Abstract
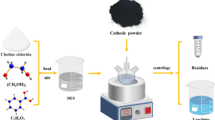
In this study, deep eutectic solvent synthesized by choline chloride (C5H14CINO) and oxalic acid dihydrate (C2H2O4·2H2O) was used to recycling LiFePO4 cathodes for spent lithium-ion batteries. The recycling process was optimized by response surface methodology. When the solid-to-liquid ratio is 0.02 and the reaction was performed at 106 °C for 110 min, the leaching efficiencies of lithium and iron are 95.3% and 85.2%, respectively. The measurement results show that iron is recycled as the form of FeC2O4·2H2O after the UV irradiation and lithium is recycled by chemical precipitation. No mineral acid or high temperature is involved in this work, which has great environmental significance to promote the healthy development of the battery recycling technology.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since commercialized by Sony in 1990s, lithium-ion batteries (LIBs) have been widely used in portable electronics, energy storage systems, power tools and electric vehicles due to their excellent performances of high energy density, long service life, minimal memory effect and low self-discharge [1,2,3]. With the large production and growing demand of LIBs, it will inevitably lead to a great number of spent LIBs generated in future [4, 5]. It was predicted that more than 11 million tons of spent LIBs will be generated in 2030 [6]. Therefore, it is necessary to develop an effective route to recycle spent LIBs because of both environmental protection and resource conservation [7].
Current methods for recycling spent LIBs mainly include hydrometallurgy, pyrometallurgy, mechanical methods, or some combination ones [8,9,10,11,12]. Among them, more than 75% studies have been done on the hydrometallurgy due to some attractive advantages, such as low consumption of electricity, high metal recovery rates [13], high product purity and low cost [14, 15]. However, sulfuric acid, hydrochloric acid, nitric acid, or other mineral acids used in the hydrometallurgical methods are extremely harmful to the environment and workers. In order to reduce the pollution, some researchers use organic acids rather than mineral acids during the recycling process, such as citric acid, oxalic acid, or formic acid, but usually combine with pyrometallurgy or mechanical methods [16]. Nowadays, the recycling of commonly used spent LiFePO4 (LFP) batteries has been reported in previous research work [17]. Qiu et al. used a green and efficient process for the recycling of spent LFP based on the oxidation leaching reaction, obtaining high leaching efficiency, and the regenerated LFP with high performance [18]. Liu et al. developed a novel process to recycle the spent LFP without adding acid or alkali and the regenerated cathode materials have high specific capacity and excellent cycle performance [19]. However, these methods require lengthy extraction steps, which still have problems for recycling spent LFP batteries because of their relatively low lithium content and the low economic value of iron. Therefore, finding new alternative methods with environmentally friendly, low cost and high efficiency to recycle the spent LFP batteries is necessary.
The conventional leaching of LiFePO4 using oxalic acid solution has many advantages. But it is extremely harmful to the environment or workers, or these methods require lengthy extraction steps, or it is not suitable for recycling batteries of relatively low economic value. Deep eutectic solvents (DESs) are an emerging class of organic solvents, which have been widely used in the field of dissolving metal oxides and metal salts, because of the characteristics of strong solubility, high thermal stability, and low vapor pressure [20]. DESs are generally formed by hydrogen bond acceptors (usually choline chloride) and hydrogen bond donors (such as organic acids, polyols, and urea) through intermolecular hydrogen bond association melting. The physical properties of DESs are dependent upon the hydrogen bond donors. Therefore, the wide range of hydrogen bond donors means that this class of DESs can be easily tailored for specific applications [21]. When carboxylic acids are chosen as the hydrogen bond donors, DESs have a higher solubility because the proton act as an oxygen acceptor [22]. Among them, oxalic acid is the simplest and cheapest dicarboxylic acid with good metal chelating properties and is a very promising hydrogen bond donor [23]. DES has been widely used in the recycling of spent lithium-ion batteries [24,25,26]. Tran et al. used DES synthesized by choline chloride and ethylene glycol to recycle the LiCoO2 batteries [27]. Although the leaching rates of Li and Co can reach more than 90%, the reaction time was more than 72 h and temperature was higher than 200 °C, which was difficult to directly apply in practice. Therefore, the recycling processes and the leaching mechanism of spent lithium-ion batteries should be investigated more deeply.
