Abstract
The incidence of cancer and its survival rate have been increasing worldwide, resulting in a greater number of cancer survivors. Since one’s job often forms a central basis for self-esteem and provides financial security, knowing whether and how cancer can affect the employment of employed cancer survivors are important issues. While the past studies generally used survey data, this study utilizes pre-existing administrative data and employs the difference-in-differences model. We take newly diagnosed cancer patients in 2012, aged from 15 to 60 years and employed upon diagnosis, as the study group (N = 12694) and the propensity-score matching-adjusted non-cancer employed population as the control group. Monthly employment information for 2011–2014 was retrieved for both groups. We define the pre-event period as the months before the diagnosis and the post-event period as the months from the diagnosis and afterwards. A pseudo-date symbolizing the index point for cancer diagnosis is assigned to the control group. The logistic regression results show that the impact of cancer on the employment status of cancer survivors is significantly negative for both genders. However, if the differences in employment status between the study and control groups prior to the incidence of cancer are significant, then some past research that used only post-period observations might have rendered biased estimates. The employment pathways indicate that 88% of female cancer survivors employed upon diagnosis continue to work during the full 12 months after diagnosis. Further analyses on earnings demonstrate the possibility of cancer survivors retaining their job, but at lower pay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of cancer globally has continued to rise, and together with innovative target therapies, the rate of cancer survival has also increased. The 5-year survival rate in the U.S. for all cancers among adults went from 55% in 1987–1989 to 69% in 2005–2011. For breast cancer, the 5-year survival rate even hit 90% [1]. As cancer increasingly changes from a terminal illness to more like a chronic disease, it is becoming more important to understand how its diagnosis and treatment affect the economic well-being of cancer patients and their families.
Cancer survivors often encounter many difficulties in various aspects, such as physical disability, psychological problems, and economic hardship. These difficulties not only affect their quality of life, but also their economic circumstances. Work is important to almost all adult people, because it often forms a central basis for self-identity and self-esteem, provides financial security, helps maintain social relationships, and represents an individual’s abilities, talents, and health [2,3,4]. Cancer survivors are no exception. Cancer-related employment issues, especially the employment of cancer survivors, have thus attracted great attention in the literature.
The empirical results in the literature have generally shown that cancer has a negative impact on employment, at least for some cancer sites or within a certain period of time. For example, about 40% of cancer survivors stop working during treatment [5]; people with cancer are less likely to work for the 6 months following their diagnosis, relative to a control sample; and regardless of which cancer [6,7,8], more than half of survivors quit working after the first year of diagnosis [5]. Moreover, 45% of colorectal cancer survivors cease or decrease work within 1 year after diagnosis, compared to 27% in a control group [7]. A logistic regression analysis reveals that cancer diagnosis reduces a person’s employment probability by 30–40% [9].
Using data on hospital-based stomach cancer survivors 28 months after their diagnosis and as representative volunteers from the general population [10], this study finds that non-working and reduced working hours are significantly higher among stomach cancer survivors (46.6%) than in the general population (36.5%). Another study using Quebec Tumor Registry and a healthy control group assessed whether there is evidence of discrimination at work (changes in job position, wages, and other conditions) and found that breast cancer survivors after 3 years are 1.29 times more likely to be unemployed [11].
One meta-analysis utilized a systematic search of studies published between 1966 and June 2008 and selected only those that include adult cancer survivors and a control group (the control group was generally matched by age, gender, and area of residence), with employment as the outcome; in the end, 26 studies were identified and utilized in the regression analysis [12]. Overall, the results indicate that cancer survivors are 1.37 times more likely to be unemployed than healthy control participants. Moreover, breast cancer and female reproductive organ survivors are 1.28 times more likely to be unemployed than the control group.
Drawing from the above empirical results, we come to the conclusion that cancer has a strong negative impact on survivors’ employment, yet studies have demonstrated that cancer survivors do return to work. A literature review on the work outcomes of cancer survivors reported the following: 72% of women with breast cancer return to work 3 months after diagnosis; 64% of individuals with a variety of cancer return to work by 18 months [13], and 75% of those who stopped work for treatment return to work [5]. Another literature review indicated that out of the 14 studies identified, the mean rate of return to work is 62% (ranging 30–93%) [4]. A more recent review covering 64 studies between 2000 and 2009 further exhibited that the rate of return depends on the period of time after cancer diagnosis. On average, 40% return to work at 6 month post-diagnosis, 62% at 12 months, 73% at 18 months, and 89% at 24 months [14]. In other words, when a longer employment path is observed, the results imply that a cancer’s negative effect on employment will be greatly reduced; for some cancers, it might even disappear.
