Abstract
Background
Hepatitis C virus (HCV) treatment can reduce the incidence of future infections through removing opportunities for onward transmission. This benefit is not captured in conventional cost-effectiveness evaluations of treatment and is particularly relevant in patient groups with a high risk of transmission, such as those people who inject drugs (PWID), where the treatment rates have been historically low. This study aimed to quantify how reduced HCV transmission changes the cost-effectiveness of new direct-acting antiviral (DAA) regimens as a function of treatment uptake rates.
Methods
An established model of HCV disease transmission and progression was used to quantify the impact of treatment uptake (10–100%), within the PWID population, on the cost-effectiveness of a DAA regimen versus pre-DAA standard of care, conducted using daclatasvir plus sofosbuvir in the UK setting as an illustrative example.
Results
The consequences of reduced disease transmission due to treatment were associated with additional net monetary benefit of £24,304–£90,559 per patient treated at £20,000/QALY, when 10–100% of eligible patients receive treatment with 100% efficacy. Dependent on patient genotype, the cost-effectiveness of HCV treatment using daclatasvir plus sofosbuvir improved by 36–79% versus conventional analysis, at 10–100% treatment uptake in the PWID population.
Conclusions
The estimated cost-effectiveness of HCV treatment was shown to improve as more patients are treated, suggesting that the value of DAA regimens to the NHS could be enhanced by improved treatment uptake rates among PWID. However, the challenge for the future will lie in achieving increased rates of treatment uptake, particularly in the PWID population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic hepatitis C virus (HCV) infection is a major cause of liver disease globally [1], and the associated mortality and morbidity is predicted to increase in many countries [2]. In the UK, the reported rate of deaths and hospital admissions associated with HCV-related end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC) continues to rise, while it is acknowledged that national data underestimate the true scope of the problem [3].
An estimated 214,000 people in the UK are chronically infected with HCV, predominantly HCV genotypes 1 and 3. Injecting drug use continues to be the principal risk factor for HCV infection [3]; 90% of the 13,758 diagnosed cases of HCV infection in 2013 were estimated to be from injecting drug use [4]. More generally, survey data suggest that approximately two in five people who inject drugs (PWID) are currently living with HCV infection in the UK [3, 4].
The established standard of care for HCV uses a pegylated interferon-alfa and ribavirin (PR) backbone, which is associated with efficacy and tolerability concerns, particularly in those with advanced disease [5, 6]. By contrast, newly available direct-acting antiviral (DAA) agents can be used as part of an oral-only, interferon-free regimen and are associated with improved tolerability and efficacy, shorter treatment durations and rates of sustained virologic response (SVR, an objective measure of cure [7]) approaching 100% [8–11]. However, these new therapies are also associated with considerable acquisition costs.
Economic evaluations of newly available DAA regimens have demonstrated that their use is largely cost-effective [12–14], due in part to the severe consequences of progression to ESLD [15, 16]. Within a UK context, the National Institute for Health and Care Excellence (NICE) has recommended for use several of the newly available DAA agents, including sofosbuvir, simeprevir, ledipasvir, and daclatasvir [17–21].
During the more recent of these appraisals, NICE recognized the importance of capturing the potential downstream benefits of cure for future disease transmission [22, 23]. By removing the opportunity of onward transmission, treatment has the potential to reduce the incidence of future infections [24]. Through modeling exercises, even modest increases in treatment uptake have been estimated to reduce disease transmission amongst high-risk populations, and in this way reduce the overall prevalence of HCV [25–31]. In line with this evidence, the European Association of the Study of the Liver (EASL) 2015 guidelines recommend prioritization of treatment for individuals at risk of transmitting HCV, including active injection drug users [7].
One recent economic evaluation assessed the cost-effectiveness of treating PWID in the UK when incorporating the downstream consequences of cure on future disease transmission, and demonstrated that this improved cost-effectiveness versus treatment in the non-PWID population [32]. The study also assessed competing treatment prioritization strategies and found that immediate treatment of PWID with chronic HCV in the UK would be prioritized ahead of patients with moderate HCV, after treating people with severe disease, due to the additional benefit of averting secondary infections. However, in order to avoid overt bias in the evaluation towards PWID treatment, the authors conservatively examined a very low treatment rate among PWID because HCV treatment rates among PWID are low in the UK and most other global settings [32]. Previous research has demonstrated that while improved SVR profiles led to reductions in modeled HCV prevalence, increased treatment uptake was the key driver of future infections avoided, which results in fewer complications, significant cost savings, and QALY gains [33].
