Abstract
The recognition and subsequent rejection of brood parasite eggs is one of the most commonly observed defensive behaviors of the host. The brown-and-yellow marshbird (Pseudoleistes virescens) is a common host of the shiny cowbird (Molothrus bonariensis). This host recognizes and rejects immaculate white eggs of the cowbird, but accepts this bird’s spotted eggs. We assessed the acceptance threshold hypothesis which proposes that parasite egg recognition is context-dependent. We experimentally parasitized host nests with a spotted cowbird egg and simultaneously added: (1) one novel egg with spots similar to those of a cowbird egg but on a blue background or (2) one immaculate white cowbird egg. In this setting, 78 % of the novel blue egg with spots and 77 % of the cowbird’s immaculate white eggs were quickly recognized and rejected. The rejection frequency of spotted cowbird eggs was also high (60 %) and was not related to the type of egg which had been added to the host nest together with the spotted cowbird egg. This rejection frequency of spotted cowbird eggs is higher than the 21 % that we previously found in a similar experimental setting but in which the spotted cowbird egg was added singly to the host nest. These results support the acceptance threshold hypothesis that predicts an adaptive modulation of the antiparasite defence when the perceived risk of parasitism is high.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brood parasitism in birds often implies a large cost to the parasitized hosts. These costs include egg losses, lower hatching success, lower chick survival and all other costs attributed to misdirected parental care (Petrie and Møller 1991; Øien et al. 1998; Rothstein and Robinson 1998; Davies 2000; Krüger 2007; Spottiswoode et al. 2012). If costs are important, natural selection should favour the evolution of host defences against brood parasites (Rothstein 1974, 1975; Briskie et al. 1992; Sealy et al. 1998; Peer and Sealy 2004).
The recognition and subsequent rejection of parasite eggs is perhaps the best studied host defensive behavior against brood parasites (Rothstein 1982; Sealy 1995; Sealy and Bazin 1995; Peer et al. 2000; Peer and Sealy 2004; Kilner and Langmore 2011). However, not all host species show the ability to recognize and reject parasite eggs (Rothstein 1974; Hauber et al. 2004; Peer and Sealy 2004). When brood parasites lay eggs of varying morphology (colour and/or pattern), hosts usually reject those eggs that are most dissimilar to their own eggs, but they fail to recognize eggs with a similar colouration and/or spotting pattern (Mason 1986; Mermoz and Reboreda 1994; Sackmann and Reboreda 2003). This variability in the response of the host to the polymorphism of the parasite’s egg is related to the host’s cognitive processes in the form of mechanisms of defence against parasites. In these cases, the matching of the pattern, size or colour between the host’s eggs (or a learned template of them) and the parasite’s eggs can be used to decide to reject the foreign egg or not (Rothsthein 1974, 1975; Moksnes 1992; Hauber and Sherman 2001; Hauber et al. 2006; Moskát and Hauber 2007; Moskát et al. 2010). It has been suggested that this assessment may involve an acceptance threshold based on similarity between the characteristics of the eggs of both, parasites and hosts (Reeve 1989; Liebert and Starks 2004; Hauber et al. 2006). This acceptance threshold should be somewhat flexible in order to maximize the benefits to the host of rejecting parasite eggs and, at the same time, minimize the costs of rejecting its own eggs due to incorrect egg recognition (Reeve 1989; Liebert and Starks 2004; Hauber et al. 2006). As the predictability of brood parasitism for a given species or population increases, the acceptance threshold should be more restrictive and, vice versa, as parasitism frequency decreases, the acceptance threshold should relax (Moksnes et al. 1993; Hauber et al. 2006).
