Abstract
We provide the first evidence for y-axis magnetic compass orientation in Alytes obstetricans. Evidence for wavelength-dependent effect of light on magnetic compass orientation is also shown. In the first series of experiments, two groups of tadpoles were tested shortly after being collected from a pond with a natural shoreline and a clearly defined y-axis under two different lighting conditions (full-spectrum and long-wavelength light). Magnetic bearings from tadpoles tested under full-spectrum natural skylight displayed bimodal distribution along an axis that coincided with the magnetic direction of the y-axis in their home pond, while those tested under long-wavelength light failed to exhibit a consistent overall direction of orientation relative to the magnetic field, although a subset of tadpoles exhibited magnetic compass orientation that was rotated about 90° counter-clockwise of the magnetic direction of the y-axis in their home pond. In the second series of experiments, tadpoles were collected from a watering trough without a defined y-axis, trained to learn the direction of the y-axis in tanks with an artificial shore at one end and tested under full-spectrum natural skylight. Tadpoles from the two trained groups showed bimodal magnetic compass orientation along the shore–deep water magnetic axis of their respective training tanks. The present findings widen the number of species with y-axis magnetic compass orientation to include Alytidae, a family of primitive semiterrestrial frogs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breeding migrations are not the only movements performed by amphibians involving precise orientation mechanisms. Particularly important is y-axis orientation, which refers to orientation by shore-dwelling amphibians on a compass course perpendicular to their home shore (Ferguson and Landreth 1966). Y-axis orientation enables amphibians to follow the most direct path to and from shore to obtain resources that vary along this axis perpendicular to their home shoreline (Wells 2007). For instance, shallow water may provide food (Álvarez and Nicieza 2002), the highest dissolved oxygen levels (Nie et al. 1999) and the warmest temperatures (Nie et al. 1999; Ultsch et al. 1999; Álvarez and Nicieza 2002; Bancroft et al. 2008), whereas deep water may provide protection from predators and access to optimal water temperatures (Werner and McPeek 1994; Vitt and Caldwell 2009). Y-axis orientation allows an efficient acquisition of these resources which affect growth, development and survival in amphibians (Travis and Trexler 1986; Álvarez and Nicieza 2002; Bekhet et al. 2014).
The use of celestial cues for y-axis compass orientation has been widely studied in anurans, including frogs (Ferguson et al. 1965; Ferguson and Landreth 1966; Landreth and Ferguson 1966; Jordan et al. 1968; Gorman and Ferguson 1970) and toads (Gorman and Ferguson 1970; Goodyear 1971), as well as newts (Landreth and Ferguson 1967) and salamanders (Tomson and Ferguson 1972). However, under total overcast skies, when cloud cover hampers the use of celestial cues (Dole 1965; Hurlbert 1969), the geomagnetic field may provide a primary source of directional information for y-axis orientation (Deutschlander et al. 2000). Experiments with alpine newts showed that the magnetic compass provided the primary source of directional information for nocturnal homing orientation under total overcast and under partly cloudy skies when the moon and large portions of the sky were obscured by clouds (Diego-Rasilla et al. 2005). Similarly, palmate newts are able to orient homewards at night using the magnetic compass as the sole source of directional information (Diego-Rasilla et al. 2008).
In previous studies, magnetic y-axis compass orientation in anuran amphibians has been demonstrated in three species of true frogs (family Ranidae), Lithobates catesbeianus (Freake et al. 2002), Pelophylax perezi (Diego-Rasilla and Phillips, 2007), and Rana temporaria (Diego-Rasilla et al. 2013), while in urodeles it has been reported in three species of newts, Notophthalmus viridescens (Phillips 1986a), Lissotriton boscai (Rodríguez-García and Diego-Rasilla 2006) and Lissotriton helveticus (Diego-Rasilla et al. 2015). Also, there is evidence that the y-axis magnetic compass is mediated by a light-dependent magnetoreception mechanism in amphibians (Phillips and Borland 1992; Deutschlander et al. 1999; Phillips et al. 2001). Furthermore, the light-dependent magnetic compass shares similar features in larval frogs and newts, and in adult newts (Deutschlander et al. 1999; Diego-Rasilla et al. 2010, 2013, 2015; Phillips et al. 2010) and is proposed to involve a light-absorbing molecule that forms radical-pair intermediates (i.e., a photoexcited-radical-pair reaction) (Phillips et al. 2010). Based on findings from behavioural studies of light-dependent magnetic compass orientation in N. viridescens (Phillips and Borland 1992), P. perezi (Diego-Rasilla et al. 2010), R. temporaria (Diego-Rasilla et al. 2013) and L. helveticus (Diego-Rasilla et al. 2015) it has been speculated that this response is caused by antagonistic short-wavelength and long-wavelength inputs, and that full-spectrum light preferentially activates the short-wavelength spectral mechanism, which has higher sensitivity than the long-wavelength spectral mechanism (Phillips et al. 2010).