In this paper, DES made of Choline chloride (ChCl) and oxalic acid (H2C2O4) was utilized to dissolve spent LFP battery cathode materials. The leaching efficiencies of Li and Fe were greater than 95.3% and 85.2% at 106 °C with the reaction time of 110 min, respectively. FeC2O4·2H2O and Li3PO4 were obtained from the leachate successfully. This study presents a simpler, greener and feasible method for the recycling of spent LFP batteries.
Experimental section
Materials and reagents
The spent LFP batteries used in this study were provided by Wuhan GEM Co., Ltd., China. The battery-grade powder of LFP was purchased from TaiYuan Lizhiyuan Battery Sales Department, China. ChCl (analytical grade), H2C2O4 (analytical grade), and potassium thiocyanate (KSCN; analytical grade) were purchased from Sinopharm Chemical Reagent Co., Ltd., China.
Pretreatment of spent LFP batteries and DES
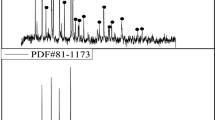
The spent LFP materials obtained from spent LFP batteries which was removed from electric vehicle. The cathode scraps were first separated from spent LFP batteries after discharging and dismantling, and then cut into small pieces with a size of about 1.0 × 1.0 cm. The spent LFP materials were separated from Al foil by ultrasonic cleaner in water lasting 30 min. After drying at 80 °C for 6 h, the spent LFP materials were dissolved in the aqua regia solution to determine the contents of valuable elements by inductively coupled plasma-optical emission spectrometry (ICP-OES). The results are given in Table 1. It can be found that around 30.6% of the materials are iron and 4.0% is lithium. The X-ray diffraction of the cathode materials (Fig. 1) shows that the presence of spent LFP is the only phase, and the result is consistent with Jin et al. [28].
The DES was prepared in a sealed glass container by mixing ChCl and H2C2O4 with a molar ratio of 1:1. Then the mixtures were stirred at 80 °C until a colorless homogeneous liquid was obtained.
Metal leaching
Measured amounts of LFP powder were added to 5 g of DES and mixed in a closed vial. The mixtures with the solid-to-liquid (S/L) ratio of 0.01, 0.02, 0.03, and 0.04 were stirred by constant temperature heating magnetic stirrer at about 800 rpm, and at the heating temperature of 80, 90, 100, 110, and 120 °C. After reacting for a certain time, the leachate was filtered at the corresponding leaching temperature, and subsequently diluted for ICP-OES analysis using 0.1 M HCl. In this study, the leaching efficiency of various elements was calculated by Eq. (1):
where ci (g L−1) and V (L) are the concentration of Li\Fe and the volume of leachate, respectively. m (g) and \({\upomega }_{{\text{i}}}\) are the mass of powder and the content of Li\Fe in the initial material, respectively.
Analytical methods
The solid samples were analyzed by X-ray diffraction (XRD, D8 Advance, Germany) with Cu K α radiation (Kα = 0.15406 nm) operating at 40 kV and 40 mA with 2 θ from 10 to 80°. The major elemental contents of the cathode powder and the concentrations of leachate were determined by ICP-OES (Prodigy 7, USA). The UV–VIS absorption spectra of leachate of spent LFP batteries cathode materials were obtained from a UV–VIS spectrophotometer (Meixi, China). The precipitates from leaching solutions of spent LFP batteries cathode materials were characterized using a Fourier-Transform Infrared Spectrometer (FI-IR, Nexus, USA). The thermos-gravimetric analysis and differential thermogravimetry (TG/DTG) were performed on a STA449C analyzer (Netzsch, Germany) at a heating rate of 10 °C/min from room temperature to 800 °C in an air atmosphere.
