Abstract
This study examines the use of fly ash, a thermal power plant waste, and the effect of diatomite, a fossil algae type, on waste-based geopolymers in the production of sustainable geopolymer binders. The effects of 1%, 2%, 3%, 4% and, 5% diatomite substitution on waste-based mortars were investigated. Mortars containing 10% and 12% Na+ by weight based on the binder material were cured at 75 °C for 48 h. The flexural and compressive strength, abrasion resistance, determination of ultrasonic pulse velocity, and resistance to high temperatures of geopolymer mortar samples were investigated. In addition, FESEM images, EDX and XRD analyses of geopolymer mortar samples were made, and their microstructures were examined. 2% diatomite substitution increased flexural and compressive strength. In parallel with this situation, it was concluded that the abrasion resistance and ultrasonic pulse velocity of the geopolymer mortar with 2% diatomite substituted increased. In addition, it has been shown in FESEM images that the microstructure has a denser morphology. All geopolymer mortars lost strength after the high temperatures of 300 °C, 600 °C and 900 °C. As a result, it was concluded that diatomite containing highly reactive silica can be used in geopolymer systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concrete is the most used building material in the construction industry. However, while producing Portland Cement (OPC), the main component of concrete, it causes environmental pollution and requires a lot of energy. In producing one ton of OPC, 1 kg of sulfur dioxide (SO2), about 810 kg of carbon dioxide (CO2) and 10 kg of waste dust are released. Since the CO2 produced during cement production causes global warming and pollution of the atmosphere, new binding materials that can be an alternative to cement are needed [1, 2]. For this purpose, alkali-activated alternative materials have been widely investigated in recent years due to their environmental benefits and improved engineering properties [3,4,5]. Natural and industrially waste produced pozzolan are used in the synthesis of geopolymers. The most widely used pozzolans are fly ash, which is the waste of thermal power plants, and blast furnace slag, which is the waste of iron and steel plants. According to the data of the Turkish Statistical Institute, almost 24.4 million tons of waste were generated from thermal power plants in 2020. Ash and slag wastes constituted 79.5% of these wastes. While 85.9% of the total waste was disposed of in ash mountains, ash dams or storage facilities, 13.2% was sent to licensed facilities and used for backfilling of mines/quarries, and 0.9% was disposed of by other methods. With the use of industrial wastes in geopolymer systems, the waste landfill problem is solved. In addition, more environmentally friendly and sustainable products have been developed to reduce the serious damage to the environment [6,7,8,9,10,11,12].
Aluminum oxide and silicon oxide compounds in the structure of binders such as fly ash, metakaolin, and blast furnace slag are dissolved by alkali activators and provide new bond formation in geopolymer structures. Looking at previous studies, alkali activators such as potassium hydroxide (KOH), sodium hydroxide (NaOH) and sodium silicate (Na2SiO3) were generally used to synthesize geopolymer structures [13,14,15,16,17,18,– 19]. The most important parameters affecting geopolymer synthesis are activator type and curing temperature. When the studies in the literature are examined, it has been observed that while the synthesis of geopolymer takes place slowly at room temperature, it develops more rapidly at high temperatures (between 60 °C and 100 °C) [20,21,22]. Atis et al. studied fly ash-based geopolymer mortar and they produced mortar samples with different Na + (4%–20%) ratios. The samples were heat cured at different temperatures (45 °C–115 °C) and for different curing times (24, 48, and 72 h), and the effects of curing conditions on mechanical properties were investigated. As a result of the study, it was stated that the mechanical strength of geopolymer mortars improved as the sodium ratio increased. They also observed that the mechanical strength increased with increasing curing temperature and time. It has been observed that the effect of thermal curing on strength decreases in cases where the thermal curing temperature is more than 75 °C and the curing time is longer than 48 h [23]. In similar studies, it was stated that the reaction rate in geopolymer synthesis increased with increases in activator molarity, curing temperature, and curing time [24,25,26]. The potential of using different materials in nano- and micro-sizes with high silica content in fly ash-based geopolymer systems has been investigated in previous studies. The strength and durability properties of mortars were investigated by substituting metakaolin, silica fume, glass powder, and nano-silica [27,28,29,30]. Recently, it has been seen that natural pozzolans are used in the production of geopolymers [31]. However, the research on diatomite, a natural pozzolan containing highly reactive silica, in geopolymer systems has been limited. Diatomite, a natural pozzolan, consists of the siliceous skeletal remains of diatoms, single-celled organisms. Diatomite contains many minerals such as silica, alumina, and iron oxide in its structure. It has many advantages, such as a porous structure, low cost, and high silica content. It is also used in the manufacture of ceramics, thermal isolation, and the elimination of heavy metal ions [32,33,34]. Thammarong et al. produced geopolymer samples in their study by substituting diatomite at the rates of 5%, 10%, 15%, 20%, and 25% by weight of metakaolin. They investigated the phase formation of geopolymers activated with NaOH and Na2SiO3 solutions. Phase formation of geopolymers was investigated using XRD and microstructures using SEM. When the compressive strength of the geopolymer samples, which were cured at room temperature for 3, 7, 14, 21, and 28 days, was examined, it was observed that the compressive strength decreased as the amount of diatomite increased in the samples containing diatomite in the range of 5 to 10% by weight. This is explained by the high dosage of diatomite containing a significant amount of Si in the sample. It has been reported that OH− ions are insufficient to completely dissolve Al+3 ions, thereby reducing the compressive strength [32].