Although four species of Ranidae have been shown to be sensitive to the earth’s magnetic field, using a magnetic compass for y-axis orientation (Freake et al. 2002; Diego-Rasilla and Phillips 2007; Diego-Rasilla et al. 2013) or in their migratory movements (Shakhparonov and Ogurtsov 2017), there is scarce evidence of this capability in other families of Anura. To the best of our knowledge, the only toad species clearly shown to use the geomagnetic field as a reference system for compass orientation is the European common toad, Bufo bufo (family Bufonidae), when migrating to breeding ponds (Sinsch 1987; Landler and Gollmann 2011). Also, tadpoles of Xenopus laevis (family Pipidae) exhibit alignment responses to low-intensity magnetic fields (Leucht 1990).
Geomagnetic field sensitivity might be widespread in other families of Anura, since the light-dependent magnetoreception mechanism could have been present in the common ancestor of the Lissamphibia (Diego-Rasilla et al. 2010) which appeared in early Permian (~ 294 Mya Zhang and Wake 2009). Therefore, we investigated this capability in common midwife toad tadpoles (Alytes obstetricans), a species from the family Alytidae. This family is a basal group of anurans that appeared regularly in fossil assemblages during the last 170 Mya (Vitt and Caldwell 2009), while the climatic and environmental changes that occurred during the Pleistocene (2.5 Mya) seem to have shaped the diversification history of A. obstetricans (Gonçalves et al. 2015).
We investigate whether tadpoles of A. obstetricans are able to use the earth’s magnetic field to orient along the natural y-axis found in their home pond, as well as along the learned direction of the y-axis in a training tank with an artificial shore. Furthermore, we investigated whether the wavelength of light influences magnetic compass orientation in this species.
Materials and methods
Subjects
The common midwife toad, Alytes obstetricans (Anura, Alytidae) exhibits an unusual reproductive cycle because they mate on land, not laying its eggs in water. The male wraps the egg-strings around his hind legs, carrying them about with him on land for 15–45 days until the eggs contain well developed tadpoles which are about to hatch; then the male enters water and the tadpoles emerge (Reading and Clarke 1988; Márquez 1993). This species has a long breeding season, with males carrying eggs during most of the year and tadpoles remaining in the water for a long time until metamorphosis is complete; in mountain areas they can remain in the water for more than a year until metamorphosis (Bosch 2014).
Tadpoles of A. obstetricans (stages 26–30 Gosner 1960) were captured in a permanent pond (Cantabria, northern Spain; 43° 14′ 8″ N, 4° 9′ 59″ W; elevation 464 m) with a clearly defined y-axis (deep end/shore 162°/342°). The pond is 11 m in length and 2.5 m in width and is surrounded by a thick forest of Quercus robur and Fagus sylvatica. It is a shallow natural pond formed by rainwater runoff on a forest path. Its depth varies progressively from a maximum of 21 cm to less than one centimetre on the shore end and it is bordered on the deep end by the hillside slope (Fig. 1). Tadpoles were captured by dip-netting from the shore end by performing perpendicular sampling to the longest axis of the pond (i.e., along the y-axis of the pond).
Two experiments were initially performed in winter (late December and early January) under a totally overcast sky. Thirty-four common midwife toad tadpoles were collected in the first experiment and 22 tadpoles in the second one. Tadpoles were transported in air-tight and light-tight plastic containers to the testing site where they were held in open tanks (54 × 35 × 21 cm) filled with water from their home pond and aligned on the north–south geomagnetic axis. Testing began about 1 h after they arrived at the testing site in an open area completely shaded by a group of hazels, Corylus avellana (43° 17′ 07″ N, 4° 4′ 44″ W; elevation 59 m), 9.06 km away from their pond.
In the third experiment tadpoles (stages 26–39 Gosner 1960) were captured in midsummer (early August) from a rectangular watering trough (Cantabria, northern Spain 43° 15′ 35″ N, 4° 4′ 45″ W; elevation 227 m) with vertical sides in all four directions. The trough (0.90 m length × 0.80 m width × 0.80 m depth) is surrounded by a forest of Eucalyptus globulus and a heavily grazed landscape. Tadpoles were transported in air-tight and light-tight plastic containers to the testing site where they were trained outdoors to learn an artificial shore direction for 6 days under full-spectrum natural skylight, in two different training configurations: (i) a training tank aligned along the magnetic north–south axis, with shore located at the south end (25 tadpoles), and (ii) a training tank aligned along the magnetic east–west axis, with shore located at the east end (28 tadpoles). The y-axis training tanks consisted of two 96 l (80 × 40 × 30 cm) all glass aquaria, each one with a sloping bottom (over 25° slope) to provide a deep end, and a shallow end with a water depth of 1 cm (see Rodríguez-García and Diego-Rasilla 2006 for a detailed description). Training tanks were filled with tap water treated with aquarium water conditioner (TetraAcqua AquaSafe®, Tetra GmbH, Germany). Tadpoles were tested for magnetic y-axis orientation after 6 days of training. Each day the aquarium water was partly replaced with fresh treated water. This was done at the time of maximum temperature (i.e., 14:00–16:00 GMT) to maintain the water temperature of the training tanks between 17 and 22 °C. Tadpoles were fed boiled lettuce, which was placed in the shallow end of the tank daily. Training and testing took place, in an open area completely shaded by a group of hazels, Corylus avellana (43° 17′ 07″ N, 4° 4′ 44″ W; elevation 59 m), 2.95 km away from their trough. Note that it is the same testing site as in the other experiments. Tadpoles were tested under a completely overcast sky.
The tadpoles were used once in each experiment and afterwards were returned to their home ponds.
