Abstract
Different strategies have evolved in response to predation pressure. Many species use acoustic signals to communicate about the presence of predators, and some of them use non-vocal sounds. Here, we evaluated the role of the non-vocal sound produced by scaled doves (Columbina squammata) during escape takeoffs. Initially, we investigated the context of the non-vocal sound production to access the effects of natural threats on individuals’ escape response. Then, we used simulated attacks (a direct running movement toward the focal individuals) to confirm the preliminary observations and to evaluate how position in the group affects escape response and vigilance. For both the observational and experimental parts, we registered, among other variables, the occurrence of takeoff flight, if it was followed by a production of non-vocal sound, the position of the individuals within the flock and their response (e.g., stay, flew, vigilance). We observed that both solitary and flocked individuals produce non-vocal sounds during takeoff flights, although it was more commonly registered for flocks. The production of the non-vocal sound elicited a faster escape response on flock members, and individuals at the center of the flock showed a higher probability to takeoff. The results suggest that the non-vocal sound may signal information about predation risk and that it may be directed both to conspecifics and to the predator itself. Our results therefore contribute to the understanding of the evolution of mechanical sound production in birds and shed some light on its function as a communication signal, especially under a predation context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation has been considered one of the main factors shaping the evolution of social behavior (Alexander 1974; Rubenstein 1978; Spieler 2003; Dittmann and Schausberger 2017). Despite other associated costs, sociality has been demonstrated to increase the chance of survival among different taxonomic groups, especially under higher risk of predation (Hill and Lee 1998; Sorato et al. 2012; Schmitt et al. 2014; Gardner et al. 2016).
The speed at which information about predation risk is exchanged in social groups depends on several factors, such as group structure, perceived predation risk, average distance between individuals, environment condition, etc. However, information exchange is expected to be more rapid in smaller groups, promoting a shorter reaction time (Lima 1994; Quinn and Cresswell 2005; but see Martín et al. 2006; Fernández-Juricic et al. 2009; Beauchamp 2012). A study conducted with flocks of common redshanks (Tringa totanus) demonstrated that birds in smaller flocks, closest to the potential predator and which were close to other group members initiate the flight earliest within a flock (Hilton et al. 1999).
Despite the vast knowledge about the advantages of social living, there is much to know about the real roles and benefits of signal production within groups, especially under predation risk. This is mainly because of the costs of this behavior to the producer (e.g., may work as a cue for predators about its location; Wheeler 2008; Putman and Clark 2015). Signals may have evolved to dissuade the predator from attacking after detecting the signaler (Hasson 1991; Clark 2005; Alvarez et al. 2006), or may be directed to conspecifics to warn about potential predators (Seyfarth et al. 1980; Fichtel and Kappeler 2002), or both. Signals produced by the bonaire whiptail lizard (Cnemidophorus murinus), for example, are interpreted as an attempt to inform the predator that it was spotted (Cooper et al. 2004). On the other hand, signals produced by Columbian ground squirrels (Spermophilus columbianus) significantly increased the vigilance of conspecifics in the presence of a potential predator, suggesting that the signal was directed to other group members (Fairbanks and Dobson 2007).
Among birds, communication signals are commonly produced by displaying parts of the body (visual stimulus), as observed in turquoise-browed motmots (Eumomota superciliosa), in elegant trogons (Trogon elegans) and in hoopoes (Upupa epops) (Murphy 2006; Bitton and Doucet 2014; Ruiz-Rodríguez et al. 2017) or through vocal structures, as observed in many species such as in red jungle fowls (Gallus gallus) (Collias 1987), in Carolina chickadees (Poecile carolinensis) (Soard and Ritchison 2009) and in tufted titmice (Baeolophus bicolor) (Courter and Ritchison 2010). In addition, some animals may use parts of their bodies to produces mechanical sounds (non-vocal sounds, e.g., whistles, trills), as observed in the club-winged manakin (Machaeropterus deliciosus) (Bostwick et al. 2010), broadbills (Smithornis rufolateralis and S. capensis) (Clark et al. 2016) and the red-billed streamertail hummingbird (Trochilus polytmus) (Clark 2008). Non-vocal sounds, considered to have a warning role during escaping behavior, have also been identified in several columbids, such as the inca dove (Columbina inca; Johnston 1960), mourning dove (Zenaida macroura; Coleman 2008), rock pigeon (Columba livia; Niese and Tobalske 2016), and crested pigeon (Ochyphaps lophotes; Murray et al. 2017).
Acoustic signals are considered as one of the main mechanisms of communication, especially for their range potential (Smith and Harper 2003). In particular, the role of non-vocal sounds seems to be dependent on adaptive selections (Prum 1998; Clark and Prum 2015), but so far this communication channel is poorly known. Recently, Murray et al. (2017) have demonstrated using playbacks and feather removal experiments that modified feathers of the crested pigeon are used as a reliable non-vocal alarm signal after a potential threat. This reinforces the need to comprehend the evolution of this type of communication among species, especially in situations of high predation risk, evaluating its effects on group coordination (Niese and Tobalske 2016).