The aim of this study is to try to develop more economical and sustainable geopolymer binder materials instead of cement, which causes high carbon emissions. Fly ash, which is the main binder of thermal power plant waste, was used instead of cement. In this way, it reduced both the damage of cement to the environment and problems such as the storage and disposal of industrial waste. Diatomite, formed by the precipitation of algae crusts, has replaced fly ash. Diatomite is a natural material and contains a high percentage of amorphous silica. Siliceous and aluminous materials are needed in the production of geopolymer binders. The contribution of diatomite substitution to the geopolymerization process in fly ash-based systems has been investigated. Diatomite was added at rates of 1%, 2%, 3%, 4%, and 5% to the fly ash geopolymer mortars activated with NaOH, which contained 10% and 12% Na+ according to the binder material. The mechanical strengths of mortars that were heat cured for 24, 48, and 72 h at 75 °C were investigated. Then, the abrasion resistance of the geopolymer mortar samples selected according to their mechanical strength, the determination of ultrasonic pulse velocities, and their resistance to high temperatures were investigated. In addition, FESEM images, EDX, and XRD analyses of geopolymer mortar samples were made, and their microstructures were examined.
Materials and method
Fly ash and diatomite
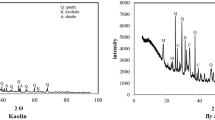
Fly ash obtained from the Sugözü thermal power plant in Adana was used in the production of geopolymer mortars. Diatomite obtained from the Hırka district of Kayseri was used as a substitute material. The chemical content and physical properties of fly ash and diatomite are given in Table 1. In addition, the SEM image and XRD model of fly ash and diatomite are shown in Fig. 1.
Sand
CEN standard sand was used, while preparing the geopolymer mortar mixes. The dry specific gravity of Rilem sand was determined as 2.61, and the water absorption rate was 0.57%.
Activator and water
The alkaline activator was prepared using NaOH and drinkable water with a purity of at least 97%.
Mixture design
In a preliminary experiment, it was started using a 0.28 water/binder ratio during mixture preparation as suggested in previous studies. However, the addition of diatomite from 1 to 5% result in a reduction in workability values of the mixture, therefore, it was decided to increase the water/binder ratio to 0.31 which provided appropriate workability. While producing the geopolymer samples, the water/binder ratio was determined as 0.31, and the sand/binder ratio was determined as 3.0. NaOH was chosen as the activator, and it was used to contain 10% and 12% Na+ by weight, depending on the binder material. In the preparation of the mixtures, diatomite was replaced with fly ash at the rates of 1%, 2%, 3%, 4%, and 5%. Mixing ratios and codes of geopolymer mortars are given in Table 2.
Experimental program
While producing geopolymer mortars, NaOH solutions containing 10% and 12% Na+ by weight, according to the binder material, were prepared 24 h ago. Fly ash-based geopolymer mortars were applied with diatomite substitutes of 1%, 2%, 3%, 4% and 5% of the total binder. The binder and NaOH solution placed in the mixing pot were mixed slowly for 30 s, and 1350 g sand was added to the pot. After continuing the mixing process at slow speed for another 60 s, the mixer was stopped and the mortar surrounding the pot was collected in the middle and rested for a total of 90 s. The mixing process was continued for 60 s at high speed, and the mixing process ended. The mortar mixtures were placed in the lubricated steel molds with dimensions of 40 × 40 × 160 mm and 71 × 71 × 71 mm by vibration to remove entrained air and bubbles, and complete compaction was obtained. This process has taken between 30 and 60 s. Then, they were placed in a preheated oven at 75 °C and subjected to thermal curing for 24 h, 48 h, and 72 h. After the curing process was completed, the samples were taken out of the oven, allowed to cool down to room temperature, and made ready for the experiment. Flexural and compressive strength tests, abrasion resistance, ultrasonic pulsed velocity, determination of high-temperature resistance, and internal structure analyses such as FESEM, EDX, and XRD were performed on the test samples. For each hardened mixture, flexural tensile strength measurements were made using three prism specimens by running a third point loading test described in TS EN 196-1 [35]. The compressive strength measurements were carried out according to relevant standard TS EN 196-1[35], using six broken pieces of the prismatic specimens obtained from the flexure test. An average of three specimens and an average of six specimens were used as data points for flexural tensile and compressive strength tests, respectively. For abrasion resistance, ultrasonic pulsed velocity, and determination of high-temperature resistance test, an average of three specimens were used.