In the study reported here we analysed the recognition and rejection behaviour of the brown-and-yellow marshbird (Pseudoleistes virescens), a species that inhabits marshy areas and humid grasslands in the pampas of Argentina and neighbouring areas of Uruguay and Brazil (Ridgely and Tudor 1989) and which is heavily parasitized by the shiny cowbird, Molothrus bonariensis (Hudson 1874; Gibson 1918; Orians 1980; Mermoz and Reboreda 1999). In our study population, frequency of parasitism has been found to average 65 % over a 15-year period (range 35–91 %; Mermoz et al. unpublished data). In addition, multiple parasitism (i.e. ≥2 cowbird eggs per parasitized nest) averaged 60 % of parasitized nests (range 50–80 %). The costs of cowbird parasitism to the host are related to the egg pecking behaviour of females (Mermoz and Reboreda 1994, 1999), lower hatching success of eggs in parasitized clutches (Mermoz et al. unpublished data) and lower survival of nestlings (Duré Ruiz et al. 2008; Mermoz et al. unpublished data). In the Southern temperate pampas shiny cowbird eggs are polymorphic in terms of spotting pattern, varying from completely immaculate white to heavily spotted (Mason 1986; Mahler et al. 2008). The brown-and-yellow marshbird lays brown-spotted eggs with a white background (Fig. 1). The results of previous studies in our study population have shown that the brown-and-yellow marshbird is able to recognize and reject those cowbird eggs which are more dissimilar to their own eggs in terms of spotting pattern (Mermoz and Reboreda 1994; Mermoz et al. 2013). Accordingly, the rejection rates of immaculate cowbird eggs are about 90 %, while those of spotted cowbird eggs are only 8 % (Mermoz et al. 2013). This variability in the rejection behaviour led to our study of the cues used by brown-and-yellow marshbirds to recognize and subsequently reject parasite eggs. We therefore designed and carried out a field experiment in which we added one mimetic egg (i.e. similar in appearance to the brown-and-yellow marshbird eggs) together with one non-mimetic (a white immaculate or a novel blue egg with spots) to the host’s nest. The addition of a novel blue egg with spots also constitutes an artificial stimulus that allowed us to assess if the egg rejection behaviour of brown-and-yellow marshbirds depends on previous experience with the phenotype of a foreign egg. Prevalent theories on the rejection of parasite eggs assume that host recognition of parasite eggs is based on the discordance of egg characteristics (rejecting the less common egg type in the clutch; Rothstein 1975, Hauber and Sherman 2001) or on the comparison of eggs with an imprinted (Rothstein 1974) or memory-based template of the characteristics of the host’s own eggs (Moksnes 1992; Lotem et al. 1995; Hauber and Sherman 2001). Both mechanisms imply that the acceptance threshold is based on dissimilarity between the host’s own eggs and the parasite eggs rather than on the aspect (phenotype) of the foreign egg. Consequently, we tested if experience with the egg phenotype of the parasite is important in determining the level of egg rejection in the brown-and-yellow marshbird. If the acceptance threshold is based on the dissimilarity between eggs, we would expect the host to have similar rejection rates for both non-mimetic foreign eggs regardless of the phenotype of the egg.
a Examples of artificial parasite eggs used in the experiments. These artificial eggs were painted to simulate white immaculate cowbird (Molothrus bonariensis) eggs (Cwl; top row), novel blue eggs with spots (NBS; middle row) and spotted cowbird eggs (CwS; bottom row). b Examples of natural spotted cowbird eggs. c A naturally parasitized brown-and-yellow marshbird (Pseudoleistes virescens) nest with three host eggs (Pv) and two spotted cowbird eggs (Cw). d An experimental nest where we had added an artificial blue egg with spots (NBS) and one spotted cowbird egg (CwS). The eggs were accepted and the nest was later parasitized naturally by a cowbird (Cw)
The simultaneous addition of a spotted and a white immaculate or blue egg with spots also allowed us to analyse if the presence of dissimilar foreign egg morphs affects the acceptance threshold of mimetic (i.e. similar in appearance) parasite eggs. According to the acceptance threshold hypothesis, the acceptance threshold should be more restrictive when hosts detect an increased risk of parasitism (Reeve 1989; Hauber et al. 2006). In this case, in the presence of strongly dissimilar egg morphs, the acceptance threshold of more mimetic eggs is expected to be reduced.