Carefully looking through past studies, we notice that most of them did not use nationally represented samples and instead concentrated on non-longitudinal data; a few attempts have been made to utilize pre-existing longitudinal surveys, such as the Health and Retirement Study (HRS) [15], the Panel Study of Income Dynamics (PSID) [16], the National Health Interview Survey (NHIS) [17], and the Current Population Survey (CPS) [6]. In recent years, the pooling of existing administrative data—such as pooling cancer registry, population register, population census, and taxes for Norway [9]; pooling cancer registry, vital statistics registry and personal income tax records for Canada [18]; pooling cancer registry and detailed skill requirement data for Denmark [19]—has opened up a new way for providing a better understanding of the impact of cancer on employment and earnings. Our study thus pools cancer registry and monthly underwriting data for Taiwan’s long-running social health insurance scheme.
Materials and methods
Taiwan started its cancer registry in 1979, and its quality has gained an international reputation over the years [20, 21]. In addition, Taiwan implemented a compulsory, single-payer National Health Insurance (NHI) system in 1995, with nearly the whole population being covered, and all insurance underwriting data are in digital format. Using the cancer registry and NHI underwriting data together with the cause of death data, we are able to both investigate the impact of cancer on employment status and to trace the possible changing patterns of cancer survivors’ employment.
Taiwan’s NHI collects premiums from the insured on a monthly basis. For those who work, the premium is based upon their regular earnings (including base pay and overtime pay, but not year-end bonus), however, only up to a ceiling (in 2012, it was NT$182,000/per month, or equivalent to 9.7 times the minimum wage). The employer’s premium share for its employees and their dependents is 60%.
We use Taiwan’s cancer registry to identify newly diagnosed cancer patients, their date of initial diagnosis, and the primary site, employ NHI underwriting data to obtain the date of birth, gender, employment status, area of residence, and regular earnings (if working), and utilize the cause of death data to identify the date of death and cause of death. These three data sets are linked by a unique scrambled identification number. Since those under 15 years of age are not allowed to work in the labor market, and those over 60 are very close to retirement, they are excluded from the analysis. Area of residence is defined as the six areas, where NHI branch offices are located. In general, northern Taiwan, including Taipei area and northern area, has more job opportunities and higher earnings, while the eastern area is less developed and thus has fewer jobs and less pay. The other three areas are in-between.
The Bureau of National Health Insurance (now the National Health Insurance Agency, NHIA) has been providing yearly underwriting data to researchers, but beginning in 2011 monthly data have been offered. Since there are 2–3 years of lag for the release of data, especially for the cancer registry, we use data for the period 2011–2014 in this study.
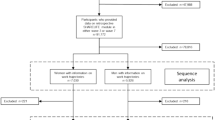
We first take newly diagnosed cancer patients in 2012 from Taiwan’s cancer registry as the baseline data and merge them with the 2012 NHI monthly underwriting data to identify those cancer patients who were employed upon diagnosis as the study group, while the non-cancer general population is selected as the control group. A pseudo-date symbolizing the index point for cancer diagnosis is assigned to the control group [22]. The propensity-score matching (PSM) method 3:1 helps select the control group from the 2012 NHI underwriting data (signifying the whole population) by matching gender, age, and area of residence for those who were employed at that pseudo-date. Those selected in the control group are further checked to see whether they have ever been in the cancer registries. Those found to be in the cancer registries are excluded. Both newly diagnosed employed cancer patients (abbreviated as cancer patients) and the non-cancer employed general population (abbreviated as non-cancer population) are cross-referenced with the cause of death data set to make sure they survived throughout the whole study period; otherwise, they are excluded.