When curing time increases from 24 to 48 h, the rate of increase in flexural and compressive strength were higher than that of while curing time increases from 48 to 72 h. Thus, it is considered that 48 h of heat curing time is optimum. Therefore, 48 h of heat curing time was used for abrasion, elevated temperature resistance test, and SEM/EDX analysis.
After the mechanical strength results, geopolymer mortar samples were determined for other tests and analyses. Accordingly, from the geopolymer samples containing 10% and 12% Na+, the reference sample, the D2 sample with the highest strength, and the D5 sample with the lowest strength were selected.
Workability
The workability of fresh mortars produced in the study was determined by the flow table according to the TS EN 1015-3 [36] standard.
Flexural and compressive strength
Flexure and compressive strength tests were carried out by TS-EN 196-1 [35] standard. Accordingly, the flexure strengths were found by loading three 40 × 40 × 160 mm specimens from a single point. After the flexure strength test, the compressive strength test was performed on the samples divided into two parts. The compressive strength was determined on a 40 × 40 mm slab.
Abrasion resistance
Abrasion resistance has been determined by TS 2824 EN 1338 [37] standard. Abrasion test samples were produced in 71 × 71 × 71 mm molds and were performed on D0, D2, and D5 samples that were heat cured at 75 °C for 48 h.
Ultrasonic pulse velocity
The ultrasonic pulse velocities of geopolymer mortar samples were determined by TS 12,504-4 [38] standard with a Proceq brand device called Pundit. Ultrasonic pulse velocity measurements of the samples were made in the direction of their 160 mm length. Ultrasonic pulse velocity test measurements were made on three samples (D0, D2, and D5) for each mortar mixture, and the average of these values was taken. Ultrasonic pulse velocities were calculated using the following equation.
UPV: ultrasonic pulse velocity, km/sec L: transition length, mm T: transition time, μs.
Elevated temperature resistance
Geopolymer binders are generally known to be elevated temperature-resistant materials. Therefore, due to the replacement of diatomite with fly ash, its current strength and resistance to the elevated temperature of geopolymer binders are thought to be investigated. Thus, diatomite-containing geopolymer mortar samples were exposed to elevated temperatures. Geopolymer mortars were subjected to high temperatures at 300 °C, 600 °C, and 900 °C after 48 h of heat curing at 75 °C. While it was subjected to elevated temperature, geopolymer samples were subjected to an elevated temperature of up to 900 °C with a heating rate of 10 °C per minute. This heating rate is consistent with the RILEM 129-MHT [39] standard recommendation. Samples were kept at the relevant temperature for 1 h. After the elevated temperature periods, the geopolymer mortars were allowed to cool down to room temperature, and then the compressive strength test was applied.
FESEM-EDX analysis
Broken surface fragments were taken from the geopolymer samples produced by thermal curing at 75 °C for 48 h, before (25 °C) and after high temperatures (300 °C, 600 °C, 900 °C). These parts were first vacuumed, and then gold–palladium plating was performed by the Erciyes University Technology Research and Application Center (TAUM). Geopolymer mortar samples were examined using a Zeiss (GeminiSEM 500) brand device. FESEM images of the geopolymer mortar samples were taken at 500 magnifications. EDX analyzes were made on the determined paste parts of the mortar samples at 500 magnification.
XRD analysis
The pieces from the produced geopolymer samples were ground into powder in an agate mortar to 63 microns. XRD analyses were made by Erciyes University Technology Research and Application Center (TAUM). The X-ray diffraction technique determined the phases of the geopolymer paste material.
Result and discussion
Workability
The flow table workability of fresh geopolymer mortar obtained in accordance with TS EN 1015-3 [36] is presented in Table 3. The workability of the reference geopolymer mortar mixture was 113 and 125 mm for mortar containing 10% and 12% Na+, respectively. It can be seen in Table 3 that the addition of diatomite as a replacement with fly ash at 1%, 2%, 3%, 4%, and 5% caused a reduction in workability values in comparison to the reference mortar mixture. For instance, the workability of the D1 mixture is 106 and 110 mm for mortar made with 10% and 12% Na+. In a similar manner, the workability of the D5 mixture is 101 and 103 mm for mortar made with 10% and 12% Na+. Increasing the Na + amount in the mixture caused better workability. Increasing the diatomite amount in the mixture results in the agglomeration of particles and reduces workability. An increasing amount of Na+ causes a lubrication effect between powder particles, thus, providing better workability.
Flexural and compressive strength
The flexural strength results of diatomite-substituted geopolymer mortars are shown in Fig. 2. When the flexural strengths of geopolymer mortars heat cured at 75 °C for 24, 48 and 72 h are examined, the highest flexural strength in mixtures containing 10% and 12% Na+ was found to be 10.6 MPa and 11.8 MPa, respectively, in the D2 mortar sample, which was heat cured for 72 h has been obtained. When the geopolymer mortars were examined at all curing times, the flexural strength values of the D1 and D2 coded mortar samples were higher in the mortars containing 10% and 12% Na+ than the reference (D0) mortar sample. The flexural strength values of the D3, D4, and D5 coded mortar samples were lower than the reference (D0) samples. The flexural strength of 12% Na+ diatomite-substituted geopolymer mortars was higher than 10% Na+ diatomite-substituted geopolymer mortars. The greatest effect of diatomite substitution on the increase in flexural strength at 75 °C was observed at the end of 72 h in mortars containing 10% Na+, at the end of 48 h in mortars containing 12% Na+. Accordingly, the contribution of high Na+ content to the flexural strength was faster with the presence of diatomite. When the flexural strength of the geopolymer mortar containing 12% Na+ and 5% diatomite was examined, 72 h of thermal curing made the strength loss more striking, and lower flexural strength was obtained than 48 h of thermal curing.