Recognizing if a species has the ability to intentionally modulate a non-vocal sound which has evolved for a communication function (i.e., voluntariness) is obviously not an easy task (Bostwick and Prum 2003; Clark 2016). The identification of specialized morphology and behavior or the use of appropriate experiments may help to produce unequivocal evidence for voluntariness. Despite that, basic information about the context of non-vocal sound production and the response of conspecifics is unknown for most species. In pigeons, the ability to produce subtle modifications of wing kinematics during the wing trill emission suggests that the production of the non-vocal sound may be voluntary (Clark 2016). The scaled dove (Columbina squammata) is a small columbid widely distributed in the Neotropical region. Among its main characteristics, its sociality stands out, together with the cryptic coloration and the production of a loud mechanical wing trill during takeoff flight, which may not be emitted under certain circumstances (Sick 1997; Dias 2006). Here, we evaluated the role of the trill produced by the scaled dove through observational recording and manipulation procedures. For this purpose, we tested the following hypotheses: (1) the non-vocal sound is used to communicate about situations of potential danger; thus, it is expected to be more frequently produced during escape takeoffs; (2) the non-vocal sound is directed to conspecifics, so it is expected to be produced in flocks but not in solitary conditions; (3) the non-vocal sound is interpreted as an alarm information by other flock members, and consequently it should promote escape responses on conspecifics; and (4), in flocks, individuals closer to the non-vocal sound producer should present faster escape responses in comparison to further ones.
Materials and methods
Study area
The study was conducted on the campus of the University of Brasilia, located in the central region of Brazil. The vegetation of the area is highly modified, composed of small grasses and scattered trees, surrounded by buildings of the institution. The presence of domestic animals (e.g., dogs and cats), car traffic and other natural predators (e.g., Elanus leucurus, Falco femoralis, Gampsonyx swainsonii) are the main threats faced by the birds in the area. The observations and recordings were conducted during the years of 2015 and 2016 between 0730 and 1100 hours, Brasilia time.
General procedures
Active searches were conducted to locate scaled dove individuals, both in flocks and solitary. After locating the individuals, we approached smoothly (trying not to disturb the natural behavior) staying around 15 m away to conduct the observations. The focal sampling initiated when the individuals were seen foraging, drinking water or resting on the ground. The data were registered both from personal observation and through video recordings. The video recordings were made with a 5-megapixel digital camera (Bright 0372 HD) supported by a tripod.
After the field procedures, all the video recordings were analyzed to assure the behavior of all flock members were individually considered. The study area has a large population of the scaled dove, and this may have helped to avoid pseudoreplication, since the birds were not individually marked. We measured to the nearest millimeter the width of the six outer primaries of two individuals to investigate the presence of any morphological adaptation for sound production. The width was measured at 2.5 cm from each feather tip.
Recording and sound analysis
We conducted audio recordings of individuals’ non-vocal sounds using a digital Marantz PMD 660 recorder (16-bit precision and 44.1 Hz sampling rate), coupled to a Sennheiser K6/ME66 unidirectional microphone. Recordings were made between 0700 and 1100 hours. We analyzed the non-vocal sounds using the Raven Pro 1.5 (Bioacoustics Research Program 2014). We selected recordings of five different individuals with low background noise for the analysis, but also filtered out background sound below 400 Hz. We measured (1) the pulse period (time between the beginning of two successive pulses), (2) the pulse rate (Hz) and (3) the peak frequency (the frequency with the highest energy). Measurements were made on spectrograms with a Hann window of 512 samples, a hop size of 2.13 ms and with an 80.1% overlap.
Part 1: Context of non-vocal sound production
To understand the natural context of the non-vocal sound production, we conducted animal and group focal observations for 30 min or until one of the individuals initiated a takeoff flight. For each focal unit, we recorded the flock size at the beginning of the observation, the presence of a potential threat (yes or no) and the occurrence of takeoff flight (yes or no). If any individual fled during the observation period, we registered whether or not it was followed by the production of a non-vocal sound (which is loud and can be heard several meters away), and if it stimulated an escape response and vigilance behavior of other group members (yes or no). Vigilance behavior was defined as any moment that the individuals lifted their head upward while looking around. Additionally, for flocks, we registered the position of the individuals within the flock to investigate the role of spatial position (central or periphery) and also the response time of each flock member to the takeoff flight. We considered response time as the interval between the takeoff of a flock member and the response of each of the other members.