Through retrieving the employment information from the insurance monthly underwriting data from 2011 to 2014 for both cancer survivors and the non-cancer population with their date of diagnosis, we are able to track the employment status before and after the diagnoses. We then employ a standard difference-in-differences (DID) model to investigate the employment effects of the study group vs. those of the control group. In addition, since the employment situation is very different for males and females, we separate the analysis by gender.
Statistical analysis
The DID method adopted herein is widely used to measure the changes induced by an event between study and control groups [23, 24]. To evaluate the possible employment changes due to the incidence of cancer, we define the pre-event period as the months before the diagnosis and the post-event period as the months from the diagnosis and afterwards. In other words, we follow the monthly employment status of the cancer survivors from the date of diagnosis and up to 36 months later.
We present the specification of the DID model as follows:
where \({\text{Emp}}\) represents employment status (1 stands for being employed in the labor market; 0 otherwise); \({\text{Ca}}\) denotes the presence of cancer (1 stands for having cancer; 0 otherwise); \({\text{Post}}\) is a dummy variable for all months after the diagnosis of cancer; \(i\) denotes the \(i{\text{th}}\) individual; \(j\) denotes the \(j{\text{th}}\) gender; \(k\) denotes the \(k{\text{th}}\) area of residence (with residence in the central area of Taiwan as the reference); \(\ell\) denotes the \(\ell\)th age group (with age 35–44 as the reference); and \(\varepsilon\) represents the error term.
The coefficient for the presence of cancer, \({r_1}\), depicts the possible differences in employment status between cancer survivors and the non-cancer population prior to the incidence of cancer, whereas \({r_2}\) depicts the difference in employment status between the pre-cancer period and post-cancer period for the non-cancer population. The coefficient \({r_3}\) captures the difference between the pre–post changes of the different groups (cancer vs. non-cancer). Thus, we are particularly interested in whether the odds ratio of this interactive term is significantly different from zero. Equation (1) is estimated by logistic regression, using software SAS 9.4. To evaluate the possible changes on regular earnings due to the incidence of cancer, Eq. (1) is re-estimated by ordinary least square regression with the dependent variable being the person’s regular earnings.
Results
Table 1 displays the characteristics of cancer survivors, the site and stage of their cancer, and the propensity-score-matching-adjusted non-cancer population. Female cancer survivors outweigh males by 6.3:3.7. Among the seven cancer sites either commonly studied in western countries [25] or special to Taiwan, female breast cancer occupies the greatest share (25.5%), followed by colorectal (12.5%), cervical (9.6%), oral (6.7%), lung (4.8%), liver (4.3%), and prostate (1%); altogether they comprise more than 64% of the total samples. As to the cancer stage, 32.4% are at stage 0 or I, 14.8% at stage II, 9.2% at stage III, and 6.3% at stage IV, while the rest (37.3%) are either others or unknown.
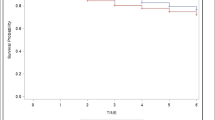
Figure 1 exhibits the employment rate for both cancer survivors and non-cancer population by gender and months since diagnosis (MSD). When MSD equals zero, it represents the month when the cancer is diagnosed. It shows that, prior to the incidence of cancer, the employment rates for cancer survivors are higher than those of the non-cancer population, and this is true for both genders. However, the employment rate for cancer survivors starts to go down right after cancer diagnosis, and the speed of the drop is higher for females than for males for the first 20 months after diagnosis; then, the trend starts to reverse. At 30 months after diagnosis, the employment rate for cancer survivors is 86.6% for males and 86.5% for females, and the corresponding figures for the control group are 92.1% for males and 92.0% for females. In other words, regardless of the gender, for those who are employed upon diagnosis, a little less than 14% of them stopped working within 30 months, while the corresponding figure for the control groups is 8%.
Figure 2 displays the average earnings of cancer survivors and non-cancer population by gender and months since diagnosis. On average, it shows that males’ earnings are more than 20% higher than females’. The average earnings of cancer survivors are also higher than those of the control groups for both genders prior to the incidence of cancer. Moreover, earnings increase over time in both cancer survivors and the non-cancer control group; however, the upward trend for survivors is not as steep as that for the latter. Therefore, the smaller positive difference in monthly earnings (it is NT$427 for males and $2473 for females, shown in Table 2) prior to the incidence of cancer between survivors and controls ultimately results in higher earnings for the controls than for survivors who are male.
