Abstract
Mirror tests have been used to test aggressiveness because they reduce the variability of the real opponent and allows for repeated measurements. Regular mirrors have also several disadvantages due to the inability to display a head-to-tail position during lateralization. Recently, a method using a non-reversing mirror was developed, which eliminated this disadvantage, but was tested in a single species only. The present study reflects the need for validating the non-reversing mirror test in other species, represented by chub (Squalius cephalus L.) in the present study, due to species-specific manifestations of aggressiveness. The prediction that a non-reversing mirror would lead to more aggressive interactions than a regular mirror in chub was confirmed. The durations of aggressive interactions were longer in fish exposed to the regular mirror test with specific significance in case of the frontal and the lateral displays. The individuals maintained the same level of aggressiveness throughout both tests, suggesting that both tests are valid for testing the aggressiveness. Nevertheless, due to higher number of interactions performed, a non-reversing mirror may offer a stronger stimulus and could represent a real contest better than the regular mirror. Therefore, the non-reversing mirror test should be recommended for use in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aggressiveness is one of the five behavioural axes with serious consequences for an individual’s reproductive success (Ariyomo and Watt 2012). Wild populations usually contain members with either significantly higher or lower levels of aggressiveness, forming active and passive behavioural strategies (Benus 1988). Laboratory tests have found that individuals with higher aggressiveness up to a certain limit have better mating success (Sih et al. 2014). However, an animal can spend only a specific amount of energy on aggressiveness and can withstand only a certain amount of injury (Briffa and Sneddon 2007; Payne 1998). Therefore, the individuals have to balance benefits and costs of their aggressiveness level to gain the most from specific situations.
The information obtained from behavioural experiment is significantly shaped by the method used (Earley et al. 2000). Several standardized tests of aggressiveness are available with the common aim to represent the conditions of real environment. If we omit the model tests or the computer-simulated stimuli (Clement et al. 2005; Earley et al. 2000; Verbeek et al. 2007), which provide a synthetic opponent with related disadvantages (Wackermannova et al. 2017), the two most frequently used tests remain. The first most commonly used test is the standard opponent test with or without a transparent barrier (Arnott et al. 2016; Budaev et al. 1999; Ruiz-Gomez and Huntingford 2012). Overall, the disadvantage of the standard opponent test is the size-mismatched opponent causing variability in aggression performed by the focal subject (Rincón and Grossman 2001). The standard opponent tests without a barrier is assumed ideal for gathering information about the types of aggressive behaviour of the species with full visual and chemical cues present in the environment, which significantly alters the behaviour (Coppock et al. 2016) but is not favoured because of the risk of injury. The second option is to use the mirror image test, which is used for animals unable to distinguish themselves in the mirror (Desjardins and Fernald 2010). The self-recognition is usually tested with mark test, which fish usually fail to pass, because they react towards its image in the same manner as towards a conspecific (Hotta et al. 2018). However, if the fish is presented to mirror over several days, its behaviour may change, which could indicate self-recognition (Kohda et al. 2019). Therefore, the mirror should not be presented to a fish over a longer period of time, if the aggressiveness towards the conspecific is the aim of the test. The mirror opponent test is independent of the characteristics of the opponent (Balzarini et al. 2014) and is safe for the tested animal (Neat et al. 1998), which has led to the vast usage of this method (Campbell et al. 2015; Chang et al. 2012; Church and Grant 2019). However, the mirror opponent test also faces criticism mainly due to inconsistent responses towards a mirror image when compared to a real opponent. Specifically, the hormonal response represented by the androgen levels have been shown not to correspond (Oliveira et al. 2005). The brain gene expression was more similar to noninteracting fish (Oliveira et al. 2016) and heightened in areas tied to fear in case of mirror image (Desjardins and Fernald 2010; Li et al. 2018b). Consequently, the mirror opponent test aggressiveness levels may not correlate to standard opponent test levels in some species (Balzarini et al. 2014) and the number of aggressive interactions or their duration may differ (Elwood et al. 2014; Hubená et al. 2020).