Compressive strength results of diatomite-substituted geopolymer mortars are shown in Fig. 3. When the compressive strengths of geopolymer mortars heat cured at 75 °C for 24, 48 and 72 h are examined, the highest compressive strength in mixtures containing 10% and 12% Na+ was found to be 55.4 MPa and 54.9 MPa, respectively, in the D2 mortar sample (containing 2% diatomite considered as optimum amount), which was heat cured for 72 h has been obtained. At optimum diatomite amount (2% in this study), provide appropriate dissolution rate of silica content of diatomite. In addition, at the optimal amount of diatomite, workability of fresh mortar was found to be favorable. Therefore, good workability and appropriate dissolution rate results with highest flexural and compressive strength regardless of either concentration of Na+ or the curing time.
It can be seen from Fig. 1 that the intensity change of XRD of diatomite shows an amorphous silica phase. Also, the BET surface area (m2/g) of diatomite is 42.74 and is considered very high (see Table 1). This high surface area results in a higher geopolymer reaction with Na+ which causes higher strength compared to the reference mixture.
When the geopolymer mortars were examined at all curing times, the compressive strength values of the D1 and D2 coded mortar samples were higher in the mortars containing 10% and 12% Na+ than the reference (D0) mortar sample. The compressive strength values of the D3, D4, and D5 coded mortar samples were lower than the reference (D0) samples. Results similar to flexural strength were also valid for compressive strength. The compressive strength of 12% Na+ diatomite-substituted geopolymer mortars was higher than 10% Na+ diatomite-substituted geopolymer mortars. The greatest effect of diatomite substitution on the increase in compressive strength at 75 °C was observed at the end of 72 h in mortars containing 10% Na+ and at the end of 48 h in mortars containing 12% Na+. Accordingly, the effect of high Na+ content on the compressive strength was faster with the presence of diatomite. When the compressive strength of the geopolymer mortar containing 12% Na+ and 5% diatomite was examined, 72 h of thermal curing made the strength loss more striking and lower flexural strength was obtained than after 48 h of thermal curing. Considering both flexural strength and compressive strength, the mechanical strength results of 5% diatomite-substituted geopolymer mortars cured at 75 °C for 72 h were lower than those of 5% diatomite-substituted geopolymer mortars cured at 75 °C for 48 h. In previous studies in the literature, as the activator ratio increased, the result of the increase in strength values [23] was seen in the flexural and compressive strength results at all curing times in this study. With the increase in alkali ratio, the reactive silica in the structure of diatomites dissolved more and supported the NASH gel structure. Thus, a compact gel structure was formed and mechanical strength was increased. In addition, the substitution of 3%, 4%, and 5% diatomite in all mixtures increased the amount of silica in the structure of the mixture more and the dissolution of Al+3 ions in the alkaline solution was insufficient. This situation has led to a decrease in mechanical strength, as previously stated in the literature [29]. In addition, the loss of strength in flexure and compression results is explained by the agglomeration of diatomite particles and reduction in the workability values. Since replacement of more than 2% of diatomite with fly ash on a mass basis results in agglomeration in the mixture thus results in a reduction in workability value (see Table 3) resulting in un-appropriate compaction during the sample casting process which causes a reduction in flexural and compressive strength. Similarly, Messina et al. [40] and Durak et al. [41] reported that the increased amount of silica fume or nano-SiO2 in the geopolymer mortar caused agglomeration or partial blockage in the microstructure. Therefore, this effect caused a decrease in strength development as observed in D3, D4 and D5 mixtures.
Abrasion resistance
Geopolymer mortar samples were abraded after heat curing at 75 °C for 48 h. Figure 4 shows the volume losses of geopolymer mortar samples after abrasion. The volume losses of the geopolymer mortars containing 10% Na+ were calculated as 21,504, 19,860, and 25,340 mm3/5000 mm2 for the D0 (reference), D2, and D5 samples, respectively. The post-abrasion volume losses of geopolymer mortars containing 12 percent Na+ were calculated as 20,553, 19,056, and 26,128 mm3/5000 mm2, respectively, when the D0 (reference), D2, and D5 samples were examined. The D2 sample lost less volume and had higher abrasion resistance than the reference (D0) sample at both activator ratios. The D5 sample, on the other hand, lost more volume and had lower abrasion resistance than the D0 sample. There is a relationship between compressive strength and abrasion resistance, according to previous studies in the literature on geopolymer, and samples with higher compressive strength are more resistant to abrasion [20, 42, 43]. This behavior has been observed in diatomite-substituted geopolymer mortars in the literature. Figure 5 shows that the compressive strength values and abrasion losses of the geopolymer mortar samples containing 10% and 12% Na+, respectively, have a strong linear relationship (R = 0.9924, R = 0.950).