Table 2 shows the regression results for employment status and monthly regular earnings for both genders. Column 1 displays the results of the logistic regression model for employment status of males; column 2 displays the results of the logistic regression model for females; column 3 displays the OLS results of monthly regular earnings for males; and column 4 displays the OLS results for females. As shown in columns 1 and 2 (columns 3 and 4), the post-diagnosis period and the presence of cancer both have significantly positive effects on employment status (on monthly regular earnings). However, the coefficient of the interaction term for cancer status and the post-diagnosis period is significantly negative.
Table 3 reports the employment pathways for both cancer survivors and the control group for both males and females. For males, we see that 89.1% of all cancer survivors employed at the time of diagnosis continued to be employed throughout the whole observation period (all 12 months after the diagnosis). Among those who stopped being employed in some months, 77.2% (upper panel, or 8.42% of all cancer survivors) did not return to work in the observation period. Only less than 3.3% (lower panel, or 0.36% of all cancer survivors) who stopped being employed actually returned to their job. The other 2.12% cancer survivors show no clear employment pattern. The figures for the control group are 92.2%, 64.9% (5.07%), 3.9% (0.31%), and 2.43%, respectively.
For females, 88.0% of all cancer survivors employed at the time of diagnosis continued to be employed throughout the whole observation period. Among those who stopped being employed in some months, 73.7% did not return to work in the observation period. Only less than 3.9% who stopped being employed actually returned to their job. The other 2.74% cancer survivors show no clear employment pattern. The figures for the control group are 92.4%, 63.7% (4.86%), 3.2% (0.24%), and 2.52%, respectively.
Conclusions and discussion
The majority of the past studies focusing on the employment effect of cancer survivors indicated that they exhibit a lower chance of being in the labor market and often work fewer hours [5,6,7,8,9,10,11,12]. However, the magnitudes of the negative effects vary among studies, depending upon the gender, cancer sites, and age groups being studied. Moreover, their employment status fluctuates, especially during the periods of 6 months after diagnosis [5,6,7,8, 14]. For example, one study that reported a high percentage (around 40%) of cancer survivors who stopped working during treatment also found a higher percentage (75%) of them had returned to work [5]. The unemployment rate is not higher for survivors of blood cancer, prostate cancer, or testicular cancer, and the likelihood of employment for prostate cancer patients and control subjects is not statistically significantly different at 12 months after diagnosis [8, 12]. A more recent review further showed that 40% on average returned to work at 6 month post-diagnosis, 62% at 12 months, 73% at 18 months, and 89% at 24 months [14].
All those results imply that cancer’s major impact on employment happens during the first few months to a year after diagnosis. By tracing the employment pathway of each cancer survivor who was working at the time of diagnosis for 12 months after diagnosis, we are able to observe not only the status of the employment for that period, but also the timing of the employment change for both the study and control groups.
The analysis shows a rather steady trend for the employment of cancer survivors for both genders in Taiwan. Taking males as an example, 89.1% of those working at the time of diagnosis continued to work over the following 12 months. Among those who stopped working in some months (accounting for 10.9%), more than 75% of them did not return to work; only 3.3% had returned to work in the observation period. The corresponding figures for the control groups are 92.2% and 3.9%. Similar results are found for females, whereby 88.0% of those working at the time of diagnosis continued to work during the following 12 months. Among those who stopped working in some months, close to 75% of them did not return to work, while less than 3.9% had returned to work in the observation period. The corresponding figures for the control groups are 92.4% and 3.2%. In other words, 88% of employed female cancer survivors continued to work during the full 12 months after diagnosis, while the respective figure for the non-cancer population is 92%.
In the real labor market, as a result of either own reasons or outside forces, individual workers often face different work decisions, such as change in employer, becoming self-employed, or dropping out of the job market. Thus, even when we trace the same group of people, we see dynamic changes. This study chooses people employed upon diagnosis of cancer, which guarantees that at the time of diagnosis, all subjects are employed workers, whereas at other times (pre-cancer period or post-cancer period), they are possibly unemployed or self-employed. In other words, the employment rate is at the peak (100%) at the time of diagnosis. They might be employed, self-employed, or even unemployed prior to cancer diagnosis; or they might change from employed to self-employed or unemployed after the diagnosis. Therefore, a mountain-shaped graph appears rather than a downward sloping curve. The employment rate increases to a peak at the time of diagnosis and then gradually decreases, as displayed in Fig. 1.