The differences in responses towards mirrors and real opponents are thought to relate to lateralization as one of the most important ways of communicating with the opponent (Arnott et al. 2011). During the regular mirror image test, the fish can see only the head-to-head lateral position, even though the fish aims to achieve the head-to-tail position (Arnott et al. 2011). An important innovation was introduced by Li et al. (2018a), who exposed the mangrove rivulus [Kryptolebias marmoratus (Poey, 1880)] to mirrors fixed in 90° angle, which reverses the image in the mirror and thus a head-to-tail stance of the mirror image was achieved. The study of Li et al. (2018a) compared the regular and non-reversing mirror test aggressive results together with the model tests results. Later, the real opponent test was performed on the same individuals to find which mirror test predicted the real contest better (Li et al. 2018a). The number of attacks in non-reversing mirror test predicted the contest outcome unlike the results from the regular mirror test (Li et al. 2018a). Therefore, it is assumed the non-reversing mirror predicts better the individual performance during real fights, because as they drove similar behavioural and neurobiological responses as the real opponent (Li et al. 2018b).
The aim of the present study was to validate the effectiveness of the non-reversing mirror compared to the mirror image test to study the aggressiveness. The performance of a non-reversing mirror has been tested only in a single species, the mangrove rivulus (Li et al. 2018a). The chub (Squalius cephalus), one of the most common riverine species in Europe, was chosen as a laboratory animal in the present study. The mangrove rivulus used in the study of Li et al. (2018a) may generally react more to the mirror tests than to standard opponent (Earley et al. 2000) and is lateralized (Li et al. 2018a). The chub on the other hand offers a good comparison for its non-lateralized performance and lower response to the regular mirror test than to the standard opponent, even though their aggressiveness level is stable across situations (Hubená et al. 2020). We predicted that the non-reversing mirror would lead to more aggressive interactions than a regular mirror in the present study, as was observed by Li et al. (2018a). The prediction was based on the fact that the head-to-tail stance in the non-reversing mirror is preferred by fish (Arnott et al. 2011; Li et al. 2018a) and the hormonal response is similar to the response to real contests (Li et al. 2018b).
Materials and methods
Model animal
The chub (Squalius cephalus) abundantly occupies almost all riverine zones in Europe (Kottelat and Freyhof 2007). The fish devour plants and animals located in their home range during their daily feeding migrations; thus, they are omnivorous (Krywult et al. 2008). The chub exhibits clear aggressive behaviour in mirror and standard opponent tests, including frontal display, lateral display, up and down swimming and circling (Hubená et al. 2020). This study required twelve juvenile chubs that were purchased from Czech Fishery Ltd., Czech Republic, at the age of 0+, as an F1 generation of wild individuals. The number of chubs used reflects the Reduction in the number of animals that can be used in laboratory studies.
Laboratory conditions
One month prior to the experiment, the chubs were transported to the laboratory and randomly divided into two tanks. The tanks contained 100 L of water with no gravel nor vegetation. During the acclimatization time, the fish were kept under standard conditions, the same as those to which they were accustomed in the hatchery. The lighting conditions were held at a regime of 12 h of day and 12 h of night. The water temperature was controlled automatically using external air conditioning and maintained at an average of 20 °C. The water was refined using biological filters with an integrated UV sterilizer (Pressure-Flo 5000, Rolf C. Hagen Inc., www.lagunaponds.com). The chubs were fed ad libitum diet of commercial pellet food (https://www.krmivahulin.cz) once a day. No health irregularities (i.e., abnormal swimming or bites) or mortality was recorded during daily controls.