Part 2: Simulation of predation risk
After concluding the first part of the study, we began to evaluate the effects of simulated attacks on dove behavior. After locating the individuals, we registered the flock size and approached them, staying at around 15 m distance. After waiting 15 s, the simulated predation attack was performed. The simulations were conducted through a direct and steady running movement toward the center of the evaluated unit (flock or solitary individual) by one of the researchers (P.P.A.). After the simulation, the same variables previously described in Part 1 were registered (the occurrence of takeoff flight, production of non-vocal sound, the position of the individuals within the flock, the response time of each flock member to the takeoff flight and whether or not it stimulated vigilance behavior in other group members). Again, for flocks, the video recordings were used to quantify the response time (s) for each flock member after the takeoff flight and were also used to record the spatial position of flock members during that moment.
Statistical analyses
To test our hypothesis, the non-vocal sound production (yes or no) was fitted as a response term in a generalized linear model (GLM) with binomial family where the occurrence of a potential threat (yes or no) and the social organization (flocks or solitary individuals) were fitted as explanatory variables. Similarly, the vigilance (yes or no) and flight response (yes or no) of the focal birds, after the takeoff of any flock member, were fitted as a response term and the production of non-vocal sound (yes or no) was fitted as an explanatory variable. The effect of the non-vocal sound production in flock response time (s) was evaluated fitting a GLM with a Poisson family. To determine whether flock position influenced the vigilance response (yes or no), flight response (yes or no) and response time (s), we fitted a generalized linear mixed-effects models (GLMMs) with flock ID included as a random effect. For this analysis, we used the lme4 package (Bates et al. 2015). We used likelihood ratio tests to compare nested models of increasing simplicity. All statistical analyses were carried out in R v.3.4.1 (R Core Team 2017).
Results
Morphology of primary feathers and wing trill structure
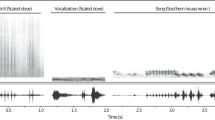
It was not possible to visually identify a clear modification on the six outermost primary feathers (P10–P5; Fig. 1a, b). Despite that, the three outermost feathers (P10–P8) are narrower than the adjacent ones and it possible to see a round shape on the central portion of the P7–P5 feathers. The wing trill produced by the scaled dove was composed of a sequence of repeated notes with an average pulse period of 0.058 ± 0.004 s (mean ± SD) and a pulse rate of 17.24 ± 1.10 (mean ± SD). The average observed peak frequency was 5205.20 ± 532.74 kHz (mean ± SD, Fig. 2).
Part 1: Context of non-vocal sound production
During this observational section, we evaluated 101 solitary individuals and 44 flocks (range 2–3). The average observation time (s) was 260.99 ± 356.15 (mean ± SD). In most cases (n = 141), the solitary individuals and flocks fled within the observation time. For those individuals and flocks, 57% (n = 80) voluntarily left the area without being disturbed. All individuals and flocks exposed to a potential threat (e.g., approaching predator) immediately left the area. The production of the non-vocal sound was influenced by whether or not the individuals were gathered in flocks (χ2 = 4.60; P = 0.031; Fig. 3), but were not directly affected by the occurrence of a potential threat (χ2 = 0.91; P = 0.337). The non-vocal sound produced by at least one of the flock members seems to positively affect the takeoff response of the other birds (χ2 = 8.68; P = 0.003), but had no influence on the vigilance response immediately after the sound production (χ2 = 0.01; P = 0.909). Flock members presented a faster response time (s) after the production of non-vocal sound (χ2 = 80.30; P < 0.001; Fig. 4) in comparison to no sound production.
Part 2: Simulation of predation risk
We simulated predation attacks on 48 solitary individuals and 53 flocks (range 2–7). In all simulations, the target individual flew away from the area, being followed by at least one member of the flock, in social conditions. Invariably, the target individuals produced the non-vocal sound during the escape takeoff. The alert response of flock members was determined by their position within the flock. Central individuals presented a higher probability to became vigilant after the production of the non-vocal sound than peripheral ones (χ2 = 30.96; P < 0.001; Fig. 5a). Similarly, central individuals were more likely to perform an escape takeoff after the non-vocal sound (χ2 = 30.97; P < 0.001; Fig. 5b). However, the response time of those central individuals was not faster than the ones in the periphery (χ2 = 0.31; P = 0.576).