The regression results exhibit that differences in employment status between cancer survivors and the non-cancer population after diagnosis are statistically significant for all months except the first one (results are not shown). The employment rate in Fig. 1 reflects a mix of people who were employed continuously as well as those who dropped out of the labor force at some point and then returned to work. Table 3 traces the employment pathways for the same group of people—those who are employed at the time of diagnosis.
Owing to the fact that an individual’s employment situation is influenced not only by individual factors, but also by macroeconomic conditions such as economic growth and unemployment rate, investigating the employment status of cancer survivors ideally should take macroeconomic circumstances into consideration. Choosing the appropriate control group is the general approach taken by most previous studies. However, there are still likely differences between cancer survivors and the non-cancer population that could impact their employment outcomes. For example, there are differences in health status [17], level of skills required [19], and workplace support [26], and information is often available in the survey data, but not in the administrative data. We, therefore, employ the DID model, and by doing so, we are able to identify whether there are any significant differences in employment status prior to the incidence of cancer between cancer survivors and the non-cancer population, the differences between the pre-diagnosis period and post-diagnosis period for the non-cancer population, and the differences between the pre–post changes of the different groups (cancer vs. non-cancer).
The coefficient of the variable “Cancer” in Table 2 captures the composite effect of the differences between cancer survivors and the non-cancer population prior to the incidence of cancer. The significant positive effects of cancer on employment status and monthly regular earnings imply that those who were diagnosed with cancer in 2012 and survived through the end of 2014 have a higher probability of being employed and have higher monthly regular earnings prior to the incidence of cancer, compared with the control groups. It is possible that those with higher earnings are equipped with greater skills/knowledge, have a better working environment, and thus have a higher probability of being employed, but they might face greater stress, live a relatively unhealthy life, and thus have a higher chance to be in ill health.
Since the past studies regarding the employment effect of cancer have almost all investigated the periods after the diagnosis, to conduct a proper comparison, we re-run the logistic regression model using only post-diagnosis observations. This results in the DID model collapsing into the regular logistic model, with only \({\text{Ca}}\) remaining in the equation and without the variables \({\text{Post}}\) and \({\text{Ca}} \times {\text{Post}}\). The results exhibit a significantly negative coefficient for \({\text{Ca}}\), however, at a larger magnitude. The odds ratio between the pre–post changes of the different groups (cancer vs. non-cancer) indicates that the effect of cancer is 0.423 for males and 0.412 for females in the DID model, whereas it is 0.548 for males and 0.528 for females in the logistic model. The larger magnitude of the odds ratio in the logistic model, signifying a smaller negative effect, is from the positive differences in employment status between cancer survivors and the non-cancer population prior to the incidence of cancer. It implies that if there is a prior positive (or negative) difference in employment status between cancer survivors and the non-cancer population, then the negative impact of cancer on employment reported in the past literature might be under-estimated (over-estimated).
The employment effect of cancer on cancer survivors is also likely influenced by legislation on sick leave and sickness benefits [25, 27]. According to the Labor Standards Act in Taiwan, a worker is allowed up to 30 days in a year for sick leave with compensation at 50% of regular earnings. Since treatments for cancer often begin right after diagnosis, the temporary drop in average earnings upon diagnosis implies that some cancer survivors take sick leave during treatment, and this phenomenon occurs in both genders. A better provision for sick leave, such as longer periods of time and a higher compensation rate, should reveal different work behaviors.
Is it possible that what we observe in this study—less than 14% of those employed upon diagnosis stopped working 30 months from the diagnosis—is actually due to the fact that cancer survivors retain their job, but at a lower pay? The OLS regression results of the DID model regarding the impact of cancer on regular earnings, shown in Table 2, display that the coefficient that captures the difference between the pre–post changes of the different groups (cancer vs. non-cancer) is significantly negative. It would imply the possibility of cancer survivors retaining their job at lower pay.