Testing apparatus and protocols
Testing protocols and testing apparatus have been described elsewhere (Diego-Rasilla and Phillips 2007; Diego-Rasilla et al. 2010, 2013). The testing apparatus was a circular, featureless arena open only to the sky (circular plastic container, 43 cm diameter and 24 cm high) enclosed within two, orthogonally aligned, double-wrapped cube-surface coils (Rubens 1945) powered by a dual DC power supply (Protek 30,158) used to alter the alignment of the earth’s magnetic field. Four different magnetic field conditions were used (see Phillips 1986a, b): the ambient magnetic field (magnetic north at north) and three altered fields (magnetic north rotated to east, south and west). The sides of the apparatus were covered with a black cotton curtain. As noted above, the testing apparatus was located under dense foliage that blocked a direct view of the overhead sky, allowing only diffuse light to reach the tadpoles in the testing arena.
In the first and third experiments tadpoles were tested for their directional preferences under full-spectrum natural skylight, while in the second one they were tested under long-wavelength light (≥ 500 nm). For full-spectrum tests, the top of the apparatus was uncovered, exposing the animals to full-spectrum natural skylight. Long-wavelength testing conditions were produced by covering the top of the testing apparatus with a long-pass spectral filter (Lee no. 101; Lee Filters©) that transmitted wavelengths ≥ 500 nm, between two transparent methacrylate sheets (105 × 105 cm side). Since the sides of the testing apparatus were opaque, only light passing through the spectral filter reached the animal in the testing arena (Diego-Rasilla et al. 2010). Light intensity in both lighting conditions varied over the course of the day and was not controlled for.
In the first (i.e., non-artificially trained tadpoles tested under full-spectrum natural skylight) and second (i.e., non-artificially trained tadpoles tested under long-wavelength light) experiments, prior to testing, each larva was removed with a small dip net from the communal tank (54 × 35 × 21 cm) in which they were held at the testing site and placed in a rectangular plastic container (34 × 24 × 16 cm) filled with water from their home pond to a depth of 1 cm and aligned on the north–south geomagnetic axis. In the third experiment (i.e., tadpoles trained and tested under full-spectrum natural skylight), each larva was removed from the training tank with a small dip net and placed in a rectangular plastic container (34 × 24 × 16 cm) filled with tap water treated with aquarium water conditioner to a depth of 1 cm and its length along the same alignment as the training tank (N–S or E–W).
After 5 min in this individual isolation tank, the tadpole was removed with the dip net, carried to the testing arena, and placed in the arena centre beneath an opaque, cylindrical plastic container (9 cm diameter, 14.5 cm high) that served as a release device. Tadpoles were held in the release device for 1 min to overcome effects of handling before the release device was lifted, allowing them to move freely about the arena. The arena was filled with water to a depth of 1 cm (water from their home pond in the first and second experiments, and tap water treated with aquarium water conditioner in the third experiment). Larvae movements were observed through tiny holes in the black curtain that surrounded the test apparatus. A tadpole’s directional response was recorded at the first point where it contacted the wall. Directional bearings were scored with respect to symmetrical radial marks drawn on the exterior edge of arena walls.
Bearings of larvae that contacted arena wall in less than 10 s were not recorded. These individuals, which moved immediately as soon as the release device was lifted, were considered to exhibit a randomly oriented escape response and were excluded from the analyses. In addition, trials were abandoned if the larva failed to score within 10 min (Freake et al. 2002; Freake and Phillips 2005; Diego-Rasilla and Phillips 2007).
The experimenter who set the horizontal alignment of the field using remote switches was not involved in scoring the tadpoles’ directional responses, while the second experimenter placed the tadpole in the testing arena and recorded its directional response without knowing the alignment of the magnetic field. The sequence of fields was not revealed to the observer recording the tadpoles’ directional responses until after experiments were completed. Therefore, the experimenter was blind to the treatment.
Absolute or “topographic” bearings (i.e., actual directions of movement ignoring the alignment of the magnetic field in testing) were recorded. Magnetie bearings were obtained by normalizing the bearings with respect to the direction of magnetic North to which each tadpole was exposed during testing, i.e., by rotating the distributions of bearings from each of the four magnetic alignments (magN = N, magN = E, magN = W, magN = S) so that the direction of magnetic North was at 0°. By pooling bearings from an approximately equal number of larvae tested in each of the four magnetic field alignments, we were able to factor out any non-magnetic bias from the resulting distribution and retain only the component of the tadpoles’ orientation that was a consistent response to the magnetic field (Phillips 1986b).