All the fish were tagged with passive integrated transponder antennae 14 days prior to the start of the experiment to facilitate individual recognition. The fish were anaesthetized with 2-phenoxy-ethanol (0.2 ml/L; Merck KGaA, Germany), measured (standard length LS; mean 55 mm, range 49–65 mm) and weighed (mean 2.78 g, range 1.93–4.56 g). Passive integrated transponders (PITs; Trovan ID100, 0.1 g in air, 12 × 2.1 mm; EID Aalten B.V., Aalten, the Netherlands) were then inserted into the abdominal cavity using a hypodermic needle attached to a syringe according to Horká et al. (2019). The length measurements were gained using a U-shaped rectangular container with straight shorter sides. The ruler to measure the fish was attached to the bottom of the container, starting from one of the straight short sides. The U-shaped container allowed to be filled with water so the fish could breathe during the procedure.
Experimental procedure
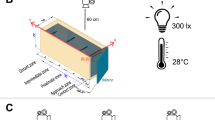
The following experiment was conducted according to the local valid law (Act No. 246/1992, §19, art 1, letter c) and with the relevant permission from the Ministry of Education, Youth and Sports of the Czech Republic (permit no. MSMT-1972/2016-5). The Departmental Expert Committee superintended the animal welfare. All twelve chubs were subjected to both aggression tests. One test was conducted using a regular mirror, and the other test was conducted using a non-reversing (90°) mirror according to the procedure outlined by Li et al. (2018a). The other details of both tests noted below were identical. One-half of the experimental fish (six individuals in total; three individuals from each tank) was subjected to the regular mirror first, while the other half (six individuals in total; three individuals from each tank) was subjected to the non-reversing mirror first to control for potential effect due to order. A one-hour recovery period, as observed by Li et al. (2018a), was preserved between the tests. During the tests, the fish were separately placed into clear experimental aquarium (footprint 30 × 15 cm, depth 20 cm). One of the longer sides of the experimental tank was covered with a grey removable barrier (RGB: 238, 238, 238) with a mirror behind it (either a regular or non-reversing mirror, depending on the test). The grey cover was also placed on the two shorter sides of the tank and the second longer side of the tank was left open for the view of the digital camera (GoPro HD HERO; GoPro Inc.). The non-reversing mirror was crafted using two same-sized mirrors glued in 90° angle (see Li et al. 2018a). After one minute of acclimatization to the environment, the barrier was removed. A contest of focal fish with the mirror was recorded using a digital camera for six minutes, which was proved to be a suitable length of recording for the evaluation of aggressiveness in chub, unless a contest outcome is required (Hubená et al. 2020).
Data analyses
The ‘types of aggressive behaviours’ were evaluated based on the study of Hubená et al. (2020), where the basic chubs’ ethogram was provided. Therefore, the types of aggressive behaviours were defined as ‘frontal display’, ‘circling’, ‘left lateral display’, ‘right lateral display’ and ‘up and down swimming’ (Fig. 1). The frontal display included various head-on activities targeted against the opponent, such as biting, ramming or mouth-wrestling. The behaviours were merged under this one label, because the camera viewed these activities from behind of the fish; therefore, there was a high risk of misinterpreting of the behaviours. Circling and up and down swimming were influenced by the barrier between the fish and their image. Circling was defined as very quick switches of the sides of body presented to the mirror image. Up and down swimming was viewed as fish swimming vertically on the glass wall in front of the image. Lateral displays have been labelled as presentations of the body of the focal fish to their image, usually with fins raised. The behaviours were evaluated as aggressive only when they were visually targeted against the mirror image. The distance to the mirror was not evaluated because of the relatively small size of the aquarium. However, the size of the testing tanks was the same as used in the previous study on chub’s aggressive ethogram (Hubená et al. 2020). The ‘number of aggressive interactions’ and their ‘duration’ were defined on the individual level for both tests, distinguishing among ‘types of aggressive behaviours’, using the ethological software Boris (https://www.boris.unito.it) and the recorded video files. The ‘test’ variable distinguished between the regular and non-reversing mirror tests.