Materials and methods
This study was carried out during 2002–2004 near the town of General Lavalle (36°26′ S, 56°25′ W) in the province of Buenos Aires, Argentina (for a detailed description of the study site see Mermoz et al. 2013).
We first assessed the decision rules used by brown-and-yellow marshbirds to recognize and reject cowbird eggs using artificial eggs made of plaster of Paris and painted with acrylic paint. Brown-and-yellow marshbirds are grasp-ejecters and do not break the egg during ejections. Their behaviour is the same with eggs constructed of plaster of Paris and natural eggs (Mermoz et al. 2013), regjecting artificial and natural eggs at similar rates (Haupt and Mermoz, unpublished data). Artificial eggs were painted to simulate a natural cowbird spotted egg [hereafter referred to as “spotted eggs” (CwS in Fig. 1)], the white morph of the parasite [hereafter referred to as “immaculate eggs” (CwI in Fig. 1)] and a novel blue egg with small brown spots [hereafter referred to as “blue eggs” (NBS in Fig. 1)]. These blue eggs did not resemble any egg morph of the parasite nor thos of the host (Fig. 1d) and constituted a novel stimulus for brown-and-yellow marshbirds.
We searched for nests during early spring (October–November) and chose early laying nests for the experiment (with one or two host eggs; clutch size of brown-and-yellow marshbirds is 4–5 eggs; Mermoz and Reboreda 1998). Nests were assigned to one of two treatments. In Treatment 1 (T1) we added simultaneously one spotted (CwS) and one blue (NBS) egg to the host nest; in Treatment 2 (T2) we added simultaneously one spotted (CwS) and one immaculate (CwI) egg to the host nest. The simultaneous occurrence of two cowbird eggs in one nest is not unusual for the hosts, as the nest-searching areas of female cowbirds overlap (Scardamaglia and Reboreda 2014) and host nests could be parasitized in the same day by two or more females (Mermoz and Reboreda 1999; Gloag et al. 2014). For example, in a study in the chalk-browed mockingbird (Mimus saturninus), the mean time interval between two parasitic events was found to be 5 min, with 31 % of the parasitic events occurring within 60 s of each other (Gloag et al. 2013; R. Gloag, personal communication).
We performed a total of 39 experiments (29 with the spotted/blue egg pair and 10 with the spotted/immaculate egg pair). All experiments were performed early in the morning (0600–0800 hours) and, to minimize disturbance to the hosts, we waited until the adults had left the vicinity of the nest to place the eggs in the nests. We monitored the focal nest (Altmann 1974) continuously for 45 min after the experimental parasitism using binoculars from a distance of 40–60 m. If the experimental eggs were not ejected during this observation period, we checked the nest 2–4 h later and then every 24 h up to a maximum of 5 days.
Data analyses
We compared the frequency of ejection of spotted cowbird eggs on each treatment with those we obtained in a previous study during 1992–1996 when an artificial spotted cowbird egg was put singly into brown-and-yellow marshbird nests (see Mermoz et al. 2013).
To facilitate the comparison of our previous results with those from the present study, we recalculated the ejection frequencies obtained in Mermoz et al. (2013) by including all experimental nests that lasted at least 2 days (see below). Our recalculated rate (21 %) differed from that reported by Mermoz et al. (2013) (8 %), as we included nests which were pecked by cowbirds or depredated. We compared the frequency of egg ejection in experimental nests between treatments using Fisher’s exact test.
We used a survival analysis using non-parametric Cox proportional hazard models to compare the time lapsed until each artificial egg (i.e. spotted, immaculate and blue eggs) was rejected (Jahn-Eimermacher et al. 2011). Eggs that remained in the nest after 5 days, or were depredated but remained in the nest at least 2 days, were considered censored data.