Ultrasonic pulse velocity
When the ultrasonic pulse velocity of the 10% Na+ containing geopolymer mortars cured at 75 °C for 48 h was examined, the D0, D2, and D5 samples were measured at 4.2, 4.7 and 3.4 km/sec, respectively. When the ultrasonic pulse velocity of geopolymer mortars containing 12% Na+ was examined, the D0 (reference), D2 and D5 samples were measured as 4.5, 4.9 and 3.7 km/sec, respectively. Ultrasonic pulse velocities of the samples are given in Fig. 6. In both activator ratios, it was observed that the ultrasonic pulse velocity of D2 samples with 2% diatomite substitution compared to the reference (D0) sample increased, and the ultrasonic pulse velocity of D5 samples with 5% diatomite substitution decreased. The ultrasonic pulse velocity of a material is related to the porous structure of that material. In previous studies in the literature, it has been reported that the ultrasonic pulse velocity decreases with the increase of the porous structure [44]. In addition, it is shown in Fig. 7 that there is a high linear relationship between the compressive strength values and the UPV values of the geopolymer mortar samples containing 10% and 12% Na+, respectively (R = 0.9999, R = 0.9672).
Elevated temperature resistance
The geopolymer D0, D2, and D5 mortar samples containing 10% and 12% Na+, which were heat cured at 75 °C for 48 h. It was brought to the target temperature with a temperature increase of 10 °C/min in the oven and exposed to high temperatures separately at 300 °C, 600 °C, and 900 °C for 60 min. In Fig. 8, compressive strengths before and after high temperatures are given. After high temperatures, there was a loss of strength in all D0, D2, and D5 coded mortar samples compared to before high temperatures. However, the compressive strength values of 2% diatomite-substituted geopolymer mortars (D2) containing 10% Na+ and 12% Na+ after high temperatures at 300 °C, 600 °C, and 900 °C were again higher than the reference (D0) mortar sample. Thus, it is seen that the appropriate ratio of diatomite substitution increases the resistance to high temperatures. In addition, the compressive strengths of geopolymer mortars substituted with 5% diatomite in both Na+ ratios after the high temperature were lower than the reference. The increase in the alkali activator ratio in the compressive strengths after the high temperature increased this compressive strength loss more. It is stated that this situation arises from the porous structure formed in the binder paste as a result of the tendency of the alkalis not included in the NASH gel structure to evaporate, especially at high temperatures. In addition, this situation is also seen in FESEM images. In previous studies in the literature, it has been stated that the use of highly reactive silica-containing materials such as rice husk ash and nano-SiO2 reduces high temperature resistance [45]. It was observed that diatomite containing highly reactive silica also showed similar behavior.
FESEM-EDX analysis
FESEM images (500 magnification) of samples containing 10% Na+ and heat cured at 75 °C for 48 h are given in Fig. 9 after 900 °C high temperature. On the FESEM images, the letter S is used to represent sand, and the letter M is used to represent the geopolymer matrix. In addition, EDX analyzes of geopolymer pastes in these areas were made and are given in Fig. 9. When the FESEM images are examined, it is seen that the general morphological structure of the D2 coded geopolymer mortar samples is denser than the reference D0 coded sample. It is seen that the morphological structure of the D5 coded geopolymer mortar sample has more voids and microcracks compared to both the D0 coded reference sample and the D2 coded sample. Thermal shrinkages and expansion occur in geopolymer samples exposed to high temperature. These thermal shrinkages and expansions cause macro cracks in the geopolymer sample [46, 47]. On the other hand, Kong et al., in this study, fly ash and metakaolin-based geopolymers were investigated after exposure to high temperatures (800 °C). They stated that while the compressive strength of fly ash-based geopolymers increased, the compressive strength of metakaolin-based geopolymers decreased. While 0.1–0.2 mm cracks were formed on the surfaces of metakaolin-based geopolymers, these cracks were not observed in fly ash-based geopolymers. More water was required in the production of metakaolin-based geopolymers compared to the production of fly ash-based geopolymers [48]. Metakaolin, a natural pozzolan, is partially similar to diatomite in these aspects. When diatomite with high porosity was substituted for by 2% in alkaline medium, NASH supported the gel structure. However, when 5% diatomite was substituted, it could not degrade sufficiently in alkaline environment and could not react. In addition, these unreacted diatomite pieces form voids in the paste structure when exposed to high temperatures due to their high porosity and humidity retention properties.
EDX analyses were obtained from the geopolymer paste sections boxed in Fig. 9. As a result of EDX analysis, mainly Al, Si, O, and Na elements were found in the geopolymer samples. The N–A–S–H (Sodium alumina silicate hydrate) gel has an important role in the development of strength in systems containing low calcareous fly ash in geopolymer structures [49, 50].