Illustrative schema of detected types of aggressive behaviours affected or unaffected by the mirror type. The frontal display (a; various head-on activities targeted against the mirror image), the up and down swimming (b; swimming vertically on the glass wall targeted towards the image), the lateral displays (c; presentations of the body sides of fish to their image) and the circling (d; very quick switches of the sides of body presented to the mirror image). The quick switching of the body sides in circling could not be accounted for as the lateral display, because the purpose of circling seemed not to be the comparison of body characteristics to the opponent
Statistical analyses
The statistical analyses were performed in SAS (SAS Institute Inc., version 9.4, https://www.sas.com). Mixed models with random factors were used to account for the repeated measures collected for the same experimental units (individual fish) across the duration of the experiment. PROC GLIMMIX for a Poisson distribution was used to analyse the number of aggressive interactions, while PROC MIXED for a normal distribution was used to analyse the duration of aggressive behaviours. Duration was log-transformed to meet normality requirements prior to the analyses. The significance of each explanatory variable (‘test’ and ‘type of aggressive interactions’) and all possible two-way interactions were assessed using F tests in both of the procedures. The fixed effects and their interactions that were not statistically significant were excluded from the final model. Least-squares means (henceforth referred to and in bar charts presented as ‘adjusted means’ of model predictions) were subsequently computed for the particular classes of class variables. The differences between classes were tested with t tests, and Tukey–Kramer adjustment was used for multiple comparisons. Association between the dependent variable and other continuous variable was estimated by fitting a random factor model as described by Tao et al. (2002). With this random coefficient model, we calculated the predicted values for the dependent variable and plotted them against the continuous variable by using the predicted regression lines in particular scatter charts. The degrees of freedom were calculated using the Kenward–Roger method (Kenward and Roger 1997).
Spearman’s correlation coefficient was used to perform additional analyses of relationship between the duration of particular aggressive behaviour and the time when it started during the course of the particular mirror test. Altogether, ten coefficients (one per particular aggressive behaviour and test; Table 1) were provided.
Results
In total, 991 aggressive interactions (individual variability 0–257 interactions during the particular test) that lasted for 845 s were documented during all the tests. The use of the non-reversing mirror resulted in an overall higher number of aggressive interactions than the regular mirror (F1,118 = 197.19, P < 0.0001; Fig. 2a; adj. P < 0.0001), but the number of performed types of aggressive behaviour between the tests did not differ. The durations of the aggressive interactions were longer when fish were exposed to the regular mirror than to the non-reversing mirror (F1,986 = 31.08, P < 0.0001; Fig. 2b; adj. P < 0.0001). An analogous trend was observed for type of aggressive interactions (F8,982 = 18.42, P < 0.0001; Fig. 3). Frontal displays, left lateral displays and right lateral displays lasted longer in the case of the regular mirror (adj. P < 0.0001), but no difference was found between the tests used for up and down swimming and circling (adj. P > 0.9). The later the up and down swimming started across the course of both tests (the regular as well as the non-reversing mirror), the longer it lasted (Table 1). The later the frontal display started, the longer it lasted, but only in case of the regular mirror test (Table 1). The duration of other aggressive interactions was not dependent on the time it started across the course of both tests (Table 1). There was a positive relationship between the number of aggressive interactions (F1,6.973 = 8.17, P < 0.0245; Fig. 4a) as well as their durations (F1,12 = 9.71, P < 0.0089; Fig. 4b) in particular tests.