Statistical analysis
Instead of only using standard circular statistics (i.e., statistical tests to determine if data support or reject a null hypothesis based on an arbitrary significance threshold, often P < 0.05) (Batschelet 1981), we also used model-selection procedures relying on the probability of the distribution of bearings given a specific model (i.e., maximum likelihood methods; Fitak and Johnsen 2017). Maximum likelihood methods are preferred for quantifying strength of evidence in favour of a hypothesis (Burnham et al. 2011; Fitak and Johnsen 2017) since the value of P has been questioned as a measure for strength of evidence (Royall 1997; Nakagawa and Cuthill 2007; Martínez-Abraín 2008; Fitak and Johnsen 2017; Bello and Renter 2018) and may suffer from low statistical power (Jennions and Møller 2003; Nakagawa 2004; Bello and Renter 2018). Schnute and Groot 1992 presented a general method for analysis of animal orientation data in which one or more directional components occur. They developed a likelihood approach using ten models to simultaneously examine multiple orientation hypotheses that has been implemented by Fitak and Johnsen 2017 in a set of functions for use in R (package CircMLE; R Core Team 2017): (1) uniform model (M1), (2) unimodal models with a preferred direction (M2A, M2B and M2C), (3) bimodal models with two axial preferred directions (M3A, M3B, M4A and M4B) and bimodal models with non-axial preferred directions (M5A and M5B). These models are based on up to five parameters: two orientation directions (φ1, φ2), a third parameter λ, that quantifies the fraction of animals orienting in direction φ1 (the size of the second distribution is thus fixed at 1 − λ). In addition, the strength of animals’ tendency to orient is defined by concentration parameters (κ1, κ2); larger values indicate greater animal clustering (Schnute and Groot 1992). Thus, if κ1 is large, animals in the first group orient strongly in the direction φ1; conversely, a small κ1 indicates almost random orientation in this group. A similar description applies to κ2 (Schnute and Groot 1992). Interestingly, Schnute and Groot 1992 demonstrated that a likelihood-based approach using these models provides objective criteria for model selection, determining the most appropriate model for a given data set and measuring uncertainty for all model parameters. Also, likelihood-based approaches enable the simultaneous testing of null and multiple alternative hypotheses (Burnham et al. 2011). As model-selection method we used the Akaike Information Criterion (AIC Akaike 1998); AIC was calculated for each model and the model with the smallest AIC, or least amount of information loss, was selected as the best model (Fitak and Johnsen 2017). The individual AIC values are not interpretable as they contain arbitrary constants and are affected by sample size. Therefore, it is necessary to rescale AIC to ∆AIC (i.e., the information loss experienced if we are using a certain fitted model rather than the best model for inference). Models with a difference in AIC relative to the best model (ΔAIC) ≤ 2 have substantial support, models in which 4 ≤ ΔAIC ≤ 7 have considerably less support, and models with ΔAIC > 10 have essentially no support (Burnham and Anderson 2004; Burnham et al. 2011). The model weights (Akaike weights or AIC weights), or probabilities, are also provided. AIC weights quantify conclusions based on AIC analyses by specifying the amount of statistical confidence for the model with the lowest AIC value (Wagenmakers and Farrell 2004). Thus, Akaike weights provide additional support for the conclusion that a model is preferred over its competitors, as well as a continuous measure of strength of evidence (Wagenmakers and Farrell 2004).
For comparison purposes, we used the most common statistical test applied to test for departure from uniformity, the Rayleigh test (Ruxton 2017; Landler et al. 2018). Statistics for bimodal distributions using the Rayleigh test were calculated by doubling each data value and reducing any greater than 360 using modulo arithmetic (Batschelet 1981). The Watson U2 test carried out on doubled bearings was used to test for differences between the distributions of magnetic bearings (Batschelet 1981; Mardia and Jupp 2000).
To determine whether the experimental conditions affected the tadpoles’ motivation to orient in the first series of experiments (i.e., tadpoles tested under full-spectrum natural skylight or tested under long-wavelength light), we compared the ability of individual tadpoles to orient within the specified time limits in both experiments using the Fisher exact test (Siegel 1956).
Results
Non-artificially trained midwife toad tadpoles tested under full-spectrum natural skylight
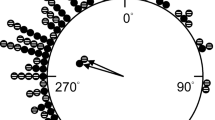
The distribution of magnetic bearings from tadpoles tested under full-spectrum natural skylight a few hours after being collected followed a homogeneous symmetric bimodal model (M3A: ∆AIC = 0, AIC weight = 0.27444; φ1 = 338°, φ2 = 158°, κ1 = 2.316, κ2 = 2.316, n = 34; Fig. 2), assuming a 50/50 split between the two modal directions (λ = 0.5). Orientation directions (i.e., φ1 and φ2) coincided with the magnetic direction of the shore-deep home pond axis (i.e., 342°-162°). The uniform model was the best model for the topographic bearings (M1: ∆AIC = 0, AIC weight = 0.62015; κ1 = 0; λ = 1; Fig. 2).
Best fit models for topographic y-axis orientation (M1) and magnetic y-axis orientation (M3A) in common midwife toad tadpoles (Alytes obstetricans) tested under full-spectrum natural skylight. Each black triangle represents the bearing of one tadpole, tested only once in one of four horizontal alignments of the magnetic field shortly after capture. Circular histogram (grey bars) represents observed orientation of tadpoles in 20° bins. The model's probability density function (dashed line) and mean directions (red dashed arrows) of the best model are shown. Triangle outside the plot indicate the magnetic direction of the shore end of the y-axis
The Rayleigh test provided the same outcome; the distribution of magnetic bearings from tadpoles experiencing same wavelength conditions in their home pond and at the testing site exhibited significant bimodal orientation that coincided with the magnetic direction of the shore-deep home pond axis (Rayleigh test; P = 0.009, N = 34, Table 1), and the 95% confidence intervals for the mean vector bearing included the magnetic direction of the shore-deep home pond axis. The topographic bearings were randomly distributed (Table 1).
