patients about their current treatment priorities.
-
4.
Shared decision-making: The GP recorded all data and worked with the patient to reach a shared decision on any medication changes.
-
1.
5. Who provided:
-
General practitioners (GPs) in Irish general practice settings.
6. How:
-
The intervention was delivered face-to-face between the GP and the patient, guided by a web-based platform.
7. Where:
-
Conducted in primary care settings across Ireland.
8. When and how much:
-
The intervention consisted of a single 30-min session.
9. Tailoring:
-
The review length was suggested but not strictly instructed, and most sessions took longer in practice. Scheduling was flexible, allowing practices to determine when and how to conduct the reviews within a given timeframe.
10. Modifications:
-
Conducted phone reviews in response to COVID-19 for one practice. Some practices performed opportunistic reviews instead of scheduled ones, although this was not part of the original plan.
11. How well (planned):
-
Implementation fidelity was monitored through a mixed methods process evaluation, with data collected on website usage and feedback from GPs and patients.
12. How well (actual):
-
Adaptations were made as necessary, such as conducting phone reviews due to COVID-19. The overall fidelity to the planned intervention was assessed through quantitative data (website usage) and qualitative data (semi-structured interviews).
-
The "brown bag" component was most effective in resulting in medication changes. Most GPs and patients did not engage with the priority-setting exercise.
See Tables 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and 15; Figs. 2, 3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gillespie, P., Moriarty, F., Smith, S.M. et al. Cost effectiveness of a GP delivered medication review to reduce polypharmacy and potentially inappropriate prescribing in older patients with multimorbidity in Irish primary care: the SPPiRE cluster randomised controlled trial. Eur J Health Econ (2024). https://doi.org/10.1007/s10198-024-01718-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10198-024-01718-7