Relationship between the number of aggressive interactions (a; y = 0.1686 + 0.0163x; r2 = 0.54) and their durations (b; y = 0.9542 + 0.6021x; r2 = 0.5) during the mirror tests using a regular mirror and a non-reversing mirror. The predicted values are from the PROC GLIMMIX (a) and PROC MIXED (b) analyses
Discussion
The non-reversing mirror test aroused more aggressive interactions with shorter durations than the regular mirror test in the present study. The increased number of aggressiveness was also achieved for the standard opponent test, when compared with the mirror test (Hubená et al. 2020). Therefore, the non-reversing mirror could be offered as a substituent for the standard opponent test in chub. The only difference between the two mirror tests is the reversed image of the opponent. Arnott et al. (2011) predicted that 66.2% of lateralized displays of convict cichlids [Amatitlania nigrofasciata (Günther, 1867)] are head-to-tail stances and only 33.8% are head-to-head stances. The head-to-head stance is not favoured because of the high risk of injury, such as jaw dislocation (Arnott et al. 2011). Thus, the regular mirror image offers an opponent, where the cost of fighting is too high for some individuals. However, Arnott et al. (2016) also found that Siamese fighting fish [Betta splendens (Regan, 1910)] are probably not as affected by the difference in laterality of the mirror image as by the inexhaustible motivation of the mirror opponent. Earley et al. (2000) found the same motivation-driven aggressiveness in mangrove rivulus, which is the only species in which the non-reversing mirror test was validated (Li et al. 2018a). We suggest that the behaviour of chubs is more similar to that of cichlid species, whose fighting vigour depends on specific aggressive interactions of the opponent. This was proven in chub for frontal display and up and down swimming, because the fish tended to respond to the opponent with the same behaviour (Hubená et al. 2020). Furthermore, on the basis of the differences in aggressiveness we could assume that the anti-parallel swimming (head-to-tail) are more common in chub, just like in cichlids (Arnott et al. 2011). Consequently, if the opponent does not behave according to the expectations of the fish, the fish express less aggressive interactions and prolong the display durations to synchronize with their mirror image. In other words, the contest does not escalate as naturally as in the non-reversing mirror in the present study or further in the standard opponent test in Hubená et al. (2020).
As the non-reversing mirror test was tested in only a single species, we gather more information from studies comparing the standard opponent and regular mirror test. The non-reversing mirror test is assumed to represent the real contest better (Li et al. 2018a, b), even though the only difference from the regular mirror test is the opposite display behaviour of the mirror image. Most of the tested species {Siamese fighting fish, zebrafish [Danio rerio (Hamilton, 1822)], and Astatotilapia burtoni (Günther, 1894)} did not express any difference in the total aggression towards the mirror or the opponent (Ariyomo and Watt 2013; Arnott et al. 2016; Desjardins and Fernald 2010). In case of the mangrove rivulus, the mirror image is a stronger stimulus than the real opponent (Earley et al. 2000). In the same species, the non-reversing mirror test triggered significantly more aggressiveness than the regular mirror test (Li et al. 2018a). The difference could be also caused by the distance between the opponents (Cattelan et al. 2017), which is lower in case of the regular than the non-reversing mirror test. However, in Hubená et al. (2020) the real opponent was as close as the regular mirror and the increase in aggressiveness of chub during the real opponent test was confirmed. This may indicate that more realistic stimulus elicits more aggressive interactions. Therefore, we believe that the non-reversed mirror image is a stronger and more realistic stimulus for chub than the regular mirror test because of the increase in aggressiveness. Due to the found significant differences between the non-reversing and regular mirror tests, the first assumption was that the actions of the image in the regular mirror test might have induced stress in the focal fish. When fish are exposed to a stressful situation, including an encounter, the physiological response of an increase in cortisol and serotonin follows (Silva et al. 2015; Summers and Winberg 2006). For subordinates, this response might cause behavioural inhibition in rainbow trout, resulting in lower aggressiveness (Øverli et al. 2004). When Nile tilapia (Oreochromis niloticus L.) were exposed to a stressful situation, a different brain activity was observed in the dorsolateral and dorsomedial telencephalon, which represent the hippocampus and amygdala and are known to process fear (Desjardins and Fernald 2010; Silva et al. 2015). Additionally, when Nile tilapia were exposed to a mirror, these brain centres were activated more than when exposed to the real opponent (Desjardins and Fernald 2010). Thus, a fish exposed to the regular mirror test might fear its image. Alternatively, the chub could have been frustrated, because the individuals could not elicit a correct head-to-tail stance with the images in the regular mirror test. We cannot reject the theory that frustration could have caused stress or fear in the fish, which was discussed before. However, we assume that the fish in the regular mirror test could suffer higher stress, because chub relieves stress through aggressiveness (Hubená et al. 2020). Therefore, the higher expression of aggressive behaviours in case of non-reversing mirror could also potentially relieve the stress of the chub in the present study and explain the differences.