All p values related to the results are two-tailed, and differences were considered to be significant at p < 0.05.
Results
All eggs had high rejection rates. Within the most dissimilar egg types, blue eggs (NBS) were ejected from 22 of 28 nests (78.6 %; one experimental nest was deserted), whereas immaculate eggs (CwI) were ejected in equal proportion to the blue (NBS) ones (77.7 %; 7/9 nests; Fisher’s exact test p = 0.64; Fig. 2; one experimental nest was depredated during the first day after the experimental introduction). The ejection rates of spotted eggs (CwS) did not differ when they were introduced with the blue eggs (CwS/NBS) and when they were introduced with the familiar immaculate (CwS/CwI) eggs (Fisher’s exact test p = 0.45; Fig. 2). The mimetic spotted egg was ejected from 22 of 37 experimental nests (59 %; Fig. 2).
Proportion of artificially added eggs rejected by brown-and-yellow marshbirds according to egg colouration. NBS Novel blue eggs with spots, CwI immaculate white cowbird eggs, Singly CwS spotted cowbird eggs added singly, CwS/NBS spotted cowbird eggs rejected when added simultaneously with the novel blue ones with spots, CwS/CwI spotted cowbird eggs rejected when added simultaneously with cowbird immaculate ones. Numbers above bars Number of experiments
Spotted cowbird eggs added to the nests with immaculate (CwS/CwI) or blue (CwS/NBS) eggs suffered a higher rejection rate than those spotted cowbird eggs which were added singly (Singly CwS) into the nests (22/37 vs. 4/19 nests, respectively; Fisher’s exact test p = 0.009; Fig. 2). Although these experiments were performed over different time periods, there is no evidence that the rejection rates of cowbird eggs by brown-and-yellow marshbirds vary among years—the rejection rates of naturally laid cowbird spotted eggs were similar between studies. During 1992–1996, brown-and-yellow marshbirds ejected at least one cowbird spotted egg in 15 over 97 naturally parasitized nests, whereas during 2002–2004 they rejected five cowbird spotted eggs over 29 nests (Fisher’s exact test p = 0.42). Furthermore, 86.2 % of white immaculate cowbird eggs (25/29) were rejected during the 1992–1996 breeding periods (including the artificially added eggs), whereas 77.7 % of the added white immaculate cowbird eggs were rejected in present experiments (7/9; Fishers’ exact test p = 0.44).
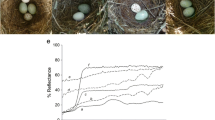
Latency to rejection was also lower for the more dissimilar blue (NBS) and immaculate eggs (CwI) than for the mimetic spotted (CwS) eggs (\(\chi_{2}^{2}\) = 9.18, p = 0.01; Fig. 3a). Mean rejection times were 1.71 ± 1.57 and 1.71 ± 1.65 days [mean ± standard deviation (SD)] for immaculate (CwI) and blue (NBS) eggs, respectively, whereas it was 2.64 ± 1.70 days for spotted eggs [2.71 ± 1.64 days when added with the blue egg (CwS/NBS) and 2.33 ± 2 days when added with the immaculate egg (CwS/CwI)]. Latency to rejection of spotted eggs was reduced when they were introduced with dissimilar eggs (\(\chi_{2}^{2}\) = 8.86, p = 0.01; Fig. 3b), compared to when they were introduced singly. The mean rejection time of spotted cowbird eggs was 2.71 ± 1.64 and 2.33 ± 2 days (mean ± SD) when added with the blue (CwS/NBS) and the immaculate eggs (CwS/CwI), respectively, whereas it was of 4.01 ± 1.46 days when they were added singly (Singly CwS; Fig. 3b).