Experimental design based on response surface methodology
The response surface methodology (RSM) is an experimental design method based on mathematical and statistical techniques. It can not only analyze the effect of different factors and their interactions, but also optimize the experimental conditions based on the regression equations. A Box–Benhnken Design (BBD) was used to study the effect of temperature, solid-to-liquid ratio and the time on the leaching efficiencies of Li and Fe. According to the experimental results, linear model, two-factor interaction model, quadratic model and cubic model were established. After the analysis and comparison of the variance and determination coefficient, the comprehensive index of each parameter of the quadratic model was better than that of other models, so the quadratic model is chosen. The design of experiment was developed using the empirical quadratic model:
where Y is the predicted value of the response variable. \({\text{X}}_{\text{i}}\) and \({\text{X}}_{\text{j}}\) are the input variables. \({\upbeta }_{{0}}\), \({\upbeta }_{{\text{i}}}\), \({\upbeta }_{{{\text{ii}}}}\), \({\upbeta }_{{{\text{ij}}}}\) and \({\upvarepsilon }\) are the offset term, the linear coefficient, the quadratic coefficient, the interactions coefficient, and the error, respectively. In this study, Y represents the leaching efficiency in the empirical models.
Results and discussion
Leaching of battery-grade LFP powder
In order to improve the research efficiency, single factor experiments were conducted to preliminarily investigate the effects of reaction temperature, S/L ratio and reaction time on the leaching efficiencies using battery-grade LFP powder. The effect of reaction temperature on the leaching efficiencies was studied in the range of 80–120 °C. The experiments were conducted under the conditions of S/L ratio of 0.02 and reaction time of 120 min. As shown in Fig. 2a, with the increase of temperature from 80 to 110 °C, the leaching efficiencies of Li and Fe increase from 71% and 72.7% to 99.3% and 85.8%, respectively. The reaction temperature has great effects on the leaching process and increasing the reaction temperature is certainly beneficial to dissolving LFP. This may be due to the fact that with the increase of temperature, more carboxylic acids are formed. Carboxylic acids provide more protons as oxygen acceptors. The kinetic of proton gets also accelerated with the increase of temperature, and which promote the dissolution of LFP. Meanwhile, increasing the temperature also helps the protons to spread and diffuse [22, 29]. However, further increasing temperature to above 120 °C shows a negligible effect on the leaching efficiencies. Therefore, 110 °C is chosen as the optimal reaction temperature.
Figure 2b shows the effect of S/L ratio on the leaching efficiencies at 110 °C for 120 min. It can be found that when the S/L ratio increases from 0.01 to 0.02, the leaching efficiency of Li or Fe has little change. When the S/L ratio increases from 0.02 to 0.04, the leaching efficiencies of Li and Fe decrease from 99.3% and 85.8% to 41.4% and 67.4%, respectively. A smaller S/L ratio means a higher available surface area per unit volume of the solution and the sufficiency of the leaching agent and this is beneficial to the dissolution of LFP powder [30]. In order to have a higher leaching efficiency without compromising the economy, the S/L ratio of 0.02 is taken as the optimal value for the rest study.
The effects of reaction time on the leaching efficiencies were studied at 110 °C with S/L ratio of 0.02 (Fig. 2c). The results indicate that the leaching efficiency of Li increases from 79.4 to 99.3% when the time prolongs from 60 to 120 min, while the leaching efficiency of Fe remains at a stable level (~ 85%). With the leaching time further going up, no obvious change is found in leaching efficiencies of Li and Fe. Considering the Li is more valuable, the reaction time of 120 min is suitable to leach metals from LFP powder.
Statistical analysis of spent LFP materials
In order to investigate the interactions of temperature, S/L ratio and time on the leaching efficiencies, the RSM was carried out based on the single factor experiments, and the levels of each variable are presented in Table 2. In order to improve the actual value of the study, the spent LFP materials were used instead of the battery-grade LFP powder. Seventeen experiments were designed and the results on the basis of Box–Benhnken Design (BBD) are listed in Table 3. Empirical quadratic models were used to predict the leaching efficiencies of Li and Fe. Table 4 and Table 5 give the analysis of variance (ANOVA). It can be seen that the P values of the two models are far less than 0.05, and the lack of fit terms are both greater than 0.1, indicating that the models are significant and the regression equations have high credibility [31]. The leaching efficiencies of predicted versus actual are shown in Fig. 3a, b. The predicted data were generated by empirical model and the actual values were obtained by experiments. It can be seen that these points are closely distributed on the both sides of the 45° diagonal, indicating the empirical model has high credibility and can be used to optimize the experiment process [32].