XRD analysis
While sodalite, mullite, and quartz peaks were found in the reference (D0) sample, which was cured at 75 °C for 48 h, as shown in Fig. 10, sillimanite peaks were also found in the (D2) sample containing 2% diatomite. When the morphological structure of geopolymer pastes and XRD results are examined, it cannot be said that the increase in mechanical strength is directly related to the crystal structure. In addition, it is not easy to determine the geopolymeric reaction products by XRD alone [20, 51,52,53,54]. In this respect, it is more possible to explain the positive increase in mechanical strength with FESEM images rather than XRD analysis.
Conclusion
The following results were obtained in the evaluation of the effect of diatomite substitution on fly ash-based geopolymer mortars:
-
1.
According to the flexural and compressive strength results of geopolymer mortars heat-cured at 75 °C for 48 h, 1% and 2% diatomite substitution increased the flexural and compressive strengths compared to the reference (DO) mortar sample. However, 3% (D3), 4% (D4), and 5% (D5) diatomite substitution decreased the flexural and compressive strength. In addition, it was observed that the rate of increase in mechanical strengths decreased with the curing time.
-
2.
The abrasion resistance results were obtained in direct proportion to the mechanical strength results. Abrasion resistance increased with the increase in flexure and compressive strength.
-
3.
At both activator ratios, the ultrasonic velocity of D2 samples, which have a compact and void-free gel structure compared to reference (D0) samples, increased, while the ultrasonic velocity of D5 samples decreased due to the voids in their structure.
-
4.
After the geopolymer mortars were exposed to high temperatures (300 °C, 600 °C, and 900 °C) for 60 min, the compressive strength of all D0, D2, and D5 coded mortar samples decreased compared to before the high temperature.
-
5.
It has been found that 2% diatomite substitution is optimum in terms of improving mechanical properties. The 5% diatomite substitution damaged the mechanical properties of the geopolymer mortars. FESEM images support these effects on mechanical properties. When 2% diatomite was substituted in an alkaline medium, diatomite substitution supported the formation of NASH gel, creating a denser and void-free dough structure. However, in the FESEM images of the geopolymer mortars with 5% diatomite substitution, insufficiently reacted diatomite pieces and a porous paste structure were observed.
-
6.
As a result of XRD analysis, although 2% diatomite substitution supported the formation of a new crystal sillimanite peak, a direct correlation could not be established with the development of compressive strength. However, this correlation could be confirmed with FESEM images.
References
Zhang P, Zheng Y, Wang K, Zhang J (2018) A review on properties of fresh and hardened geopolymer mortar. Compos Part B Eng 152:79–95. https://doi.org/10.1016/J.COMPOSITESB.2018.06.031
Malhotra VM (2002) Introduction: sustainable development and concrete technology. Concr Int 24(7):22
Ma Y, Ye G (2015) The shrinkage of alkali activated fly ash. Cem Concr Res 68:75–82. https://doi.org/10.1016/J.CEMCONRES.2014.10.024
Morsy MS, Alsayed SH, Al-Salloum Y et al (2014) Effect of sodium silicate to sodium hydroxide ratios on strength and microstructure of Fly ash geopolymer binder. Arab J Sci Eng 39(6):4333–4339. https://doi.org/10.1007/S13369-014-1093-8
Duan P, Yan C, Zhou W (2017) Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cem Concr Compos 78:108–119. https://doi.org/10.1016/j.cemconcomp.2017.01.009
John SK, Nadir Y, Girija K (2021) Effect of source materials, additives on the mechanical properties and durability of fly ash and fly ash-slag geopolymer mortar: a review. Constr Build Mater 280:122443. https://doi.org/10.1016/J.CONBUILDMAT.2021.122443
Saha S, Rajasekaran C (2017) Enhancement of the properties of fly ash based geopolymer paste by incorporating ground granulated blast furnace slag. Constr Build Mater 146:615–620. https://doi.org/10.1016/j.conbuildmat.2017.04.139
Pasupathy K, Berndt M, Sanjayan J et al (2017) Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cem Concr Res 100:297–310. https://doi.org/10.1016/J.CEMCONRES.2017.07.010
Kumar EM, Perumal P, Ramamurthy K (2022) Alkali-activated aerated blends: interaction effect of slag with low and high calcium fly ash. J Mater Cycles Waste Manag 24:1378–1395. https://doi.org/10.1007/s10163-022-01434-5