Given the proven clinical benefits of new DAA regimens, including improved tolerability and efficacy [8–11], an increase in treatment uptake is plausible, particularly in the context of low historical treatment rates. However, an assessment of the impact of increasing treatment uptake on the cost-effectiveness of DAAs in PWID has not been undertaken.
The aim of this study was to build on existing research, using an established model of HCV disease transmission and disease progression [33] to demonstrate how the cost-effectiveness of DAAs differs based on treatment uptake as a result of reduced disease transmission. As an example, treatment of the PWID population in the UK setting is presented, using daclatasvir plus sofosbuvir versus pre-DAA standard of care.
Methods
Combined HCV progression/transmission model
A previously published model was utilized [33], based on a combination of established HCV disease progression and disease transmission models [29, 33–37].
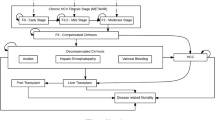
Figure 1 depicts the progression of an individual patient.
The disease transmission component was based on the HCV transmission model published by Martin et al. [25, 29], and is comparable with other published HCV transmission models [28]. The deterministic compartment model simulates susceptible PWID individuals who become acutely infected with HCV, and may either spontaneously clear the infection or progress to chronic HCV. The PWID population is stratified by transmission risk (low versus high risk) and receipt of opiate substitution therapy (OST); rates of onward transmission are controlled by relative risks of acquiring HCV in each subgroup (Table 1).
If individuals with chronic HCV infection receive treatment, they may achieve SVR and re-enter the uninfected state; uninfected patients can no longer transmit the virus to others, but become susceptible to reinfection. Patients who fail treatment remain chronically infected and at risk of passing infection to others, with no option to re-treat modeled following treatment failure.
The progression of new chronically infected patients was modeled from F0 (assuming age 20 years), through liver fibrosis stages, and from the F4 disease stage to decompensated cirrhosis, HCC, liver transplant, or death. Dynamic, fibrosis stage-specific transition rates from a meta-regression analysis from a multi-country, multi-center study using data from 33,121 individuals chronically infected with HCV were utilized [38]. Additional transition rates were obtained from Martin et al. [74]. Genotype 3-specific multipliers (relating to fibrosis stage progression and the progression from compensated cirrhosis and decompensated cirrhosis to HCC) were utilized [39].
Data and assumptions
A prevalent population of PWID was established according to historical data regarding treatment prior to 2015 as described previously [33], and the impact of new therapies was assessed thereafter. Data inputs were based upon the PWID population in Edinburgh (Table 1); the size of the modeled PWID population was assumed to be fixed.
Costs and utilities associated with HCV disease progression and treatment were based on a review of the published literature, and are in line with inputs reported by Townsend et al. [40]. Costs and utilities for health states, complications, and treatment regimens are described in Table 2; monitoring costs were obtained from a previous HCV economic analysis conducted within the UK [41]. In the absence of a published utility value for patients achieving SVR from fibrosis stage F4, it was assumed that utility following SVR was the same for patients in F3 and F4 pre-treatment. This assumption was based on (1) SVR being accepted as a cure, and (2) cirrhosis has been shown to regress in up to 75% of patients achieving SVR [7, 42–44]. Where required, costs were inflated to 2013 values using the Hospital and Community Health Services (HCHS) Index [45].
Clinical data inputs [SVR, extended rapid virologic response (eRVR) and discontinuation rates] extracted for each of the assessed treatments are summarized in Table 3. Predominantly, clinical data were obtained from naive comparisons of daclatasvir + sofosbuvir with relevant treatment regimens. It was assumed that all discontinuations for the respective regimen occur in week 4 (the earliest point of discontinuation that the model allows).
Analysis
In order to quantify the health economic value of HCV treatment unaccounted for in conventional economic analyses, the cost offsets and quality-adjusted life-year (QALY) gains associated with reduced disease transmission following treatment (SVR rate 50–100%) in the PWID population were estimated versus no treatment; no costs of treatment were modeled. The predicted cost offsets and QALY gains were then combined to present the incremental net monetary benefit (iNMB), at a willingness-to-pay threshold of £20,000/QALY. In this context, iNMB may be interpreted as the maximum monetary investment that could be allocated to the new treatment (drug acquisition, administration, monitoring etc.) without exceeding the willingness-to-pay threshold.
Treatment uptake rates of 10–100% of chronically infected patients not previously treated were applied at the beginning of 2015, with no treatment over subsequent years. Treatment uptake was applied proportionally across all subgroups on or off OST, at high or low risk of transmission. Results were accumulated over a 50-year horizon (2015–2064) and presented per patient treated; due to the uncertainty in future dynamics of disease, shorter time horizons were assessed in sensitivity analyses.