Are there possibilities that workers continue to work so as to keep their health insurance? [28] As Taiwan’s NHI is a compulsory single-payer healthcare system, those who are employed are covered through their employer, while those who are not employed are covered through a government district office. Therefore, the job lock phenomenon observed in the environment of employment-based health insurance is not a practical constraint for cancer survivors in Taiwan.
In sum, the impact of cancer incidence on employment is negative, and the magnitudes of its negative effects vary by individual factors such as gender, cancer sites, and age groups. Through choosing an appropriate control group, the past studies also implicitly take macroeconomic conditions into consideration, but it seems that there is no guarantee that the control group is really no different from the study group in terms of employment status prior to cancer incidence. Therefore, in this study, we utilize pre-existing administrative data and employ the DID model. In doing so, we are able to decompose the differences in employment status between cancer survivors and the non-cancer population into three parts: the differences between the two groups prior to the incidence of cancer, the differences between pre-period and post-period for the control group, and the differences between the pre–post changes of the two groups. What we are interested in knowing is the sign and magnitude of the third component. By comparing the results of the DID model with the results from using only the post-period observations, we find that some past studies might have rendered biased estimates.
The employment effect of cancer on cancer survivors is also influenced by legislation on sick leave and sickness benefits. The less generous provision for sick leave, such as 30 days in a year with compensation at 50%, resulted in a temporary drop in average earnings upon diagnosis in Taiwan. The analyses regarding regular earnings with the DID model further demonstrate the possibility of cancer survivors retaining their job at lower pay.
Aside from all cancers, we also conduct separate analyses for common cancers: colorectal, liver, and lung cancers for both genders, and female breast cancer, using common control groups. The employment pathways for those cancer survivors employed at the time of diagnosis who continued being employed throughout the following 12 months for both genders are 89.1% and 88% for all cancers; 91.2% and 88.8% for colorectal cancer; 87.4% and 89.7% for liver cancer; 87.5% and 84.3% for lung cancer; and 88.8% for female breast cancer. The significant positive differences in employment status prior to the incidence of cancer between cancer survivors and the non-cancer population as well as the significant negative effect on the employment status due to cancer incidence are also consistent across cancer sites.
Compared with other studies that use administrative data or adopt a similar analytical approach, the magnitudes of our estimates are similar to those in [29]. They used Danish administrative data for breast and colorectal cancer survivors and found a declining probability of employment over a 3-year period, with a 3.6–4.5 percentage-point drop in the first year. Our estimate of a 4 percentage-point drop is in line with that in [18], who used Canada administrative data for all cancers and found that cancer lowers the probability of working by 3 percentage points in the first year.
As to the earnings reduction in the first year after diagnosis, it was 10% in [18], close to 20% in [16], and less than 3% in [29]. In our study, since earnings increase over time in both cancer survivors and non-cancer controls, the reduction in earnings due to cancer is very low, around 2%.
Study limitations
First, an individual’s employment status is defined by whether he/she was insured by the NHI system through an employer, and thus, we include those who took sick leave. Second, we only report the employment pathways for employees and exclude those who are self-employed due to their flexibility at work. Third, the NHI underwriting data are constructed primarily for the collection of premiums; detailed information such as hours worked is not available. Fourth, since we are interested in knowing whether having cancer presents any differences in terms of people’s employment decision, we, therefore, excluded those who died during the study period, as for obvious reasons, they did not remain in the labor market. In doing so, we observe a higher percentage of workers who maintain their work status vs. without this exclusion.