a Effect of flock position on the vigilance state (mean ± SE) of flock members of the scaled dove (Columbina squammata) and b effect of flock position on the probability of taking-off (mean ± SE) by flock members of the scaled dove (Columbina squammata) after an experimental simulation of predation risk
Discussion
The production and function of mechanical sounds have been studied in several taxonomic groups including arthropods, mammals and birds. These non-vocal sounds were selected under different contexts and signal specific messages used during mate choice (Van Staaden and Römer 1997; Prum 1998; Bostwick and Prum 2003; Hebets 2008; Koch et al. 2015), territory defense (Miller and Inouye 1983; Bowen et al. 2008; Schuppe et al. 2016) and under perceived predation risk situations (Randall et al. 2000; Cristaldo et al. 2015). In some columbids, non-vocal sounds have been demonstrated to transmit relevant information about predation risk (Coleman 2008; Hingee and Magrath 2009; Murray et al. 2017). Here, we observed that the production of non-vocal sound during takeoffs is not mandatory in scaled doves. The results suggest that the non-vocal sound production may function mainly as a predation avoidance mechanism, but it is highly context-dependent. The non-vocal sound may have multiple signaling functions such as communicating information about predation risk and possibly group coordination (e.g., changing foraging area). This multiple signaling function on the same trait has been observed for other species, such as the lined rainbow-skink (Carlia jarnoldae), which performs tail displays more frequently during competition with same-sex conspecifics and when interacting with potential predators (Langkilde et al. 2005).
In both natural and simulated contexts, potential threats seem to influence scaled dove escape decision. When a potential threat was present, all focal solitary individuals and at least one individual of each observed focal flocks fled. These results suggest that the perceived risk may differ between solitary and flocked birds, with solitary individuals responding promptly to any direct threat. A study that analyzed the effects of predatory threat on perches (Perca fluviatilis) demonstrated that solitary individuals were less bold than individuals in groups (Goldenberg et al. 2014). When evaluating the role of the non-vocal sound as an alarm information, we observed that, although the trill production was statistically more common in flocks, solitary individuals also produced non-vocal sound during escape takeoffs, especially under simulated attacks. This result indicates that trill production in scaled doves may have the function to warn conspecifics about a potential danger, but it might also communicate to the predator that it has been spotted, as suggested for the wag-display of the turquoise-browed motmot (Murphy 2006). An investigation of acoustic directionality of antipredator calls from different bird species demonstrated that alarm calls usually have low directionality, suggesting that they function to communicate with surrounding conspecifics. However, some species can increase the directionality in the presence of predators, indicating that some antipredator calls are aimed at communicating with both conspecifics and predators (Yorzinski and Patricelli 2010). Warning conspecifics is the more common function of alarm signals (Burton and Yasukawa 2001; Dabelsteen 2005; Patricelli et al. 2008), but signals may carry other important information that could also influence predator decision (Curio 1978; Sherman 1985). For example, in Thomson’s gazelles (Gazella thomson), it is likely that stotting serves to alert the predator that it has been detected (Caro 1986) or to advertise to the predator their escaping ability (Fitzgibbon and Fanshawe 1988). Alternatively, wing sound production may distract the predator, leaving time for individuals to escape, especially when the perceived danger is more intense (Sherman 1977).
In flocks of scaled doves, the production of the non-vocal sound during takeoffs elicited more escape responses in other flock members in comparison to takeoffs without sound production. Despite that, trill production did not affect the vigilance response of those individuals that chose to stay. The results suggest that some receivers interpret the non-vocal sound as a trigger to escape while others may incorporate different environment information about the potential risk of predation to formulate the decision whether to escape or stay. The risk sensitivity hypothesis predicts that several factors may influence prey response in encounters with predators (Lovegrove and Wissel 1988; Lovegrove 1991; Cooper 2009). This is because the risk varies as a function of the distance to the predator (Helfman 1989) in relation to group size (Helfman and Winkelman 2010) and may also be associated with cost reduction, such as avoid stopping foraging (Ydenberg and Dill 1986).
The escape response time was correlated with the production of the non-vocal sound. In natural conditions, individuals showed faster escape responses after the trill was produced. Variation in the speed of escape response may be associated with physical constraints related to signal detection or to the cost–benefit tradeoff of escaping (Ydenberg and Dill 1986; Quinn and Cresswell 2005). Possibly, the absence of other stimuli (e.g., a predator attack) may have made the escape decision less advantageous in some cases. Considering the context of non-vocal sound signaling, playback experiments on crested pigeons demonstrated that the mechanical wing whistle promoted an escape response in most analyzed flocks (Hingee and Magrath 2009). Additionally, wing whistle sproduced by zenaida doves (Zenaida aurita) increased vigilance of conspecifics, but seems to be less informative than the predator vocalization, suggesting that other clues from predators may influence predation risk perception (Barrera et al. 2011).
In the simulated attacks, central individuals presented a faster response after the wing trill production. Likewise, individuals in the center of the flock showed a higher probability to become vigilant after a potential threat, if they have not fled. Perceived predation risk and economic decisions may also explain the effect of the position within the flock on the escape response time and vigilance (Cooper, 2009; Quinn and Cresswell 2005). Movement coordination during escaping behavior is especially important to avoid collisions (Nudds and Bryant 2003; Usherwood et al. 2011) and to maximize the speed of response (Hilton et al. 1999). Here, due to the fact that the groups are small, loosely distributed and that the sound started at the edge of the flock, the faster speed of escape of central individuals could simply be due to their, on average, closer spatial distance in relation to the non-vocal sound sourcei (but see Beauchamp 2012).