To explore the impact of this underestimation of treatment benefit on cost-effectiveness estimates, a conventional cost-effectiveness evaluation of daclatasvir + sofosbuvir versus established standard of care in genotypes 1 and 3 was undertaken over a lifetime horizon (80 years), before adjusting for onward transmission. The population evaluated in the conventional analysis was aged 50 years, 67% male and distributed across fibrosis stages based on data reported in the hepatitis C in the UK: 2013 report (F0: 30.89%; F1: 30.89%; F2: 16.82%; F3: 16.82%; F4: 4.57%) [46, 47]. Age among treated PWID may be lower than the general population; any bias introduced by this limitation is likely to bias against the strategy to treat.
Treatment uptake rates of 10–100% were evaluated under the assumption that hypothetical cohorts were comprised of the specified genotype only. For patients infected with HCV genotype 1, established standard of care was modeled as PR, telaprevir + PR or no treatment. Established standard of care in patients with HCV genotype 3 was modeled as PR or no treatment.
The analysis was performed from the perspective of the UK NHS and Personal Social Services (PSS), and both costs and benefits were discounted annually at a rate of 3.5%, in line with UK guidelines [48].
Results
Underestimation of value of SVR in conventional health economic models
Figure 2 graphically illustrates the cost savings, QALY gains, and iNMB at a willingness-to-pay threshold of £20,000/QALY associated with the disease transmission consequences of HCV treatment, dependent on treatment uptake and SVR rate. In the modeled scenario, where all previously untreated patients were treated with an antiretroviral therapy with a 100% SVR rate, the predicted iNMB was estimated to be £90,559 per patient treated (cost savings: £20,733, QALY gains: 3.49), as a result of reduced opportunity for future transmission of infection. In contrast, treating 10% of patients produced a lower iNMB of £29,188 per patient treated, at the same SVR rate (100%). Treating only 10% of patients using a less effective therapy (SVR 50%) further reduced the iNMB to £24,304 per treated patient.
Cost offsets, QALY gains, and incremental net monetary benefit associated with increased HCV treatment uptake and SVR. The effect of varying HCV treatment uptake and SVR is graphically illustrated (light grey lines represent low uptake/SVR; dashed lines in a and b represent costs, while solid lines represent QALYs). The effect of improving treatment uptake (10–100%) causes greater QALY gains, cost offsets, and net monetary benefit (a, b), and this is dependent on SVR rate. Similarly, improving SVR rate (from 50–100%) causes greater QALY gains, cost offsets, and net monetary benefit (b, d), which can be elevated further by increasing treatment uptake
Impact on cost-effectiveness of newly available DAA therapies
The conventional evaluation of daclatasvir + sofosbuvir versus no treatment in genotype 1 produces incremental benefits of 3.05 QALYs at an incremental cost of £36,764, corresponding to iNMB of £24,165 and an incremental cost-effectiveness ratio (ICER) of £12,068. The value associated with reduced disease transmission as a consequence of 95% of patients achieving SVR was estimated to be £81,652 at 100% treatment uptake. Adding this value to the conventional estimates increases iNMB (£105,817) and decreases the ICER (£2911) by a factor of approximately 4.
Figure 3 shows the impact of incorporating the estimated health and economic benefits associated with reduced onward transmission into the ICER calculations for the illustrative comparison of daclatasvir + sofosbuvir versus established comparators in genotype 1 and genotype 3 patients. The estimated cost-effectiveness of the daclatasvir-based regimens improves with the inclusion of future transmission consequences, and this improvement increases with greater treatment uptake. In patients with HCV genotype 1, ICERs associated with daclatasvir + sofosbuvir versus no treatment, PR, telaprevir + PR fell by £6812–£9157, £12,015–£19,208, and £13,846–£25,924, respectively, dependent on treatment uptake rate (10–100%), compared to the base case (conventional analysis, no benefit of reduced onward transmission); this corresponds to relative reductions of 36–76% in ICERs. Similarly, in the HCV genotype 3 scenario, base case ICERs associated with daclatasvir + sofosbuvir fell by £6314–£8334 and £18,016–£28,841 in comparisons against no treatment and PR, respectively, depending on treatment uptake rate (10–100%) corresponding to relative reductions of 41–79% in the ICER.