Latency to rejection of artificially added eggs by brown-and-yellow marshbirds according to egg colour morph. a Comparison of rejection time of spotted cowbird (CwS), novel blue egg with spots (NBS) and immaculate cowbird (CwI) eggs. The CwS eggs were added simultaneously either with a NBS or a CwI egg. b Comparison of rejection times for spotted cowbird eggs when they were added singly (Singly CwS), simultaneously with one blue egg with spots (CwS/NBS, and simultaneously with one immaculate cowbird egg (CwS/CwI)
Discussion
We found that brown-and-yellow marshbirds rejected non-mimetic blue and white immaculate eggs at higher rejection rates, and more quickly, than they did cowbird spotted eggs. Although we had no knowledge of whether the individual hosts assessed in our study had any previous experience with immaculate cowbird eggs, all were naive to the novel blue egg. In addition, rejection behaviour towards the novel and the more familiar egg phenotype did not differ, providing evidence that experience with the parasite egg is not directly involved in the decision rule. The acceptance threshold in this species may be set only on a relative dissimilarity gradient in egg appearance, maximizing the benefits of rejecting parasite eggs relative to the costs of rejecting own eggs (Hauber et al. 2006). The notion that the acceptance threshold is based on dissimilarity rather than on the specific phenotype of the parasite eggs is implicit in all studies performed to date which have assessed the recognition cues of hosts by using artificially painted eggs to simulate non-mimetic parasite eggs (e.g., Móskat et al. 2008; Samaš et al. 2011; Bán et al. 2013; Hauber et al. 2014). Therefore, parasite egg rejection in most hosts may be based on a single and non-specific decision rule that requires only knowledge of the aspect of the host’s own eggs (either learned or imprinted) and which is based on the difference between own eggs and foreign eggs or objects (sensu Moskát et al. 2003).
The major result of our study is related to the shift in the acceptance threshold of mimetic parasite eggs when they were presented together with non-mimetic eggs. It has been proposed that this recognition threshold could vary depending on the predictability of brood parasitism (Reeve 1989; Hauber et al. 2006). According to this view, as the probability of brood parasitism increases, the recognition threshold should be more restrictive, and parasite egg rejection should increase. Our results support this prediction. When spotted cowbird eggs were added to the brown-and-yellow marshbirds nests singly, the percentage of egg ejection was 21 %. However, this percentage increased to approximately 60 % when they were added together with a dissimilar blue or white immaculate egg. Also, the latency to rejection of similar spotted cowbird eggs decreased in the presence of dissimilar eggs in the nest. Together, our results lead us to propose that brown-and-yellow marshbirds have a flexible capability to discriminate and reject parasite eggs. We did not specifically test the cognitive mechanisms underlying this rejection behaviour, but the shift of the acceptance threshold implies that the detection of a non-mimetic egg could trigger a more detailed inspection of nest content. As we parasitized nests when the host had one to two own eggs, birds saw their own and the parasite eggs simultaneously and, therefore, both a template-based recognition and an online self-referencing process (sensu Hauber and Sherman 2001) could be acting in a complementary manner. However, specific experiments should be conducted to test this hypothesis.
This study provides the first evidence of a shiny cowbird host having a recognition system for parasite eggs that is characterized by a relatively flexible acceptance threshold. However, there is experimental evidence indicating that not all of the hosts of the shiny cowbird have a flexible acceptance threshold. In an experiment similar to the one reported here, chalk-browed mockingbirds that were artificially sequentially parasitized with a novel egg without spots and with a spotted cowbird one rejected 45 % of eggs of the novel morph, but they never rejected the spotted cowbird eggs (de la Colina et al. 2012). The chalk-browed mockingbird apparently did not adaptively vary its acceptance threshold as breeding pairs did not modify their acceptance of spotted cowbird eggs, even if they had been previously exposed to a novel egg.