Non-artificially trained midwife toad tadpoles tested under long-wavelength light (≥ 500 nm)
When a second group of tadpoles was tested under long-wavelength light (≥ 500 nm) a few hours after being captured, the best model was the symmetric modified unimodal (M2B: ∆AIC = 0, AIC weights = 0.41014, n = 15; Fig. 3) in which larvae comprise two groups (λ = 0.5). Half of them were magnetically oriented with a mean direction (φ1) of 273° and concentration (κ1) of 18.645, and the other half exhibited a random orientation (κ2 = 0; Fig. 3). Those magnetically oriented exhibited an orientation rotated approximately 90° counter-clockwise from the home pond shoreward direction. The topographic bearings were randomly distributed, since the uniform model gave the best fit (M1: ∆AIC = 0; AIC weight = 0.31161; κ1 = 0; λ = 1, n = 15; Fig. 3).
Best fit models for topographic y-axis orientation (M1) and magnetic y-axis orientation (M2B) in common midwife toad tadpoles (Alytes obstetricans) tested under long-wavelength light (≥ 500 nm) shortly after capture. Each black triangle represents the bearing of one tadpole, tested only once in one of four horizontal alignments of the magnetic field. Circular histogram (grey bars) represents observed magnetic orientation of tadpoles in 20° bins. The density (dashed line) and mean directions (red dashed arrows) of the models are shown. Triangles outside the plots indicate the magnetic direction of the shore end of the y-axis
A detailed observation of Fig. 3 shows that responses of tadpoles were split into three clusters, two of them on either side (approximately ± 45°) of the 90° rotated axis of orientation. In contrast to the model-based approach, the Rayleigh test failed to detect this clustered pattern of orientation (P = 0.263, N = 15, Table 1). Standard circular statistics also showed that the topographic bearings were randomly distributed (Table 1).
Seven of 22 non-artificially trained tadpoles tested under long-wavelength light (31.82%) failed to orient within the specified time limits, whereas not a single non-artificially trained tadpole tested under full-spectrum natural skylight failed to orient (Fisher exact test; P = 0.0007).
Midwife toad tadpoles trained and tested under full-spectrum natural skylight
We used a model-based approach to the analysis of distribution of magnetic bearings from larvae held in both training tanks. For the tadpoles trained in the tank that was aligned along the north–south axis with shore facing south (n = 21), an axial bimodal model (M4B; ∆AIC = 0, AIC weight = 0.42122; Fig. 4c) was the best fit. This model suggests two modes 180° apart (168°/348°) and concentration parameters of 0.870 and 55.075, respectively; the proportional size of the first distribution (λ = 0.358) indicates that 35.8% of animals oriented in direction 168° and 64.2% oriented in direction 348°. The unimodal model gave the best fit for the topographic bearings (M2A: ∆AIC = 0, AIC weight = 0.31469, φ1 = 151°, κ1 = 0.806, λ = 1, n = 21; Fig. 4a).
Best fit models for topographic and magnetic Y-axis orientation in common midwife toad tadpoles (Alytes obstetricans) trained and tested under natural skylight. Each black triangle represents the bearing of one tadpole, tested only once in one of four horizontal alignments of the magnetic field. a Topographic bearings from tadpoles trained in the tank that was aligned along the north–south axis; (b) topographic bearings from tadpoles trained in the tank that was aligned along the east–west axis; (c) magnetic bearings from tadpoles trained in the tank that was aligned along the north–south axis; (d) magnetic bearings from tadpoles trained in the tank that was aligned along the east–west axis; (e) bearings from c, d pooled with respect to the magnetic direction of shore (180°). Circular histogram (grey bars) represents observed orientation of tadpoles in 20° bins. The density (dashed line) and mean directions (red dashed arrows) of the best model are shown. Triangles outside the plots indicate the magnetic direction of the shore end of the y-axis
Standard circular statistics provided similar results, showing that tadpoles exhibited a significant bimodal orientation relative to the direction of the magnetic field (Rayleigh test 161–341 ± 18°, r = 0.448, n = 21, P = 0.013, Table 2). Also, the 95% confidence limits for the mean vector bearing included the trained y-axis. The Rayleigh test indicated that topographic bearings were randomly distributed (Table 2).
For the tadpoles trained in the tank that was aligned along the east–west axis with shore facing east (n = 28), the symmetric bimodal model (M3B; ∆AIC = 0, AIC weight = 0.26538; Fig. 4d) was the best fit, with half the individuals magnetically oriented with a mean direction (φ1) of 283° and the second half 180° different, φ2 = 103°. Model M3B predicted parameters κ1 = 1.898, κ2 = 7.032, and λ = 0.5. The topographic bearings were randomly distributed, since the uniform model gave the best fit (M1: ∆AIC = 0; AIC weight = 0.25899; κ1 = 0; λ = 1; n = 28; Fig. 4b).
Standard circular statistics provided identical results. Tadpoles showed significant bimodal orientation relative to the direction of the magnetic field of the trained y-axis (Rayleigh test 101–281 ± 18°, r = 0.401, n = 28, P = 0.01, Table 2) and the 95% confidence limits for the mean vector bearing included the trained y-axis. The topographic bearings were randomly distributed (Table 2).