Saxena R, Gupta T (2022) Assessment of mechanical, durability and microstructural properties of geopolymer concrete containing ceramic tile waste. J Mater Cycles Waste Manag 24:725–742. https://doi.org/10.1007/s10163-022-01353-5
Irshidat MR, Al-Nuaimi N, Rabie M (2022) Sustainable alkali-activated binders with municipal solid waste incineration ashes as sand or fly ash replacement. J Mater Cycles Waste Manag 24:992–1008. https://doi.org/10.1007/s10163-022-01374-0
Altanlar A (2021) (TÜİK) Türkiye İstatistik Kurumu. 37198:3–4
Somna K, Jaturapitakkul C, Kajitvichyanukul P, Chindaprasirt P (2011) NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 90:2118–2124. https://doi.org/10.1016/j.fuel.2011.01.018
Pacheco-Torgal F, Castro-Gomes J, Jalali S (2008) Alkali-activated binders: a review. Part 2. About materials and binders manufacture. Constr Build Mater 22:1315–1322. https://doi.org/10.1016/J.CONBUILDMAT.2007.03.019
Palomo A, Grutzeck MW, Blanco MT (1999) Alkali-activated fly ashes: a cement for the future. Cem Concr Res 29:1323–1329. https://doi.org/10.1016/S0008-8846(98)00243-9
Fernández-Jiménez A, Palomo JG, Puertas F (1999) Alkali-activated slag mortars: mechanical strength behaviour. Cem Concr Res 29:1313–1321. https://doi.org/10.1016/S0008-8846(99)00154-4
Xu H, Van Deventer JSJ (2002) Geopolymerisation of multiple minerals. Miner Eng 15:1131–1139. https://doi.org/10.1016/S0892-6875(02)00255-8
Brough AR, Holloway M, Sykes J, Atkinson A (2000) Sodium silicate-based alkali-activated slag mortars: Part II. The retarding effect of additions of sodium chloride or malic acid. Cem Concr Res 30:1375–1379. https://doi.org/10.1016/S0008-8846(00)00356-2
Durak U (2022) The improvement of strength and microstructural properties of fly ash-based geopolymer by adding elemental aluminum powder. J Mater Cycles Waste Manag. https://doi.org/10.1007/s10163-022-01520-8
Durak U, İlkentapar S, Karahan O et al (2021) A new parameter influencing the reaction kinetics and properties of fly ash based geopolymers: a pre-rest period before heat curing. J Build Eng. https://doi.org/10.1016/J.JOBE.2020.102023
Bakharev T (2005) Geopolymeric materials prepared using class F fly ash and elevated temperature curing. Cem Concr Res 35:1224–1232. https://doi.org/10.1016/J.CEMCONRES.2004.06.031
Rattanasak U, Chindaprasirt P (2009) Influence of NaOH solution on the synthesis of fly ash geopolymer. Miner Eng 22:1073–1078. https://doi.org/10.1016/j.mineng.2009.03.022
Atiş CD, Görür EB, Karahan O et al (2015) Very high strength (120 MPa) class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr Build Mater 96:673–678. https://doi.org/10.1016/j.conbuildmat.2015.08.089
Provis JL, Palomo A, Shi C (2015) Advances in understanding alkali-activated materials. Cem Concr Res 78:110–125. https://doi.org/10.1016/J.CEMCONRES.2015.04.013
Nath SK, Mukherjee S, Maitra S, Kumar S (2017) Kinetics study of geopolymerization of fly ash using isothermal conduction calorimetry. J Therm Anal Calorim 127:1953–1961. https://doi.org/10.1007/s10973-016-5823-x
Nath SK, Maitra S, Mukherjee S, Kumar S (2016) Microstructural and morphological evolution of fly ash based geopolymers. Constr Build Mater 111:758–765. https://doi.org/10.1016/J.CONBUILDMAT.2016.02.106
Manikandan P, Vasugi V (2021) A critical review of waste glass powder as an aluminosilicate source material for sustainable geopolymer concrete production. SILICON. https://doi.org/10.1007/s12633-020-00929-w
Pascual AB, Tognonvi TM, Tagnit-Hamou A (2021) Optimization study of waste glass powder-based alkali activated materials incorporating metakaolin: activation and curing conditions. J Clean Prod 308:127435. https://doi.org/10.1016/J.JCLEPRO.2021.127435
Durak U, Karahan O, Uzal B, et al (2018) The ınvestigation of mechanical effects of nano sio 2 particles for different sodium ıon concentrations on fly ash based geopolymer mortar. pp 4–9
Okoye FN, Prakash S, Singh NB (2017) Durability of fly ash based geopolymer concrete in the presence of silica fume. J Clean Prod 149:1062–1067. https://doi.org/10.1016/J.JCLEPRO.2017.02.176
Barış KE, Tanaçan L (2021) Improving the geopolymeric reactivity of Earth of Datça as a natural pozzolan in developing green binder. J Build Eng 41:102760. https://doi.org/10.1016/J.JOBE.2021.102760
Thammarong S, Lertcumfu N, Jaita P et al (2019) The effects of replacement metakaolin with diatomite in geopolymer materials. Key Eng Mater 798:267–272. https://doi.org/10.4028/WWW.SCIENTIFIC.NET/KEM.798.267
Akhtar F, Rehman Y, Bergström L (2010) A study of the sintering of diatomaceous earth to produce porous ceramic monoliths with bimodal porosity and high strength. Powder Technol 201:253–257. https://doi.org/10.1016/J.POWTEC.2010.04.004