References
American Cancer Society: Cancer facts and figures (2016)
Wells, M., et al.: Supporting ‘work-related goals’ rather than ‘return to work after cancer? A systematic review and meta-synthesis of 25 qualitative studies. Psycho-Oncology 22(6), 1208–1219 (2013)
Nachreiner, N.M., et al.: Successful return to work for cancer survivors. AAOHN J. 55(7), 290–295 (2007)
Spelten, E.R., et al.: Factors reported to influence the return to work of cancer survivors: a literature review. Psycho-Oncology 11(2), 124–131 (2002)
Short, P.F., et al.: Employment pathways in a large cohort of adult cancer survivors. Cancer 103(6), 1292–1301 (2005)
Bradley, C.J., et al.: Short-term effects of breast cancer on labor market attachment: results from a longitudinal study. J. Health Econ. 24(1), 137–160 (2005)
Beesley, V.L., et al.: Association between change in employment participation and quality of life in middle-aged colorectal cancer survivors compared with general population controls. Psycho-Oncology 26(9), 1354–1360 (2016)
Bradley, C.J., et al.: Employment outcomes of men treated for prostate cancer. J. Natl. Cancer Inst. 97(13), 958–965 (2005)
Syse, A., et al.: Cancer’s impact on employment and earnings—a population-based study from Norway. J. Cancer Surviv. 2(3), 149–158 (2008)
Lee, M.K., et al.: Employment status and work-related difficulties in stomach cancer survivors compared with the general population. Br. J. Cancer 98, 708–715 (2008)
Maunsell, E., et al.: Work situation after breast cancer: results from a population-based study. J. Natl. Cancer Inst. 96(24), 1813–1822 (2004)
de Boer, A.G., et al.: Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA 301(7), 753–762 (2009)
Steiner, J.F., et al.: Assessing the impact of cancer on work outcomes: what are the research needs? Cancer 101(8), 1703–1711 (2004)
Mehnert, A.: Employment and work-related issues in cancer survivors. Crit. Rev. Oncol. Hematol. 77(2), 109–130 (2011)
Bradley, C.J., et al.: Breast cancer survival, work, and earnings. J. Health Econ. 21(5), 757–779 (2002)
Zajacova, A., et al.: Employment and income losses among cancer survivors: estimates from a national longitudinal survey of American families. Cancer 121(24), 4425–4432 (2015)
Clarke, T.C., et al.: Working with cancer: health and employment among cancer survivors. Ann. Epidemiol. 25(11), 832–838 (2015)
Jeon, S.H.: The long-term effects of cancer on employment and earnings. Health Econ. 26(5), 671–684 (2017)
Heinesen, E., et al.: Employment, job skills and occupational mobility of cancer survivors. J. Health Econ. 58, 151–175 (2018)
Chiang, C.J., et al.: Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol 45(3), 291–296 (2015)
Chen, C.J., et al.: Cancer epidemiology and control in Taiwan: a brief review. Jpn. J. Clin. Oncol. 32(Suppl), 66–81 (2002)
Hornbrook, M.C., et al.: When does an episode of care for cancer begin? Med. Care 51(4), 324–329 (2013)
Lo, J.C., et al.: Healthcare utilization of bereaved family members following the 1999 Chi-chi earthquake: evidence from administrative data. J. Psychosom. Res. 75(5), 484–490 (2013)
Ayyagari, P., Shane, D.M.: Does prescription drug coverage improve mental health? Evidence from Medicare Part D. J. Health Econ. 41, 46–58 (2015)
Mehnert, A., et al.: Employment challenges for cancer survivors. Cancer 119(S11), 2151–2159 (2013)
Lindbohm, M.L., et al.: Early retirement and non-employment after breast cancer. Psychooncology. 23(6), 634–641 (2014)
Veenstra, C.M., et al.: Employment benefits and job retention: evidence among patients with colorectal cancer. Cancer Med. 7(3), 736–745 (2018)
Veenstra, C.M., et al.: Long-term economic and employment outcomes among partners of women with early-stage breast cancer. J. Oncol. Pract. 13(11), e916–e926 (2017)
Heinesen, E., et al.: Effects of breast and colorectal cancer on labour market outcomes—average effects and educational gradients. J. Health Econ. 32, 1028–1042 (2013)
Acknowledgements
Helpful comments from Prof. Tzu-Ting Yang are deeply appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical approval
This study has been approved by IRB for the biomedical science research of Academia Sinica, R.O.C. under approval number AS-IRB02-105306.
Informed consent
Since the study subjects are from the whole population and the results are in statistical formats, patient consent is waived.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lo, J.C. Employment pathways of cancer survivors—analysis from administrative data. Eur J Health Econ 20, 637–645 (2019). https://doi.org/10.1007/s10198-018-1025-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-018-1025-8
Keywords
- Administrative data
- Cancer survivors
- Difference-in-differences model
- Employment pathways
- National Health Insurance
- Taiwan