In addition to its anti-predator proprieties, non-vocal sound production may be relevant for group coordination in order to maintain group cohesion (Conradt and Roper 2003). Individuals may use the mechanical sound to inform other group members that they are leaving the area. The timing and directionality of group movement are relevant to avoid reducing the benefits of group living (Krause and Ruxton 2002; Conradt and Roper 2007). Factors other than a predator attack (e.g., food depletion) may be responsible for initiatng the movement of some individuals of the flock. Groups of meerkats (Suricata suricatta), for example, use individual calls as an assessment of food patch quality to decide whether or not to move (Bousquet et al. 2011).
Predation is one of the main forces shaping the evolution of survival strategies. Despite being a potential source of information, there is little evidence about the use of non-vocal sounds to alert concerning predation risk. The effects of the non-vocal sound production on the behavior of aggregated conspecifics, and the suggestion, still to be confirmed, of a voluntary sound production, weaken the possibility that the non-vocal sound is merely a non-intentional cue. Under an evolutionary perspective, it is probable that the non-vocal sound production must have become an important source of information through its accumulated benefits acquired by social individuals and its dissuasive potential, considering its possible effects on predators. Here, we suggest that the mechanical sound produced by scaled doves during escape takeoffs might be used for intraspecific communication about predation risk and during predator–prey interactions. Future manipulations based on feather removal and playback experiments may help to clarify some of the gaps not addressed in this study. Several questions are still open for future studies such as (1) how does the mechanism of sound production work in scaled doves? (2) Do non-vocal sound characteristics (e.g., sound amplitude) influence group members’ response? And (3) what is the effect of the non-vocal sound on predator behavior? In addition, much more research is still needed to help elucidate the role of non-vocal communication in birds.
References
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383. https://doi.org/10.1146/annurev.es.05.110174.001545
Alvarez F, Sanchez C, Angulo S (2006) Relationships of tail-flicking, morphology and body condition in moorhens. J Field Ornithol 77:1–6. https://doi.org/10.1111/j.1557-9263.2006.00001.x
Barrera JP, Chong L, Judy KN, Blumstein DT (2011) Reliability of public information: predators provide more information about risk than conspecifics. Anim Behav 81:779–787. https://doi.org/10.1016/j.anbehav.2011.01.010
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beauchamp G (2012) Flock size and density influence speed of escape waves in semipalmated sandpipers. Anim Behav 83:1125–1129. https://doi.org/10.1016/j.anbehav.2012.02.004
Bioacoustics Research Program (2014) Raven pro: interactive sound analysis software (Version 1.5) [Computer software]. The Cornell Lab of Ornithology, Ithaca, NY. http://www.birds.cornell.edu/raven
Bitton PP, Doucet SM (2014) A multifunctional visual display in elegant trogons targets conspecifics and heterospecifics. Behav Ecol 25:27–34. https://doi.org/10.1093/beheco/art065
Bostwick KS, Prum RO (2003) High-speed video analysis of wing-snapping in two manakin clades (Pipridae: Aves). J Exp Biol 206:3693–3706. https://doi.org/10.1242/jeb.00598
Bostwick KS, Elias DO, Mason A, Montealegre-Z F (2010) Resonating feathers produce courtship song. Proc R Soc Lond B 277:835–841. https://doi.org/10.1098/rspb.2009.1576
Bousquet CAH, Sumpter DJT, Manser MB (2011) Moving calls: a vocal mechanism underlying quorum decisions in cohesive groups. Proc R Soc Lond B 278:1482–1488. https://doi.org/10.1098/rspb.2010.1739
Bowen JI, Mahony SJ, Mason AC, Yack JE (2008) Vibration-mediated territoriality in the warty birch caterpillar Drepana bilineata. Physiol Entomol 33:238–250. https://doi.org/10.1111/j.1365-3032.2008.00627.x
Burton N, Yasukawa K (2001) The “predator early warning system” of red-winged blackbirds. J Field Ornithol 72:106–112. https://doi.org/10.1648/0273-8570-72.1.106
Caro TM (1986) The functions of stotting in Thomson’s gazelles Gazella thomsoni: some tests of the predictions. Anim Behav 34:663–684. https://doi.org/10.1016/S0003-3472(86)80052-5