The regular mirror promoted a longer duration of frontal and lateral displays. The frontal display in the regular mirror test lasted longer, if the behaviour started later in the contest. Perhaps, the longer duration of frontal display by the end of contest was also a result of the inability of fish to reach head-to-tail stance. The frontal display in the present study is one of the most aggressive behaviours, whereas lateral displays are low-risk interactions (Hsu and Wolf 2001; Neat et al. 1998; Noleto-Filho et al. 2017). The lateral display difference has been discussed above. The frontal display is usually expressed more often by winners because of its high energy cost (Earley et al. 2000; Hsu and Wolf 2001; Neat et al. 1998). Elwood et al. (2014) suggested two overall theories regarding how aggressive interactions and their durations would be modified between the regular mirrors and the standard opponent tests. The first theory suggested that a regular mirror test would cause an increase in the number of lateral displays but a decrease in their duration as a cause of switching between sides (Elwood et al. 2014). As the behaviour is specie-dependent, Li et al. (2018a) found a higher number of switches between sides of the lateral display in mangrove rivulus in case of the regular mirror. However, if we adopt the suggestion of Li et al. (2018a) that the non-reversing mirror represents more real contest, then this theory does not match the results in the present study similarly as the real contest compared to the regular mirror test in the study of Elwood et al. (2014). This theory was also rejected in the study of Elwood et al. (2014) on the basis of their results from convict cichlids (Amatitlania nigrofasciata). The second theory suggested that the regular mirror test would cause a decrease in the number of attacks lasting a longer time, because the fish would wait for their opponent to make a move (Elwood et al. 2014). This hypothesis matches the behaviour of convict cichlids better (Elwood et al. 2014) and the behaviour of chub in case of the regular mirror compared to the non-reversing mirror in the present study. Therefore, the non-reversing mirror test offers a more naturally behaving opponent, indicated by the shorter duration of specific aggressive behaviours.
The comparison of the data of individual aggressiveness has suggested that aggressiveness is stable across the two distinct testing methods. It is thus plausible to assume that the regular and non-reversing mirror tests measure the same personality trait. In both tests the fish performed longer up and down swimming, the longer the behaviour started. Overall, the studies of Ariyomo and Watt (2013), Balzarini et al. (2014) and Arnott et al. (2016) noted that the levels of aggression were also consistent between the regular mirror and the standard opponent test in zebrafish, Neolamprologus pulcher (Trewavas & Poll, 1952) and Siamese fighting fish; therefore, the regular mirror test may be employed as an index of aggressiveness in real fights in these species. In some cases, the mirror test did not correlate with the standard opponent test [Telmatochromis vittatus (Boulenger, 1898); Lepidiolamprologus elongatus (Boulenger, 1898); Balzarini et al. 2014], suggesting either self-recognition of the fish in the mirror or inadequate responses of the mirror image. Various fish fail the self-recognition test (usually a mark test), especially if the exposure time to the mirror is short (Ariyomo and Watt 2013; Hotta et al. 2018); therefore, the first option is diminished. Both mirror aggressiveness tests offer size- and motivation-matched opponents with a known set of behavioural repertoires towards the focal individual, because the mirror image cannot behave differently than the focal fish (Arnott et al. 2016). The data suggest that both mirror tests may be used to measure the aggressiveness trait in chubs with similar comparable results; however, a non-reversing mirror should be preferred due to the reasons discussed above. The mirror image tests could be further improved by addition of urine signals of conspecifics (Bayani et al. 2017). Because the contest between two individuals is thought to defend a resource such as food or hideout (Silva et al. 2013), then addition of this resource to the experimental aquarium may increase the aggressiveness of the fish.