The two distributions of magnetic bearings (north–south and east–west training configurations) were significantly different (Watson U2 test; U2 = 0.311, P < 0.005) and their mean axes of orientation differed by approximately 90°. When the two distributions of magnetic bearings were normalized with respect to the magnetic direction of shore (i.e., pooling all bearings so that shore direction is rotated to 180°), we did not find a significant difference (Watson U2 test; U2 = 0.125, 0.2 > P > 0.1), indicating that the distribution of bearings relative to the trained magnetic-axis was not different for tadpoles trained along the north–south and east–west axes.
A similar axial model, homogeneous symmetric bimodal (M3A; ∆AIC = 0, AIC weight = 0.38086, φ1 = 176°, φ2 = 356°, κ1 = 2.338, κ2 = 2.338, n = 49; Fig. 4e), was the best fit for bearings pooled with respect to the magnetic direction of shore (180°), assuming a 50/50 split between the two modal directions (λ = 0.5). Again, the results were identical using standard circular statistics (Table 2).
Discussion
Regarding statistical analyses, the results obtained are substantially the same using model-based approach (Schnute and Groot 1992) or standard circular statistics (Batschelet 1981), although model-based approach is more informative because it is based on up to five maximum likelihood parameter estimates (φ1, φ2, κ1, κ2 and λ; see Materials and methods: statistical analysis) improving our understanding of how tadpoles produce oriented responses (Fitak and Johnsen 2017). Furthermore, since a P value is not a criterion of strength of evidence or magnitude of a treatment effect of interest (Fitak and Johnsen 2017; Bello and Renter 2018), using maximum likelihood methods when analysing circular data helps to address criticisms and controversies regarding the use of statistical significance tests in research assessments (Schneider 2013). Therefore, both types of statistical analyses complement each other.
Our findings demonstrate the capability of Alytes obstetricans tadpoles to perform y-axis magnetic compass orientation and broaden not only the number of species but also the number of families of anurans using the geomagnetic field to orient their movements. In our research we used two different approaches; in the first one, tadpoles were tested shortly after being collected from their home pond without exposure to an artificial shoreline, while in the second approach tadpoles were trained to orient in the direction of an artificial shore before being tested. Results from both approaches were consistent. Magnetic bearings from the non-artificially trained tadpoles tested under full-spectrum natural skylight displayed bimodal distribution along an axis that coincided with the magnetic direction of their home pond y-axis. Likewise, the distribution of magnetic bearings from the two trained groups, even though both groups of tadpoles lacked previous y-axis orientation experience, coincided with the shore-deep water magnetic axes of their respective training tanks; bearings pooled with respect to the magnetic direction of shore were axially distributed with half the individuals magnetically oriented shoreward and the second half in the opposite direction. These findings suggest that the tadpole’s magnetic compass response could be learned in the training tanks, as well as in their home pond.
Tadpoles experiencing different wavelength conditions in their home pond and in testing (i.e., exposed to natural skylight in their home pond and tested under long-wavelength light) showed magnetic compass orientation that was rotated about 90° counter-clockwise to the magnetic direction of their home pond y-axis. Results from previous research have shown a similar shift in the direction of magnetic compass orientation that were tested under long-wavelength light after being trained under full-spectrum light in other species of anurans (Freake and Phillips 2005; Diego-Rasilla et al. 2010, 2013) and urodeles (Deutschlander et al. 1999; Diego-Rasilla et al. 2015). Also, the 90° shift has been indicated to be due to a direct effect of light on the underlying magnetoreception mechanism (Phillips and Borland 1992; Diego-Rasilla et al. 2010, 2013). Therefore, our findings provide additional support for a wavelength-dependent effect of light on magnetic compass orientation in anuran larvae. The best model (M2B; i.e., symmetric modified unimodal model) showed that half of tadpoles exhibited magnetic compass orientation that was rotated approximately 90° from the shoreward magnetic direction. However, the other half (i.e., bearings distributed on either side of the 90° rotated axis of orientation) failed to exhibit a consistent axis of orientation relative to the magnetic field. In addition, the ability of tadpoles to orient within the specified time limits was smaller under long-wavelength light than under full-spectrum natural skylight; this raises the possibility that long-wavelength light negatively affects their ability to obtain directional information from the magnetic field.
The findings reported here show that tadpoles in the family Alytidae can use the magnetic field to orient bimodally along an axis perpendicular to shore (y-axis). The magnetic compass orientation of the tadpoles held in the training tanks show that this response can be learned, as demonstrated in previous studies of the use of magnetic compass cues for y-axis orientation in other amphibians.
The responses of tadpoles tested under long-wavelength light is consistent with the underlying magnetoreception mechanism being sensitive to the wavelength of light; given that natural illumination varies by several orders of magnitude over the course of the day, the observed change in behaviour is unlikely to be primarily caused by the change in overall light intensity.
Although exposure to long-wavelength light clearly altered the tadpole’s reliance on magnetic compass cues, the nature of this effect is less clear than the effects observed in earlier studies of both urodele and anuran amphibians. Use of symmetric modified unimodal approach (Schnute and Groot 1992) suggests that roughly half of the tadpoles tested under long-wavelength light exhibited magnetic compass orientation along an axis rotated by 90° from the trained magnetic axis, as observed in the earlier studies, consistent with reliance on a light-dependent magnetic compass. The failure of the remaining tadpoles to exhibit a consistent direction of orientation relative to the magnetic field is also consistent with an effect of lighting conditions in testing, but leaves unanswered the question of why these tadpoles failed to orient relative to the magnetic field as in the other half of the tadpoles and in earlier studies of the magnetic compass orientation of amphibians tested under long-wavelength light.