Clark RW (2005) Pursuit-deterrent communication between prey animals and timber rattlesnakes (Crotalus horridus): the response of snakes to harassment displays. Behav Ecol Sociobiol 59:258–261. https://doi.org/10.1007/s00265-005-0032-9
Clark CJ (2008) Fluttering wing feathers produce the flight sounds of male streamertail hummingbirds. Biol Lett 4:341–344. https://doi.org/10.1098/rsbl.2008.0252
Clark CJ (2016) Locomotion-induced sounds and sonations: mechanisms, communication function, and relationship with behavior. In: Suthers R, Fitch T (eds) Vertebrate sound production and acoustic communication. Springer handbook of auditory research, vol 53, pp 83–117
Clark CJ, Prum RO (2015) Aeroelastic flutter of feathers, flight and the evolution of non-vocal communication in birds. J Exp Biol 218:3520–3527. https://doi.org/10.1242/jeb.126458
Clark CJ, Kirschel ANG, Hadjioannou L, Prum RO (2016) Smithornis broadbills produce loud wing song by aeroelastic flutter of medial primary wing feathers. J Exp Biol 219:1069–1075. https://doi.org/10.1242/jeb.131664
Coleman SW (2008) Mourning dove (Zenaida macroura) wing-whistles may contain threat-related information for con- and hetero-specifics. Naturwissenschaften 95:981–986. https://doi.org/10.1007/s00114-008-0404-x
Collias NE (1987) The vocal repertoire of the red jungle fowl: a spectrographic classification and the code of communication. The Condor 89:510–524. https://doi.org/10.2307/1368641
Conradt L, Roper TJ (2003) Group decision-making in animals. Nature 421:155–158. https://doi.org/10.1038/nature01294
Conradt L, Roper TJ (2007) Democracy in animals: the evolution of shared group decisions. Proc R Soc Lond B 274:2317–2326. https://doi.org/10.1098/rspb.2007.0186
Cooper WE (2009) Fleeing and hiding under simultaneous risks and costs. Behav Ecol 20:665–671. https://doi.org/10.1093/beheco/arp049
Cooper WE, Pérez-Mellado V, Baird TA, Caldwell JP, Vitt LJ (2004) Pursuit deterrent signalling by the Bonaire whiptail lizard Cnemidophorus murinus. Behaviour 141:297–311. https://doi.org/10.1163/156853904322981860
Courter JR, Ritchison G (2010) Alarm calls of tufted titmice convey information about predator size and threat. Behav Ecol 21:936–942. https://doi.org/10.1093/beheco/arq086
Cristaldo PF, Jandak V, Kutalová K, Rodrigues VB, Brothánek M, Jiřı́ček O, DeSouza O, Šabotnı́k J (2015) The nature of alarm communication in Constrictotermes cyphergaster (Blattodea: Termitoidea: Termitidae): the integration of chemical and vibroacoustic signals. Biol Open 4:1649–1659. https://doi.org/10.1242/bio.014084
Curio E (1978) The adaptive significance of avian mobbing. I. Teleonomic hypotheses and predictions. Z Tierpsychol 48:175–183. https://doi.org/10.1111/j.1439-0310.1978.tb00254.x
Dabelsteen T (2005) Public, private or anonymous? Facilitating and countering eavesdropping. In: Mcgregor PK (ed) Animal communication networks. Cambridge University Press, Cambridge, pp 38–62
Dias RI (2006) Effects of position and flock size on vigilance and foraging behaviour of the scaled dove Columbina squammata. Behav Processes 73:248–252. https://doi.org/10.1016/j.beproc.2006.06.002
Dittmann L, Schausberger P (2017) Adaptive aggregation by spider mites under predation risk. Sci Rep 7:01–09. https://doi.org/10.1038/s41598-017-10819-8
Fairbanks B, Dobson FS (2007) Mechanisms of the group-size effect on vigilance in Columbian ground squirrels: dilution versus detection. Anim Behav 73:115–123. https://doi.org/10.1016/j.anbehav.2006.07.002
Fernández-Juricic E, Delgado JA, Remacha C, Jiménez MD, Garcia V, Hori K (2009) Can a solitary avian species use collective detection? An assay in semi-natural conditions. Behav Processes 82:67–74
Fichtel C, Kappeler PM (2002) Anti-predator behavior of groupliving Malagasy primates: mixed evidence for a referential alarm call system. Behav Ecol Sociobiol 51:262–275. https://doi.org/10.1007/s00265-001-0436-0
Fitzgibbon CD, Fanshawe JH (1988) Stotting in Thomson’s gazelles: an honest signal of condition. Behav Ecol Sociobiol 23:69–74. https://doi.org/10.1007/BF00299889
Gardner MG, Pearson SK, Johnston GR, Schwarz MP (2016) Group living in squamate reptiles: a review of evidence for stable aggregations. Biol Rev Camb Philos Soc 91:925–936. https://doi.org/10.1111/brv.12201