İlkentapar S, Örklemez E, Üniversitesi E et al (2020) Uçucu Kül Esaslı Geopolimer Harçlara Diatomit İkamesinin Isı İletkenliğe Etkisi the effect of diatomite addition on fly ash based geopolymer mortars on thermal conductivity values. Erciyes Univ J Institue Sci Technol 36:2020
TS EN 196–1 (2016) Methods of testing cement—part:1 determination of strength. TSE
TS EN 1015–3 (2000) Methods of test for mortar for masonry: Part 3. Determination of consistence of fresh mortar (by flow table). TSE, Ankara
Standard T (2005) Turkish Standard Ts 2824 En 1338
TSE- EN (2017) Turkish Standard 12504–4. Türk Stand 15189:
de la Rilem PDR (1995) 129-MHT: test methods for mechanical properties of concrete at high emperatures. Mater Struct 28:410–414
Messina F, Colangelo F et al (2018) Alkali activated waste fly ash as sustainable composite: Influence of curing and pozzolanic admixtures on the early-age physico-mechanical properties and residual strength after exposure at elevated temperature. Compos Part B Eng 132:161–169. https://doi.org/10.1016/j.compositesb.2017.08.012
Durak U, Karahan O, Uzal B et al (2021) Influence of nano SiO2 and nano CaCO3 particles on strength, workability, and microstructural properties of fly ash-based geopolymer. Struct Concr 22:E352–E367. https://doi.org/10.1002/SUCO.201900479
Bilim C, Karahan O, Atiş CD, Ilkentapar S (2013) Influence of admixtures on the properties of alkali-activated slag mortars subjected to different curing conditions. Mater Des 44:540–547. https://doi.org/10.1016/J.MATDES.2012.08.049
İlkentapar S, Atiş CD, Karahan O, Görür Avşaroğlu EB (2017) Influence of duration of heat curing and extra rest period after heat curing on the strength and transport characteristic of alkali activated class F fly ash geopolymer mortar. Constr Build Mater 151:363–369. https://doi.org/10.1016/J.CONBUILDMAT.2017.06.041
Whitehurst EA (1951) Soniscope tests concrete structures. J Proc 47:433–444. https://doi.org/10.14359/12004
Nuaklong P, Jongvivatsakul P, Pothisiri T et al (2020) Influence of rice husk ash on mechanical properties and fire resistance of recycled aggregate high-calcium fly ash geopolymer concrete. J Clean Prod 252:119797. https://doi.org/10.1016/j.jclepro.2019.119797
Lahoti M, Wong KK, Yang EH, Tan KH (2018) Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram Int 44:5726–5734. https://doi.org/10.1016/J.CERAMINT.2017.12.226
Junaid MT, Khennane A, Kayali O et al (2014) Aspects of the deformational behaviour of alkali activated fly ash concrete at elevated temperatures. Cem Concr Res 60:24–29. https://doi.org/10.1016/J.CEMCONRES.2014.01.026
Kong DLY, Sanjayan JG, Sagoe-Crentsil K (2007) Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem Concr Res 37:1583–1589. https://doi.org/10.1016/J.CEMCONRES.2007.08.021
Khedmati M, Alanazi H, Kim Y et al (2018) Effects of Na 2 O/SiO 2 molar ratio on properties of aggregate-paste interphase in fly ash-based geopolymer mixtures through multiscale measurements. Constr Build Mater 191:564–574. https://doi.org/10.1016/j.conbuildmat.2018.10.024
Xiao R, Ma Y, Jiang X et al (2020) Strength, microstructure, ef fl orescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J Clean Prod 252:119610. https://doi.org/10.1016/j.jclepro.2019.119610
Komljenović M, Baščarević Z, Bradić V (2010) Mechanical and microstructural properties of alkali-activated fly ash geopolymers. J Hazard Mater 181:35–42. https://doi.org/10.1016/J.JHAZMAT.2010.04.064
Lee B, Kim G, Kim R et al (2017) Strength development properties of geopolymer paste and mortar with respect to amorphous Si/Al ratio of fly ash. Constr Build Mater 151:512–519. https://doi.org/10.1016/J.CONBUILDMAT.2017.06.078
Ryu GS, Lee YB, Koh KT, Chung YS (2013) The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr Build Mater 47:409–418. https://doi.org/10.1016/J.CONBUILDMAT.2013.05.069
Shao NN, Liu Z, Xu YY et al (2015) Fabrication of hollow microspheres filled fly ash geopolymer composites with excellent strength and low density. Mater Lett 161:451–454. https://doi.org/10.1016/J.MATLET.2015.09.016
Acknowledgements
This work with project code FLY-2019-948 is supported by Erciyes University Scientific Research Project Coordination Unit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Özsoy, A., Örklemez, E. & İlkentapar, S. Effect of addition diatomite powder on mechanical strength, elevated temperature resistance and microstructural properties of industrial waste fly ash-based geopolymer. J Mater Cycles Waste Manag 25, 2338–2349 (2023). https://doi.org/10.1007/s10163-023-01692-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01692-x