The efficiency of the adaptive flexible system of egg recognition in the brown-and-yellow marshbird as a defence mechanism is low. This species suffers a high parasitism rate (65 %; Mermoz et al., unpublished data) and only rejects a low proportion of parasite eggs (11 %), most of which are white immaculate eggs (Mermoz et al. 2013). It remains paradoxical why this species does not develop a more efficient recognition system. The brown-and-yellow marshbird is a grasp-ejecter that does not damage its own eggs during rejection (Mermoz et al. 2013). However, recognition errors with cowbird eggs having the spotted morph would be potentially high due to their similarity with host eggs (Fig. 1). A “dilution benefit” has also been proposed for chalk-browed mockingbirds which are parasitized multiple times. Egg-pecking by shiny cowbird females is one of the main costs of brood parasitism to large hosts (Reboreda et al. 2003) and, therefore, the presence of cowbird eggs in the nest would reduce the chance of host egg losses due to cowbird pecking (Gloag et al. 2012). Other possible explanations involve the existence of an important gene flow between host populations supporting different brood parasitism rates that might avoid the fixation of a more efficient recognition system (Takasu 1998; Barabás et al. 2004; Røskaft et al. 2006; Stokke et al. 2008). In the shiny cowbird 260 hosts have been recorded (Lowther 2013), and the metapopulation dynamics of those hosts could provide a marked advantage for this brood parasite. Together, the high similarity with most parasite eggs, the potential costs of rejection, the potential benefits of parasite egg acceptance and the existence of an important gene flow between host populations that differ in parasitism rates might preclude the evolution of perfect egg recognition in brown-and-yellow marshbirds.
References
Altmann J (1974) Observational study of behaviour: sampling methods. Behaviour 49:227–267
Bàn M, Moskàt C, Barta Z, Hauber ME (2013) Simultaneous viewing of own and parasitic eggs is not required for egg rejection by a cuckoo host. Behav Ecol 24:1014–1021
Barabás L, Gilicze B, Takasu F, Moskát C (2004) Survival and anti-parasite defense in a host metapopulation under heavy brood parasitism: a source–sink dynamic model. J Ethol 22:143–151
Briskie JV, Sealy SG, Hobson KA (1992) Defenses against avian brood parasites in sympatric and allopatric populations. Evolution 46:334–340
Davies NB (2000) Cuckoos, cowbirds and other cheats. T & AD Poyser, London
de la Colina MA, Pompilio L, Hauber ME, Reboreda JC, Mahler B (2012) Different recognition cues reveal the decision rules used for egg rejection by hosts of a variably mimetic avian brood parasite. Anim Cogn 15:881–889
Duré Ruiz NM, Fernández GJ, Mermoz ME (2008) Effects of cowbird parasitism in the brood reduction of the brown-and-yellow marshbird Pseudoleistes virescens. Condor 110:507–513
Gibson E (1918) Further ornithological notes from the neighbourhood of Cape San Antonio, province of Buenos Aires. Part I: Passeres. Ibis 60:363–415
Gloag R, Fiorini VD, Reboreda JC, Kacelnik A (2012) Brood parasite eggs enhance egg survivorship in a multiple parasitized host. Proc R Soc B 279:1831–1839
Gloag R, Fiorini VD, Reboreda JC, Kacelnik A (2013) The wages of violence: mobbing by mockingbirds as a frontline defence against brood-parasitic cowbirds. Anim Behav 86:1023–1029
Gloag R, Fiorini VD, Reboreda JC, Kacelnik A (2014) Shiny cowbirds share foster mothers but not true mothers in multiply parasitized mockingbird nest. Behav Ecol Sociobiol 68:681–689
Hauber ME, Sherman PW (2001) Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci 24:609–616