References
Akaike H (1998) Information theory and an extension of the maximum likelihood principle. In: Parzen E, Tanabe K, Kitgawa G (eds) Selected papers of Hirotugu Akaike. Springer, New York, pp 199–213
Álvarez D, Nicieza AG (2002) Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct Ecol 16:640–648
Bancroft BA, Baker NJ, Searle CL, Garcia TS, Blaustein AR (2008) Larval amphibians seek warm temperatures and do not avoid harmful UVB radiation. Behav Ecol 19:879–886
Batschelet E (1981) Circular statistics in biology. Academic Press, New York
Bekhet GA, Abdou HA, Dekinesh SA, Hussein HA, Sebiae SS (2014) Biological factors controlling developmental duration, growth and metamorphosis of the larval green toad, Bufo viridis viridis. J Basic Appl Zool 67:67–82
Bello NM, Renter DG (2018) Invited review: reproducible research from noisy data: revisiting key statistical principles for the animal sciences. J Dairy Sci 101:5679–5701
Bosch J (2014) Sapo partero común–Alytes obstetricans (Laurenti, 1768). In: Salvador A, Martínez-Solano I (eds) Enciclopedia virtual de los vertebrados españoles. CSIC–Museo Nacional de Ciencias Naturales (MNCN), Madrid
Burnham KP, Anderson DR (2004) Multimodel inference: Understanding AIC and BIC in model selection. Sociol Methods Res 33:261–304
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Deutschlander ME, Borland SC, Phillips JB (1999) Extraocular magnetic compass in newts. Nature 400:324–325
Deutschlander ME, Phillips JB, Borland SC (2000) Magnetic compass orientation in the eastern red-spotted newt, Notophthalmus viridescens: rapid acquisition of the shoreward axis. Copeia 2000:413–419
Diego-Rasilla FJ, Luengo RM, Phillips JB (2005) Magnetic compass mediates nocturnal homing by the alpine newt, Triturus alpestris. Behav Ecol Sociobiol 58:361–365
Diego-Rasilla FJ, Luengo RM, Phillips JB (2008) Use of a magnetic compass for nocturnal homing orientation in the palmate newt, Lissotriton helveticus. Ethology 114:808–815
Diego-Rasilla FJ, Luengo RM, Phillips JB (2010) Light-dependent magnetic compass in Iberian green frog tadpoles. Naturwissenschaften 97:1077–1088
Diego-Rasilla FJ, Luengo RM, Phillips JB (2013) Use of a light-dependent magnetic compass for y-axis orientation in European common frog (Rana temporaria) tadpoles. J Comp Physiol A 199:619–628
Diego-Rasilla FJ, Luengo RM, Phillips JB (2015) Evidence of light-dependent magnetic compass orientation in urodele amphibian larvae. Behav Processes 118:1–7
Diego-Rasilla FJ, Phillips JB (2007) Magnetic compass orientation in larval iberian green frogs, Pelophylax perezi. Ethology 113:474–479
Dole JW (1965) Summer movements of adult leopard frogs, Rana pipiens, in northern Michigan. Ecology 46:236–255
Ferguson DE, Landreth HF (1966) Celestial orientation of Fowler’s toad (Bufo fowleri). Behaviour 26:105–123
Ferguson DE, Landreth HF, Turnipseed MR (1965) Astronomical orientation of the southern cricket frog, Acris gryllus. Copeia 1:58–66
Fitak RR, Johnsen S (2017) Bringing the analysis of animal orientation data full circle: model-based approaches with maximum likelihood. J Exp Biol 220:3878–3882
Freake MJ, Borland SC, Phillips JB (2002) Use of a magnetic compass for y-axis orientation in larval bullfrogs, Rana catesbeiana. Copeia 2002:466–471
Freake MJ, Phillips JB (2005) Light-dependent shift in bullfrog tadpole magnetic compass orientation: Evidence for a common magnetoreception mechanism in anuran and urodele amphibians. Ethology 111:241–254
Gonçalves H, Maia-Carvalho B, Sousa-Neves T, García-París M, Sequeira F, Ferrand N, Martínez-Solano I (2015) Multilocus phylogeography of the common midwife toad, Alytes obstetricans (Anura, Alytidae): contrasting patterns of lineage diversification and genetic structure in the Iberian refugium. Mol Phylogenet Evol 93:363–379
Goodyear CP (1971) Y-axis orientation of the oak toad, Bufo quercicus. Herpetologica 27:320–323
Gorman RR, Ferguson JH (1970) Sun-compass orientation in the western toad, Bufo boreas. Herpetologica 26:34–45
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hurlbert SH (1969) The breeding migration and interhabitat wandering of the vermillion red-spotted newt, Notophthalmus viridescens (Rafinesque). Ecol Monogr 39:465–488
Jennions MD, Møller AP (2003) A survey of the statistical power of research in behavioral ecology and animal behavior. Behav Ecol 14:438–445
Jordan OR, Byrd WW, Ferguson DE (1968) Sun-compass orientation in Rana pipiens. Herpetologica 24:335–336
Landler L, Gollmann G (2011) Magnetic orientation of the common toad: establishing an arena approach for adult anurans. Front Zool 8:6
Landler L, Ruxton GD, Malkemper EP (2018) Circular data in biology: advice for effectively implementing statistical procedures. Behav Ecol Sociobiol 72:128