Effect of accounting for 100% treatment uptake rate within a cost-effectiveness evaluation of daclatasvir + sofosbuvir versus established standard of care, contrasted with a conventional analysis (no inclusion of onward transmission, illustrated as solid markers at 0% uptake). Willingness-to-pay thresholds of £20,000 and £30,000 per QALY are represented (grey boundaries). Incremental cost-effectiveness ratios for daclatasvir + sofosbuvir versus standard of care for patients with HCV genotypes 1 or 3 are improved when accounting for the downstream benefits of increasing treatment uptake, resulting in ratios below the willingness-to-pay thresholds of £20,000 and £30,000 per QALY
Reducing the time horizon over which disease transmission was assessed led to the capture of fewer transmissions and also fewer long-term ESLD complications among newly infected patients; as a result, the magnitude of estimated cost and QALY savings associated with reduced transmission and their consequent impact on cost-effectiveness diminished in these scenarios. In genotype 1 patients, depending on treatment uptake rate, ICERs were subject to relative reductions of 0–4% over 10 years and 11–28% over 20 years (compared to 36–76% over 50 years). Similarly, in genotype 3 patients, ICERs were subject to reductions of 0–4% over 10 years and 14–24% over 20 years (compared to 41–79% over 50 years).
Discussion
Curing patients with HCV benefits both the incident population infected with HCV and wider society by reducing the opportunity for infected individuals to transmit infection to others [24]. Conventional cost-effectiveness evaluations in HCV typically account only for the former (in terms of reduced progression to ESLD) and thus likely underestimate the cost-effectiveness of treatment, particularly amongst patient subgroups subject to high transmission risk, such as PWID. In people undertaking risky behavior, the value of cure is to some extent independent of the individual’s disease stage; unfortunately, the proportion of the prevalent HCV population who are active PWID is uncertain.
This study is the first attempt to quantify the impact of reduced HCV transmission on the cost-effectiveness of DAA regimens as a function of increased treatment uptake, applying daclatasvir plus sofosbuvir for treatment of the high-risk PWID population in a UK setting as an example. The presented data support previously published research demonstrating that the cost-effectiveness of DAA treatment improves with the inclusion of future transmission consequences in the PWID population (compared to ex/non-PWID) [32]. However, this analysis also demonstrated that improvements in estimated cost-effectiveness were directly related to increased treatment uptake, due to increased cost offsets and QALY gains associated with the avoidance of greater numbers of future chronic infections. Assuming an optimal treatment uptake (all patients receiving immediate therapy), base-case ICERs associated with daclatasvir + sofosbuvir were reduced by up to 79% compared to standard of care.
Several assessments of the cost-effectiveness of new DAA regimens have been undertaken, focusing solely on benefits for individual patients without assessment of potential downstream benefits of reduced transmission [13, 14] and often demonstrating that it is more cost-effective to treat those with severe liver disease [49, 50]. This conclusion was supported by a recent evaluation that incorporated the consequences of HCV transmission, which found that treatment of ex/non-PWID with mild HCV was not cost-effective in contrast to delaying treatment [32]. By contrast, in the PWID population, there were substantial benefits in most scenarios for treating patients with mild disease due to the onward disease transmission and prevention benefits [32]. This conclusion is supported by the evidence presented within this analysis, which has demonstrated that the benefits of HCV treatment associated with onward disease transmission can be maximized by improving treatment uptake in high-risk populations such as PWID.
The evidence presented here demonstrates that when a small treatment uptake is applied, there is limited impact on onward HCV transmission dynamics even within a high-risk population, such as PWID. Thus, the economic benefit of HCV treatment that could be realized in clinical practice is likely to be only slightly underestimated by exclusion of onward disease transmission from evaluations focused on individual patient-level benefit, such as those conducted by NICE. However, increasing treatment uptake improves the cost-effectiveness of DAAs, and hence value to the NHS would be maximized when treatment rates are improved.
Within the context of historical low treatment rates and reluctance to treat PWID [51, 52], an increase in treatment uptake may be plausible given the proven clinical benefits of new DAA regimens, including the improved tolerability and efficacy [8–11]. However, modeling the associated service implications and the affordability of treating more patients with antiviral therapies were beyond the scope of this analysis. Increased uptake of treatment would inevitably place additional financial pressure on existing healthcare budgets with respect to access and delivery of care to a larger number of PWID, in addition to considerable drug acquisition costs. Although the costs of treating PWID patients should be weighed against the benefits of reduced onward HCV transmission to society, these long-term benefits will not offset the financial burden of increased treatment uptake on healthcare budgets fully or in the short term.