References
Ariyomo TO, Watt PJ (2012) The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim Behav 83:41–46
Ariyomo TO, Watt PJ (2013) Aggression and sex differences in lateralization in the zebrafish. Anim Behav 86:617–622
Arnott G, Ashton C, Elwood RW (2011) Lateralization of lateral displays in convict cichlids. Biol Lett 7:683–685
Arnott G, Beattie E, Elwood RW (2016) To breathe or fight? Siamese fighting fish differ when facing a real opponent or mirror image. Behav Proc 129:11–17
Balzarini V, Taborsky M, Wanner S, Koch F, Frommen JG (2014) Mirror, mirror on the wall: the predictive value of mirror tests for measuring aggression in fish. Behav Ecol Sociobiol 68:871–878
Bayani DM, Taborsky M, Frommen JG (2017) To pee or not to pee: urine signals mediate aggressive interactions in the cooperatively breeding cichlid Neolamprologus pulcher. Behav Ecol Sociobiol. https://doi.org/10.1007/s00265-016-2260-6
Benus RF (1988) Differences in behavioural strategies between aggressive and non-aggressive male mice. Aggression and coping. University of Groningen, Groningen
Briffa M, Sneddon LU (2007) Physiological constraints on contest behaviour. Funct Ecol 21:627–637
Budaev SV, Zworykin DD, Mochek AD (1999) Consistency of individual differences in behaviour of the lion-headed cichlid, Steatocranus casuarius. Behav Proc 48:49–55
Campbell JM, Carter PA, Wheeler PA, Thorgaard GH (2015) Aggressive behavior, brain size and domestication in clonal rainbow trout lines. Behav Genet 45:245–254
Cattelan S, Lucon-Xiccato T, Pilastro A, Griggio M (2017) Is the mirror test a valid measure of fish sociability? Anim Behav 127:109–116
Chang C, Li CY, Earley RL, Hsu Y (2012) Aggression and related behavioral traits: the impact of winning and losing and the role of hormones. Integr Comp Biol 52:801–813
Church KD, Grant JW (2019) Ideal despotic distributions in convict cichlids (Amatitlania nigrofasciata)? Effects of predation risk and personality on habitat preference. Behav Proc 158:163–171
Clement TS, Parikh V, Schrumpf M, Fernald RD (2005) Behavioral coping strategies in a cichlid fish: the role of social status and acute stress response in direct and displaced aggression. Horm Behav 47:336–342
Coppock AG, Gardiner NM, Jones GP (2016) Sniffing out the competition? Juvenile coral reef damselfishes use chemical cues to distinguish the presence of conspecific and heterospecific aggregations. Behav Proc 125:43–50
Desjardins JK, Fernald RD (2010) What do fish make of mirror images? Biol Lett 6:744–747
Earley RL, Hsu Y, Wolf LL (2000) The use of standard aggression testing methods to predict combat behaviour and contest outcome in Rivulus marmoratus dyads (Teleostei: Cyprinodontidae). Ethology 106:743–761
Elwood RW, Stoilova V, McDonnell A, Earley RL, Arnott G (2014) Do mirrors reflect reality in agonistic encounters? A test of mutual cooperation in displays. Anim Behav 97:63–67
Horká P, Horký P, Slavík O (2019) Effect of implanting a passive integrated transponder tag in juvenile chub, Squalius cephalus (L.), on their condition, growth and survival. Eur J Environ Sci 9:102–105
Hotta T, Komiyama S, Kohda M (2018) A social cichlid fish failed to pass the mark test. Anim Cogn 21:127–136
Hsu Y, Wolf LL (2001) The winner and loser effect: what fighting behaviours are influenced? Anim Behav 61:777–786
Hubená P, Horký P, Slavík O (2020) Test-dependent expression of behavioral syndromes: a study of aggressiveness, activity and stress of chub. Aggress Behav 46:412–424
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997
Kohda M, Hotta T, Takeyama T, Awata S, Tanaka H, Asai JY, Jordan AL (2019) If a fish can pass the mark test, what are the implications for consciousness and self-awareness testing in animals? PLOS Biol 17:e3000021