Landreth HF, Ferguson DE (1966) Evidence of sun-compass orientation in the chorus frog, Pseudacris triseriata. Herpetologica 22:106–112
Landreth HF, Ferguson DE (1967) Newt orientation by sun-compass. Nature 215:516–518
Leucht T (1990) Interactions of light and gravity reception with magnetic fields in Xenopus laevis. J Exp Biol 148:325–334
Mardia KV, Jupp PE (2000) Directional statistics. Wiley, New York
Márquez R (1993) Male reproductive success in two midwife toads, Alytes obstetricans and A. cisternasii. Behav Ecol Sociobiol 32:283–291
Martínez-Abraín A (2008) Statistical significance and biological relevance: a call for a more cautious interpretation of results in ecology. Acta Oecologica 34:9–11
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Nie M, Crim JD, Ultsch GR (1999) Dissolved oxygen, temperature, and habitat selection by bullfrog (Rana catesbeiana) tadpoles. Copeia 1999:153–162
Phillips JB (1986a) Two magnetoreception pathways in a migratory salamander. Science 233:765–767
Phillips JB (1986b) Magnetic compass orientation in the eastern red-spotted newt (Notophthalmus viridescens). J Comp Physiol A 158:103–109
Phillips JB, Borland SC (1992) Behavioural evidence for use of a light-dependent magnetoreception mechanism by a vertebrate. Nature 359:142–144
Phillips JB, Deutschlander ME, Freake MJ, Borl SC (2001) The role of extraocular photoreceptors in newt magnetic compass orientation: evidence for parallels between light–dependent magnetoreception and polarized light detection in vertebrates. J Exp Biol 204:2543–2552
Phillips JB, Jorge PE, Muheim R (2010) Light-dependent magnetic compass orientation in amphibians and insects: candidate receptors and candidate molecular mechanisms. J R Soc Interface 7:S241–S256
Reading CJ, Clarke RT (1988) Multiple clutches, egg mortality and mate choice in the mid-wife toad, Alytes obstetricans. Amphibia-Reptilia 9:357–364
Rodríguez-García L, Diego-Rasilla FJ (2006) Use of a magnetic compass for y-axis orientation in premetamorphic newts (Triturus boscai). J Ethol 24:111–116
Royall R (1997) Statistical evidence: a likelihood paradigm. Chapman and Hall, London
Rubens SM (1945) Cube–surface coil for producing a uniform magnetic field. Rev Sci Instrum 16:243–245
Ruxton GD (2017) Testing for departure from uniformity and estimating mean direction for circular data. Biol Lett 13:20160756
Schneider JW (2013) Caveats for using statistical significance tests in research assessments. J Informetr 7:50–62
Schnute JT, Groot K (1992) Statistical analysis of animal orientation data. Anim Behav 43:15–33
Shakhparonov VV, Ogurtsov SV (2017) Marsh frogs, Pelophylax ridibundus, determine migratory direction by magnetic field. J Comp Physiol A 203:35–43
Siegel S (1956) Nonparametric statistics for the behavioral sciences. MacGraw-Hill Book Company, New York
Sinsch U (1987) Orientation behaviour of toads (Bufo bufo) displaced from the breeding site. J Comp Physiol A 161:715–727
Tomson OH, Ferguson DE (1972) Y-axis orientation in larvae and juveniles of three species of Ambystoma. Herpetologica 28:6–9
Travis J, Trexler JC (1986) Interactions among factors affecting growth, development and survival in experimental populations of Bufo terrestris (Anura: Bufonidae). Oecologia 69:110–116
Ultsch GR, Bradford DF, Freda J (1999) Physiology: coping with the environment. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. The University of Chicago Press, Chicago, pp 189–214
Vitt LJ, Caldwell JP (2009) Herpetology: an introductory biology of amphibians and reptiles. Academic Press, New York
Wagenmakers E-J, Farrell S (2004) AIC model selection using Akaike weights. Psychon Bull Rev 11:192–196
Wells DK (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Werner EE, McPeek MA (1994) Direct and indirect effects of predators on two anuran species along an environmental gradient. Ecology 75:1368–1382
Zhang P, Wake DB (2009) Higher-level salamander relationships and divergence dates inferred from complete mitochondrial genomes. Mol Phylogenet Evol 53:492–508
Acknowledgements
We sincerely thank John B. Phillips for helpful comments and suggestions regarding the manuscript and Marcos Diego-Gutiérrez for their valuable assistance during the study. We thank two anonymous reviewers for their helpful comments. The Cantabria autonomous government granted the necessary permits for the study.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standard
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Diego-Rasilla, F.J., Luengo, R.M. Magnetic compass orientation in common midwife toad tadpoles, Alytes obstetricans (Anura, Alytidae). J Ethol 38, 289–299 (2020). https://doi.org/10.1007/s10164-020-00653-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-020-00653-3