One possible approach to address concerns around affordability would be to prioritize, or target, treatment among patients with HCV with highest unmet medical need. Such patients would include those at greatest risk of progressing to advanced liver disease, and most likely to transmit their infection to others [53]. The aim of targeted therapy in this context would be to address areas of high unmet medical need, while obtaining the maximum economic benefits (i.e., avoided infections) with the minimum expenditure (number of patients treated).
Although the relationship between cost-effectiveness and treatment uptake (and efficacy) may be generalized to other settings, this analysis evaluated a PWID population from a UK perspective, which is limited by the available epidemiological and behavioral data for the PWID population, with inputs derived from previous studies in the UK setting by Martin et al. [25, 29]. The analysis also focused on HCV genotype 1 and 3 only, since these genotypes have the highest prevalence within PWID [54]. As such, the generalizability of this research to other settings and patient groups should be considered within this context; indeed, previous research demonstrated that the cost-effectiveness of treatment strategies may differ in settings with a high HCV prevalence (>60%) among PWIDs [32].
In conclusion, using daclatasvir plus sofosbuvir for treatment of the high-risk PWID population in the UK setting to illustrate, this study is the first to demonstrate the relationship between the cost-effectiveness of HCV treatment and rate of treatment uptake. Cost-effectiveness of DAA treatment was shown to improve with increased treatment uptake, due to increased cost offsets and health gains associated with the avoidance of greater numbers of future chronic infections. Indeed, a reimbursement decision could be substantially influenced by incorporating the potential benefits of increased treatment uptake and the resulting reduced onward transmission. Further, if treatment uptake rates in high-risk populations could be increased, DAAs would represent better value for money in clinical practice. The challenge for the future will lie in achieving increased rates of treatment uptake among high-risk populations.
References
Shepard, C.W., Finelli, L., Alter, M.J.: Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5(9), 558–567 (2005)
Hatzakis, A., Chulanov, V., Gadano, A.C., et al.: The present and future disease burden of hepatitis C virus (HCV) infections with today’s treatment paradigm—volume 2. J. Viral Hepat. 22(Suppl 1), 26–45 (2015)
Public Health England, Health Protection Scotland, Public Health Wales, Public Health Agency: Hepatitis C in the UK: 2014 report. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/337115/HCV_in_the_UK_2014_24_July.pdf (2014). Accessed Jan 2015
Public Health England, Health Protection Scotland, Public Health Wales, Public Health Agency: Shooting up: infections among people who inject drugs in the United Kingdom 2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/370707/Shooting_Up_2014.pdf (2014). Accessed Jan 2015
Afdhal, N.H.: Hepatitis C viral infection in difficult-to-treat populations: an overview. Clin. Liver Dis. 1(3), 63–64 (2012)
Kemmer, N., Neff, G.W.: Managing chronic hepatitis C in the difficult-to-treat patient. Liver Int. 27(10), 1297–1310 (2007)
European Association for the Study of the Liver: EASL clinical practice guidelines: management of hepatitis C virus infection. J. Hepatol. 60(2), 392–420 (2014)
Sulkowski, M.S., Gardiner, D.F., Rodriguez-Torres, M., et al.: Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N. Engl. J. Med. 370(3), 211–221 (2014)
Lawitz, E., Mangia, A., Wyles, D., et al.: Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 368(20), 1878–1887 (2013)
Gilead Sciences International Ltd: Summary of product characteristics. Sovaldi® 400 mg film-coated tablets. Updated June 2014. http://www.medicines.org.uk/emc/medicine/28539/SPC/Sovaldi+400+mg+film+coated+tablets/ (2014). Accessed Feb 2015
Janssen-Cilag Ltd: Summary of product characteristics. OLYSIO® 150 mg hard capsules. https://www.medicines.org.uk/emc/medicine/28888 (2014). Accessed Feb 2015
Scottish Medicines Consortium: Daclatasvir 30 and 60 mg film-coated tablets (Daklinza®). SMC No. 1002/14. https://www.scottishmedicines.org.uk/SMC_Advice/Advice/1002_14_daclatasvir_Daklinza/daclatasvir_Daklinza (2014). Accessed Jan 2015