Hauber ME, Yeh PJ, Roberts JO (2004) Patterns and coevolutionary consequences of repeated brood parasitism. Proc R Soc B 271[Suppl]:S317–S320
Hauber ME, Moskát C, Bán M (2006) Experimental shift in hosts’ acceptance threshold of inaccurate-mimic brood parasite eggs. Biol Lett 2:177–180
Hauber ME, Samaš P, Anderson MG, Rutila J, Low J, Cassey P, Grim T (2014) Life-history theory predicts host behavioural responses to experimental brood parasitism. Ethol Ecol Evol 26:349–364
Hudson WH (1874) Notes on the procreant instincts of the three species of Molothrus found in Buenos Aires. Proc Zool Soc London 11:153–174
Jahn-Eimermacher A, Lasarzik I, Raber J (2011) Statistical analysis of latency outcomes in behavioural experiments. Behav Brain Res 221:271–275
Spottiswoode CN, Kilner RM, Davies NB (2012) Brood parasitism. In: Royle NJ, Smiseth PT, Kölliker M (eds) The evolution of parental care. Oxford University Press, Oxford, pp 226–243
Kilner RM, Langmore NE (2011) Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol Rev 86:836–852
Krüger O (2007) Cuckoos, cowbirds and hosts: adaptations, trade-offs and constraints. Phil Trans R Soc B 362:1873–1886
Liebert A, Starks PT (2004) The action component of recognition systems: a focus on the response. Ann Zool Fenn 41:747–764
Lotem A, Nakamura H, Zahavi A (1995) Constraints on egg discrimination and cuckoo-host co-evolution. Anim Behav 49:1185–1209
Lowther P (2013) Lists of victims and hosts of the parasitic cowbird (Molothrus). Available at: http://fieldmuseum.org/sites/default/files/Molothrus_hosts-26aug2013.pdf
Mahler B, Confalonieri VA, Lovette IJ, Reboreda JC (2008) Eggshell spotting in brood parasitic shiny cowbird (Molothrus bonariensis) is not linked to the female sex chromosome. Behav Ecol Sociobiol 62:1163–1169
Mason P (1986) Brood parasitism in a host generalist, the shiny cowbird: I. The quality of different species as hosts. Auk 103:52–60
Mermoz ME, Reboreda JC (1994) Brood parasitism of the shiny cowbird, Molothrus bonariensis, on the brown-and-yellow marshbird, Pseudoleistes virescens. Condor 96:716–721
Mermoz ME, Reboreda JC (1998) Nesting success in brown-and-yellow marshbirds: effects of timing, nest site and brood parasitism. Auk 115:871–878
Mermoz ME, Reboreda JC (1999) Egg-laying behaviour by shiny cowbirds parasitizing brown-and-yellow marshbirds. Anim Behav 58:873–882
Mermoz ME, Reboreda JC, Fernández GJ (2013) High rates of shiny cowbird parasitism on brown-and-yellow marshbird select for complementary host defenses. Condor 115:910–920
Moksnes A (1992) Egg recognition in chaffinches and bramblings. Anim Behav 44:993–995
Moksnes A, Røskaft E, Korsnes L (1993) Rejection of cuckoo (Cuculus canorus) eggs by meadow pipits (Anthus pratensis). Behav Ecol 4:120–127
Moskát C, Hauber ME (2007) Conflict between egg recognition and egg rejection decisions in common cuckoo (Cuculus canorus) hosts. Anim Cogn 10:377–386
Moskát C, Székely T, Kisbenedek T, Karcza Z, Bártol I (2003) The importance of nest cleaning in egg rejection behaviour of great reed warblers Acrocephalus arandinaceas. J Avian Biol 34:16–19
Moskát C, Székely T, Cuthill IC, Kisbenedek T (2008) Hosts’ responses to parasitic eggs: which cues elicit hosts’ egg discrimination? Ethology 114:186–194
Moskát C, Bán M, Székely T, Komdeur J, Lucassen RWG, van Boheemen AL, Hauber ME (2010) Discordancy or template based recognition? Dissecting the cognitive basis of the rejection of foreign eggs in hosts of avian brood parasites. J Exp Biol 213:1976–1983
Øien IJ, Moksnes A, Røskaft E, Honza M (1998) Costs of cuckoo Cuculus canorus parasitism to reed warblers Acrocephalus scirpaceus. J Avian Biol 29:209–215