Kottelat M, Freyhof J (2007) Handbook of European freshwater fishes. Kottelat and Freyhof, Cornol and Berlin
Krywult M, Klich M, Szarek-Gwiazda E (2008) Metal concentrations in chub, Leuciscus cephalus, from a submontane river. Acta Ichthyol Piscat 38:47–53
Li CY, Curtis C, Earley RL (2018) Nonreversing mirrors elicit behaviour that more accurately predicts performance against live opponents. Anim Behav 137:95–105
Li CY, Hofman HA, Harris ML, Earley RL (2018) Real or fake? Natural and artificial social stimuli elicit divergent behavioural and neural responses in mangrove rivulus, Kryptolebias marmoratus. Proc R Soc B 285:20181610
Neat FC, Taylor AC, Huntingford FA (1998) Proximate costs of fighting in male cichlid fish: the role of injuries and energy metabolism. Anim Behav 55:875–882
Noleto-Filho EM, dos Santos Gauy AC, Pennino MG, Gonçalves-de-Freitas E (2017) Bayesian analysis improves experimental studies about temporal patterning of aggression in fish. Behav Proc 145:18–26
Oliveira RF, Carneiro LA, Canário AV (2005) No hormonal response in tied fights. Nature 437:207–208
Oliveira RF, Simões JM, Teles MC, Oliveira CR, Becker JD, Lopes JS (2016) Assessment of fight outcome is needed to activate socially driven transcriptional changes in the zebrafish brain. Proc Natl Acad Sci 113:654–661
Øverli Ø, Korzan WJ, Höglund E, Winberg S, Bollig H, Watt M, Forster GL, Barton BA, Øverli E, Renner KJ, Summers CH (2004) Stress coping style predicts aggression and social dominance in rainbow trout. Horm Behav 45:235–241
Payne RJ (1998) Gradually escalating fights and displays: the cumulative assessment model. Anim Behav 56:651–662
Rincón PA, Grossman GD (2001) Intraspecific aggression in rosyside dace, a drift-feeding stream cyprinid. J Fish Biol 59:968–986
Ruiz-Gomez ML, Huntingford FA (2012) Boldness and aggressiveness in early and late hatched three-spined sticklebacks Gasterosteus aculeatus. J Fish Biol 81:966–976
Sih A, Chang AT, Wey TW (2014) Effects of behavioural type, social skill and the social environment on male mating success in water striders. Anim Behav 94:9–17
Silva AC, Perrone R, Zubizarreta L, Batista G, Stoddard PK (2013) Neuromodulation of the agonistic behavior in two species of weakly electric fish that display different types of aggression. J Exp Biol 216:2412–2420
Silva PI, Martins CI, Khan UW, Gjøen HM, Øverli Ø, Höglund E (2015) Stress and fear responses in the teleost pallium. Physiol Behav 141:17–22
Summers CH, Winberg S (2006) Interactions between the neural regulation of stress and aggression. J Exp Biol 209:4581–4589
Tao J, Littell R, Patetta M, Truxillo C, Wolfinger R (2002) Mixed model analyses using the SAS system course notes. SAS Institute Inc., Cary
Verbeek P, Iwamoto T, Murakami N (2007) Differences in aggression between wild-type and domesticated fighting fish are context dependent. Anim Behav 73:75–83
Wackermannova MA, Horky P, Amorim MCP, Fonseca PJ (2017) Computer-manipulated stimuli as a research tool in Mozambique tilapia Oreochromis mossambicus. Acta Ethologica 20:85–94
Acknowledgements
The work was supported by the Czech Science Foundation (project No. 20-09951S) and European Regional Development Fund-Project "Centre for the investigation of synthesis and transformation of nutritional substances in the food chain in interaction with potentially harmful substances of anthropogenic origin: comprehensive assessment of soil contamination risks for the quality of agricultural products" (No. CZ.02.1.01/0.0/0.0/16_019/0000845).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hubená, P., Horký, P. & Slavík, O. Performance of cyprinids in non-reversing mirrors versus regular mirrors in tests of aggressiveness. J Ethol 39, 97–105 (2021). https://doi.org/10.1007/s10164-020-00679-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-020-00679-7