Goldenberg SU, Borcherding J, Heynen M (2014) Balancing the response to predation—the effects of shoal size, predation risk and habituation on behaviour of juvenile perch. Behav Ecol Sociobiol 68:989–998. https://doi.org/10.1007/s00265-014-1711-1
Hasson O (1991) Pursuit deterrent signals: the communication between prey and predator. Trends Ecol Evol 6:325–329. https://doi.org/10.1016/0169-5347(91)90040-5
Hebets EA (2008) Seismic signal dominance in the multimodal courtship display of the wolf spider Schizocosa stridulans Stratton 1991. Behav Ecol 19:1250–1257. https://doi.org/10.1093/beheco/arn080
Helfman GS (1989) Threat-sensitive predator avoidance in damselfish–trumpetfish interaction. Behav Ecol Sociobiol 24:47–58. https://doi.org/10.1007/BF00300117
Helfman GS, Winkelman DL (2010) Threat sensitivity in bicolor damselfish: effects of sociality and body size. Ethology 103:369–383. https://doi.org/10.1111/j.1439-0310.1997.tb00153.x
Hill RA, Lee PC (1998) Predation risk as an influence on group size in cercopithecoid primates: implications for social structure. J Zool 245:447–456. https://doi.org/10.1111/j.1469-7998.1998.tb00119.x
Hilton GM, Cresswell W, Ruxton GD (1999) Intraflock variation in the speed of escape-flight response on attack by an avian predator. Behav Ecol 10:391–395. https://doi.org/10.1093/beheco/10.4.391
Hingee M, Magrath RD (2009) Flights of fear: a mechanical wing whistle sounds the alarm in a flocking bird. Proc R Soc Lond B 276:4173–4179. https://doi.org/10.1098/rspb.2009.1110
Johnston RF (1960) Behavior of the Inca Dove. Condor 62:7–24. https://doi.org/10.2307/1365655
Koch RE, Krakauer AH, Patricelli GL (2015) Investigating female mate choice for mechanical sounds in the male Greater Sage-Grouse. Auk 132:349–358. https://doi.org/10.1642/AUK-14-63.1
Krause J, Ruxton G (2002) Living in groups. Oxford University Press, Oxford
Langkilde T, Schwarzkoff L, Alford RA (2005) The function of tail displays in male Rainbow Skinks (Carlia jarnoldae). J Herpetol 39:325–328. https://doi.org/10.1670/0022-1511(2005)39%5b0325:TFOTDI%5d2.0.CO;2
Lima SL (1994) Collective detection of predatory attack by birds in the absence of alarm signals. J Avian Biol 25:319–326. https://doi.org/10.2307/3677279
Lovegrove BG (1991) The evolution of eusociality in molerats (Bathyergidae): a question of risks, numbers, and costs. Behav Ecol Sociobiol 28:37–45
Lovegrove BG, Wissel C (1988) Sociality in molerats: metabolic scaling and the role of risk sensitivity. Oecologia 74:600–606
Martín J, Luque-Larena JJ, Lopez P (2006) Collective detection in escape responses of temporary groups of Iberian green frogs. Behav Ecol 17:222–226. https://doi.org/10.1093/beheco/arj024
Miller S, Inouye DW (1983) Roles of the wing whistle in the territorial behaviour of male broad-tailed hummingbirds (Selasphorus platycercus). Anim Behav 31:689–700. https://doi.org/10.1016/S0003-3472(83)80224-3
Murphy TG (2006) Predator-elicited visual signal why the turquoise-browed motmot wag-displays its racketed tail. Behav Ecol 17:547–553. https://doi.org/10.1093/beheco/arj064
Murray TG, Zeil J, Magrath RD (2017) Sounds of modified flight feathers reliably signal danger in a pigeon. Curr Biol 27:3520–3525. https://doi.org/10.1016/j.cub.2017.09.068
Niese RL, Tobalske BW (2016) Specialized primary feathers produce tonal sounds during flight in rock pigeons (Columba livia). J Exp Biol 219:2173–2181. https://doi.org/10.1242/jeb.131649
Nudds RL, Bryant DM (2003) Zebra finches, Taeniopygia guttata, with elongated tails delay taking flight during group take-offs. Behav Ecol Sociobiol 55:122–128. https://doi.org/10.1007/s00265-003-0686-0
Patricelli GL, Dantzker MS, Bradbury JW (2008) Acoustic directionality of red-winged blackbird (Agelaius phoeniceus) song relates to amplitude and singing behaviours. Anim Behav 76:1389–1401. https://doi.org/10.1016/j.anbehav.2008.07.005
Prum RO (1998) Sexual selection and the evolution of mechanical sound production in the manakins (Aves: Pipridae). Anim Behav 55:977–994. https://doi.org/10.1006/anbe.1997.0647
Putman BJ, Clark RW (2015) The fear of unseen predators: ground squirrel tail flagging in the absence of snakes signals vigilance. Behav Ecol 26:185–193. https://doi.org/10.1093/beheco/aru176
Quinn JL, Cresswell W (2005) Escape response delays in wintering redshank, Tringa totanus, flocks: perceptual limits and economic decisions. Anim Behav 69:1285–1292. https://doi.org/10.1016/j.anbehav.2004.10.007