Hagan, L.M., Sulkowski, M.S., Schinazi, R.F.: Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology 60(1), 37–45 (2014)
Petta, S., Cabibbo, G., Enea, M., et al.: Cost-effectiveness of sofosbuvir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology 59(5), 1692–1705 (2014)
Gordon, S.C., Pockros, P.J., Terrault, N.A., et al.: Impact of disease severity on healthcare costs in patients with chronic hepatitis C (CHC) virus infection. Hepatology 56(5), 1651–1660 (2012)
Westbrook, R.H., Dusheiko, G.: Natural history of hepatitis C. J. Hepatol. 61(1), S58–S68 (2014)
National Institute for Health and Care Excellence: NICE technology appraisal guidance [TA331]. Simeprevir in combination with peginterferon alfa and ribavirin for treating genotypes 1 and 4 chronic hepatitis C. https://www.nice.org.uk/guidance/ta331 (2015). Accessed 16 Mar 2016
National Institute for Health and Care Excellence: NICE technology appraisal guidance [TA364]. Daclatasvir for treating chronic hepatitis C. https://www.nice.org.uk/guidance/ta364 (2015). Accessed 16 Mar 2016
National Institute for Health and Care Excellence: NICE technology appraisal guidance [TA330]. Sofosbuvir for treating chronic hepatitis C. https://www.nice.org.uk/guidance/ta330 (2015). Accessed 16 Mar 2016
National Institute for Health and Care Excellence: NICE technology appraisal guidance [TA363]. Ledipasvir–sofosbuvir for treating chronic hepatitis C. https://www.nice.org.uk/guidance/ta363 (2015). Accessed 16 Mar 2016
National Institute for Health and Care Excellence: NICE technology appraisal guidance [TA365]. Ombitasvir–paritaprevir–ritonavir with or without dasabuvir for treating chronic hepatitis C. https://www.nice.org.uk/guidance/ta365 (2015). Accessed 16 Mar 2016
National Institute for Health and Care Excellence: Single technology appraisal. Daclatasvir for treating chronic hepatitis C: final scope. http://www.nice.org.uk/guidance/gid-tag487/documents/hepatitis-c-chronic-daclatasvir-final-scope2 (2014). Accessed Feb 2015
National Institute for Health and Care Excellence: Single technology appraisal. Ledipasvir–sofosbuvir for treating chronic hepatitis C: final scope. http://www.nice.org.uk/guidance/gid-tag484/documents/hepatitis-c-chronic-ledipasvirsofosbuvir-final-scope2 (2014). Accessed Feb 2015
World Health Organization: Guidelines for the screening, care and treatment of persons with hepatitis C infection. http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/ (2014). Accessed Jan 2015
Martin, N.K., Vickerman, P., Foster, G.R., Hutchinson, S.J., Goldberg, D.J., Hickman, M.: Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J. Hepatol. 54(6), 1137–1144 (2011)
Harris, R.J., Thomas, B., Griffiths, J., et al.: Increased uptake and new therapies are needed to avert rising hepatitis C-related end stage liver disease in England: modelling the predicted impact of treatment under different scenarios. J. Hepatol. 61(3), 530–537 (2014)
Martin, N.K., Foster, G.R., Vilar, J., et al.: HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. J. Viral Hepat. 22(4), 399–408 (2015)
Cousien, A., Tran, V.C., Deuffic-Burban, S., Jauffret-Roustide, M., Dhersin, J.S., Yazdanpanah, Y.: Dynamic modelling of hepatitis C virus transmission among people who inject drugs: a methodological review. J. Viral Hepat. 22(3), 213–229 (2015)
Martin, N.K., Vickerman, P., Grebely, J., et al.: Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 58(5), 1598–1609 (2013)
Hellard, M.E., Jenkinson, R., Higgs, P., et al.: Modelling antiviral treatment to prevent hepatitis C infection among people who inject drugs in Victoria, Australia. Med. J. Aust. 196(10), 638–641 (2012)
Duberg, A.S., Blach, S., Falconer, K., Kaberg, M., Razavi, H., Aleman, S.: The future disease burden of hepatitis C virus infection in Sweden and the impact of different treatment strategies. Scand. J. Gastroenterol. 50(2), 233–244 (2015)
Martin, N.K., Vickerman, P., Dore, G.J., et al.: Prioiritization of HCV treatment in the direct-acting antiviral era: an economic evaluation. J. Hepatol. 65(1), 17–25 (2016)
Bennett, H., McEwan, P., Sugrue, D., Kalsekar, A., Yuan, Y.: Assessing the long-term impact of treating hepatitis C virus (HCV)-Infected people who inject drugs in the UK and the relationship between treatment uptake and efficacy on future infections. PLoS One 10(5), e0125846 (2015)