Orians GH (1980) Some adaptations of marsh-nesting blackbirds. Princeton University Press, Princeton
Peer BD, Sealy SG (2004) Correlates of egg rejection in hosts of the brown-headed cowbird. Condor 106:580–599
Peer BD, Robinson SK, Herkert JR (2000) Egg rejection by cowbird hosts in grasslands. Auk 117:892–901
Petrie M, Møller AP (1991) Laying eggs in other’s nests: intraspecific brood parasitism in birds. Trends Ecol Evol 6:315–320
Reboreda JC, Mermoz ME, Massoni V, Astié AA, Rabufetti FL (2003) Impacto del parasitismo de cría del Tordo Renegrido (Molothrus bonariensis) sobre el éxito reproductivo de sus hospedadores. Hornero 18:77–88 (in Spanish)
Reeve H (1989) The evolution of conspecific acceptance thresholds. Am Nat 133:407–435
Ridgely RS, Tudor G (1989) The birds of South America, vol. 1: the oscine passerines. University of Texas Press, Austin
Røskaft E, Takasu F, Moksnes A, Stokke BG (2006) Importance of spatial habitat structure on establishment of host defenses against brood parasitism. Behav Ecol 17:701–708
Rothstein SI (1974) An experimental and teleonomic investigation of avian brood parasitism. Condor 77:250–271
Rothstein SI (1975) Mechanisms of avian egg-recognition: do birds know their own eggs? Anim Behav 23:268–278
Rothstein SI (1982) Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 11:229–239
Rothstein SI, Robinson SK (1998) The evolution and ecology of avian brood parasitism. In: Rothstein SI, Robinson SK (eds) Parasitic birds and their hosts. Oxford University Press, New York, pp 3–56
Sackmann P, Reboreda JC (2003) A comparative study of shiny cowbird parasitism in two large hosts: chalk-browed mockingbird and rufous-belied thrush. Condor 105:728–736
Samaš P, Hauber ME, Cassey P, Grim T (2011) Repeatability of foreign egg rejection: testing the assumptions of co-evolutionary theory. Ethology 117:606–619
Scardamaglia RC, Reboreda JC (2014) Ranging behavior of female and male shiny cowbirds and screaming cowbirds while searching for host nests. Auk 131:610–618
Sealy SG (1995) Burial of cowbird eggs by parasitized yellow warblers: an empirical and experimental study. Anim Behav 49:877–889
Sealy SG, Bazin RC (1995) Low frequency of observed cowbird parasitism on Eastern Kingbirds: host rejection, effective nest defense, or parasite avoidance? Behav Ecol 6:140–145
Sealy SG, Neudorf DL, Hobson KA, Gill SH (1998) Nest defense by potential hosts of the brown-headed cowbird. In: Rothstein SI, Robinson SK (eds) Parasitic birds and their hosts. Oxford University Press, New York, pp 194–211
Stokke BG, Hafstad I, Rudolfsen G, Moksnes A, Møller AP, Røskaft E, Soler M (2008) Predictors of resistance to brood parasitism within and among reed warbler populations. Behav Ecol 19:613–620
Takasu F (1998) Why do all host species not show defense against avian brood parasitism: evolutionary lag or equilibrium? Am Nat 151:193–205
Acknowledgments
We thank M. Beade, and M. Busai for logistical support at the study area. We especially thank V. Ferretti for comments on an earlier version of this manuscript for checking the English grammar. Daniel Hanley and an anonymous reviewer provided useful comments to a previous version of the manuscript. This study was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina (PIP112-200901-00011 to GJF and PICT 11420100100016 to MEM).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mermoz, M.E., Haupt, C. & Fernández, G.J. Brown-and-yellow marshbirds reduce their acceptance threshold of mimetic brood parasite eggs in the presence of non-mimetic eggs. J Ethol 34, 65–71 (2016). https://doi.org/10.1007/s10164-015-0447-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-015-0447-3