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.Rproject.org/
Randall JA, Rogovin KA, Shier D (2000) Antipredator behavior of a social desert rodent: footdrumming and alarm calling in the great gerbil, Rhombomys opimus. Behav Ecol Sociobiol 48:110–118. https://doi.org/10.1007/s002650000199
Rubenstein DI (1978) On predation, competition, and the advantages of group living. In: Bateson PPG, Klopfer PH (eds) Social behavior. Perspectives in ethology, vol 3. Springer, Boston, pp 205–231
Ruiz-Rodríguez M, Martín-Vivaldi M, Avilés JM (2017) Multi-functional crest display in Hoopoes (Upupa epops). J Avian Biol 48:1425–1431. https://doi.org/10.1111/jav.01402
Schmitt MH, Stears K, Wilmers CC, Shrader AM (2014) Determining the relative importance of dilution and detection for zebra foraging in mixed species herds. Anim Behav 96:151–158. https://doi.org/10.1016/j.anbehav.2014.08.012
Schuppe ER, Sanin GD, Fuxjager MJ (2016) The social context of a territorial dispute differentially influences the way individuals in breeding pairs coordinate their aggressive tactics. Behav Ecol Sociobiol 70:673–682. https://doi.org/10.1007/s00265-016-2088-0
Seyfarth RM, Cheney DL, Marler P (1980) Monkey responses to three different alarm calls: evidence of predator classification and semantic communication. Science 210:801–803. https://doi.org/10.1126/science.7433999
Sherman PW (1977) Nepotism and the evolution of alarm calls. Science 197:1246–1253. https://doi.org/10.1126/science.197.4310.1246
Sherman PW (1985) Alarm calls of Belding’s ground squirrels to aerial predators: nepotism or self-preservation? Behav Ecol Sociobiol 17:313–323. https://doi.org/10.1007/BF00293209
Sick H (1997) Ornitologia Brasileira. Nova Fronteira, Rio de Janeiro
Smith MJ, Harper D (2003) Animal signals. Oxford University Press, Oxford
Soard CM, Ritchison G (2009) ‘Chick-a-dee’ calls of Carolina chickadees convey information about degree of threat posed by avian predators. Anim Behav 78:1447–1453. https://doi.org/10.1016/j.anbehav.2009.09.026
Sorato E, Gullet PR, Griffith SC, Russell AF (2012) Effects of predation risk on foraging behaviour and group size: adaptations in a social cooperative species. Anim Behav 84:823–834. https://doi.org/10.1016/j.anbehav.2012.07.003
Spieler M (2003) Risk of predation affects aggregation size: a study with tadpoles of Phrynomantis microps (Anura: Microhylidae). Anim Behav 65:179–184. https://doi.org/10.1006/anbe.2002.2030
Usherwood JR, Stavrou M, Lowe JC, Roskilly K, Wilson AM (2011) Flying in a flock comes at a cost in pigeons. Nature 474:494–497. https://doi.org/10.1038/nature10164
Van Staaden MJ, Römer H (1997) Sexual signalling in bladder grasshoppers: tactical design for maximizing calling range. J Exp Biol 200:2597–2608
Wheeler BC (2008) Selfish or altruistic? An analysis of alarm call function in wild capuchin monkeys, Cebus apella nigritus. Anim Behav 76:1465–1475. https://doi.org/10.1016/j.anbehav.2008.06.023
Ydenberg RC, Dill LM (1986) The economics of fleeing from predators. Adv Stud Behav 16:229–249. https://doi.org/10.1016/S0065-3454(08)60192-8
Yorzinski JL, Patricelli GL (2010) Birds adjust acoustic directionality to beam their antipredator calls to predators and conspecifics. Proc R Soc Lond B 277:923–932. https://doi.org/10.1098/rspb.2009.1519
Acknowledgements
We would like to thank the Centro Universitário de Brasília (UniCEUB) and the Institutional Scientific Initiation Scholarship Program (PIC) for the financial support.
Funding
This study was funded by the Institutional Scientific Initiation Scholarship Program (PIC) of the Centro Universitário de Brasília.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P S. Amorim and R. I. Dias declare that they have no conflicts of interest.
Ethical statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The study complied with the current laws of Brazil under permit 50793 from Instituto Brasileiro de Recursos Renováveis (IBAMA).
Human participants
This article does not contain any studies with human participants performed by any of the authors.
About this article
Cite this article
Amorim, P.S., Dias, R.I. Non-vocal communication as an anti-predator strategy in scaled doves (Columbina squammata). J Ethol 37, 157–165 (2019). https://doi.org/10.1007/s10164-018-0583-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-018-0583-7