McEwan, P., Kim, R., Yuan, Y.: Assessing the cost utility of response-guided therapy in patients with chronic hepatitis C genotype 1 in the UK using the MONARCH model. Appl. Health Econ. Health Policy 11(1), 53–63 (2013)
McEwan, P., Ward, T., Chen, C.-J., et al.: Estimating the incidence and prevalence of chronic hepatitis C infection in Taiwan using back projection. Value Health Reg. Issues 3, 5–11 (2014)
McEwan, P., Ward, T., Yuan, Y., Kim, R., L’Italien, G.: The impact of timing and prioritization on the cost-effectiveness of birth cohort testing and treatment for hepatitis C virus in the United States. Hepatology 58(1), 54–64 (2013)
McEwan, P., Ward, T., Bennett, H., et al.: Estimating the clinical and economic benefit associated with incremental improvements in sustained virologic response in chronic hepatitis C. PLoS One 10(1), e0117334 (2015)
Thein, H.H., Yi, Q., Dore, G.J., Krahn, M.D.: Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 48(2), 418–431 (2008)
Kanwal, F., Kramer, J.R., Ilyas, J., Duan, Z., El-Serag, H.B.: HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of US Veterans with HCV. Hepatology 60(1), 98–105 (2014)
Townsend, R., McEwan, P., Kim, R., Yuan, Y.: Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health 14(8), 1068–1077 (2011)
Shepherd, J., Jones, J., Hartwell, D., Davidson, P., Price, A., Waugh, N.: Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol. Assess. 11(11), 1–205 (2007)
Pearlman, B.L., Traub, N.: Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin. Infect. Dis. 52(7), 889–900 (2011)
Akhtar, E., Manne, V., Saab, S.: Cirrhosis regression in hepatitis C patients with sustained virological response after antiviral therapy: a meta-analysis. Liver. Int. 35(1), 30–36 (2014)
Im, G.Y., Dieterich, D.T.: Direct-acting antiviral agents in patients with hepatitis C cirrhosis. Gastroenterol. Hepatol. (N. Y.) 8(11), 727–765 (2012)
Personal Social Services Research Unit: Unit costs of Health and Social Care 2013. http://www.pssru.ac.uk/project-pages/unit-costs/2013/ (2013). Accessed Oct 2015
Public Health England: Commissioning template for estimating HCV prevalence by DAT and numbers eligible for treatment. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/HepatitisC/EpidemiologicalData/ (2014). Accessed Feb 2015
Public Health England, Health Protection Scotland, Public Health Wales, Public Health Agency: Hepatitis C in the UK: 2013 report. http://www.hpa.org.uk/Publications/InfectiousDiseases/BloodBorneInfections/HepatitisCInTheUK/1307HepatitisCintheUK2013report/ (2013). Accessed Feb 2015
National Institute for Health and Care Excellence: Guide to the methods of technology appraisal 2013. http://www.nice.org.uk/article/pmg9 (2013). Accessed Feb 2015
Leidner, A.J., Chesson, H.W., Xu, F., Ward, J.W., Spradling, P.R., Holmberg, S.D.: Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology 61(6), 1860–1869 (2015)
Leleu, H., Blachier, M., Rosa, I.: Cost-effectiveness of sofosbuvir in the treatment of patients with hepatitis C. J. Viral Hepat. 22(4), 376–383 (2015)
The All-Party Parliamentary Hepatology Group: In the dark: an audit of hospital hepatitis C services across England. http://www.appghep.org.uk/wp-content/uploads/2014/02/In-The-Dark-An-audit-of-hospital-hepatitis-C-services-across-England-APPHG-2010.pdf (2010). Accessed Feb 2015
Arain, A., Robaeys, G.: Eligibility of persons who inject drugs for treatment of hepatitis C virus infection. World J. Gastroenterol. 20(36), 12722–12733 (2014)
Innes, H., Goldberg, D., Dillon, J., Hutchinson, S.J.: Strategies for the treatment of hepatitis C in an era of interferon-free therapies: what public health outcomes do we value most? Gut. 64(11), 1800–1809 (2014)
Hope, V.D., Hickman, M., Ngui, S.L., et al.: Measuring the incidence, prevalence and genetic relatedness of hepatitis C infections among a community recruited sample of injecting drug users, using dried blood spots. J Viral Hepat. 18(4), 262–270 (2011)
Sweeting, M.J., Hope, V.D., Hickman, M., et al.: Hepatitis C infection among injecting drug users in England and Wales (1992–2006): there and back again? Am. J. Epidemiol. 170(3), 352–360 (2009)
Kimber, J., Copeland, L., Hickman, M., et al.: Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. BMJ 341, c3172 (2010)
Hickman, M., Hope, V., Coleman, B., et al.: Assessing IDU prevalence and health consequences (HCV, overdose and drug-related mortality) in a primary care trust: implications for public health action. J. Public Health (Oxf.) 31(3), 374–382 (2009)
Cornish, R., Macleod, J., Strang, J., Vickerman, P., Hickman, M.: Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ 341, c5475 (2010)
Health Protection Scotland and University of the West of Scotland: Needle Exchange Surveillance Initiative (NESI): Prevalence of HCV, HIV and injecting risk behaviours among injecting drug users attending needle exchanges in Scotland, 2007. http://www.hps.scot.nhs.uk/search/atozdetail.aspx?source=8&subject=93 (2008). Accessed Feb 2015
Allen, E.J., Palmateer, N.E., Hutchinson, S.J., Cameron, S., Goldberg, D.J., Taylor, A.: Association between harm reduction intervention uptake and recent hepatitis C infection among people who inject drugs attending sites that provide sterile injecting equipment in Scotland. Int. J. Drug Policy 23(5), 346–352 (2012)
University of the West of Scotland: The Needle Exchange Surveillance Initiative (NESI): Prevalence of HCV and injecting risk behaviours among people who inject drugs attending injecting provision services in Scotland, 2008/2009 and 2010. http://www.documents.hps.scot.nhs.uk/bbvsti/hepatitis-c/publications/nesi-needle-exchange.pdf (2011). Accessed Feb 2015
Vickerman, P., Martin, N., Turner, K., Hickman, M.: Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction 107(11), 1984–1995 (2012)
Kemp, P.A., Neale, J., Robertson, M.: Homelessness among problem drug users: prevalence, risk factors and trigger events. Health Soc. Care Community 14(4), 319–328 (2006)
Innes, H.A., Hutchinson, S.J., Allen, S., et al.: Ranking predictors of a sustained viral response for patients with chronic hepatitis C treated with pegylated interferon and ribavirin in Scotland. Eur. J. Gastroenterol. Hepatol. 24(6), 646–655 (2012)
Micallef, J.M., Kaldor, J.M., Dore, G.J.: Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J. Viral Hepat. 13(1), 34–41 (2006)
Mondelli, M.U., Cerino, A., Cividini, A.: Acute hepatitis C: diagnosis and management. J. Hepatol. 42(1), S108–S114 (2005)
Turner, K.M.E., Hutchinson, S., Vickerman, P., et al.: The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 106(11), 1978–1988 (2011)
Aspinall, E.J., Corson, S., Doyle, J.S., et al.: Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin. Infect. Dis. 57(suppl 2), S80–S89 (2013)
Jacobson, I.M., McHutchison, J.G., Dusheiko, G., et al.: Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364(25), 2405–2416 (2011)
Poordad, F., McCone Jr., J., Bacon, B.R., et al.: Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 364(13), 1195–1206 (2011)
National Institute for Health and Care Excellence: Technology appraisal TA106: peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C. http://guidance.nice.org.uk/TA106 (2006). Accessed Feb 2015
Wright, M., Grieve, R., Roberts, J., Main, J., Thomas, H.: Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health. Technol. Assess. 10(21), 1–113, iii (2006)
Grieve, R., Roberts, J., Wright, M., et al.: Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut 55(9), 1332–1338 (2006)
Martin, N.K., Vickerman, P., Miners, A., et al.: Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology 55(1), 49–57 (2012)
National Institute for Health and Care Excellence: Technology appraisal 252. Telaprevir for the treatment of genotype 1 chronic hepatitis C. Updated June 2014. http://www.nice.org.uk/ta252 (2012). Accessed Feb 2015
Personal Social Services Research Unit: Unit Costs of Health and Social Care. http://www.pssru.ac.uk/project-pages/unit-costs/2014/index.php (2014). Accessed Mar 2015
Haymarket Media Group Ltd: Monthly Index of Medical Specialities (MIMS). http://www.mims.co.uk/ (2015) Accessed Feb 2015
Bristol-Myers Squibb Pharmaceuticals Ltd: Final clinical study report AI444040 [data on file]. (2013)
Hadziyannis, S.J., Sette Jr., H., Morgan, T.R., et al.: Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140(5), 346–355 (2004)
Nelson, D.R., Cooper, J.N., Lalezari, J.P., et al.: All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 61(4), 1127–1135 (2015)
Acknowledgements
This study was funded by an unrestricted grant from Bristol-Myers Squibb Pharmaceuticals Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bennett, H., Gordon, J., Jones, B. et al. Hepatitis C disease transmission and treatment uptake: impact on the cost-effectiveness of new direct-acting antiviral therapies. Eur J Health Econ 18, 1001–1011 (2017). https://doi.org/10.1007/s10198-016-0844-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-016-0844-8