Predicted leaching efficiencies vs. actual leaching efficiencies of spent LiFePO4 materials for a Li and b Fe. 3D surface maps of the interactive effects of temperature and S/L Fe with the time of 2 h for c Li and d Fe. Contour plots of the interactive effects of temperature and S/L with the time of 2 h for e Li and f Fe
The 3D surface map and the corresponding contour map of the interaction of temperature and S/L ratio on the leaching efficiencies are shown in Fig. 3c–f. When the S/L ratio is low, increasing the temperature can significantly increase the leaching efficiencies of Li and Fe. But when the S/L ratio is large, too much LFP powder will increase the viscosity of the solution, which is not conducive to the diffusion of protons, and increasing temperature will only slightly improve the leaching efficiencies of Li and Fe.
The process was optimized on the basis of the empirical model. The results show that the predicted leaching efficiencies of Li and Fe were 94.0% and 86.1% at 106 °C with the S/L ratio of 0.02 and the reaction time of 110 min. Three parallel experiments were performed under this condition. The average values of the leaching efficiencies of Li and Fe are 95.3% and 85.2%, which is almost consistent with the predicted results.
Leaching mechanism
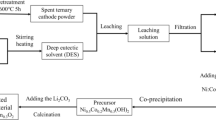
UV–VIS analysis of the leachate of spent LFP batteries cathode materials shows the signature bands of [Fe(C2O4)2]2− with the absorption bands at 336 and 347 nm [33] (Fig. 4a). As shown in Fig. 4b, the dark green leaching solution transforms into sanguine after adding KSCN solution, indicating the presence of Fe3+. Oxalic acid, due to its good metal chelating properties, is known to be a good ligand for many di- and trivalent metal cations [34]. Therefore, the existence of [Fe(C2O4)2]2− in the DES is not surprising. The presence of Fe3+ may be ascribed to the oxidation of [Fe(C2O4)2]2− by the dissolved O2 in DES, and it is likely to further complex with C2O42− to form [Fe(C2O4)3]3− [35]. Other products, such as Li2C2O4 and Li3PO4, may exist in the solution, which are soluble in an acidic media. The leaching reaction mechanisms of LFP with DES may be represented as follows:
Precipitation of Fe and Li
Since Islam et al. suggested that the Fe3+ to Fe2+ redox reaction may find applications in photochemistry, the [Fe(C2O4)3]3− has attracted intense research interest due to its high photochemical activity [36]. The leaching solutions of spent LFP cathode materials were first diluted 1:10 with deionized water, then left in the dark environment (named as sol-dark) and irradiated under the ultraviolet light (named as sol-UV) for 8 h. A high recovery efficiency is obtained by the dilution of leaching solution with deionized water. If the leaching solution is not diluted, the time of photochemistry irradiation will be extended and the recovery efficiency will reduce. A little precipitate forms in the sol-dark and the solution remains yellow-green (Fig. 5a). However, the color of sol-UV becomes lighter gradually with the consumption of time (Fig. 5b). Finally, it becomes colorless and yellow precipitate appears at the bottom. The ICP-OES results in Table 1 show that the concentration of Fe in the sol-dark remains almost unchanged. However, the UV radiation reduces the content of iron from 776.9 to 69.3 mg L−1 in the sol-UV. Most iron becomes to precipitates after the treatment for 8 h. As shown in Fig. 5c, XRD patterns of the precipitates in sol-UV indicate that their characteristic peaks agree well with the standard FeC2O4·2H2O phase.
To further confirm the composition of the precipitates from the sol-UV, FT-IR spectrum was used and the results are shown in Fig. 5d. The absorption peak at 3341 cm−1 corresponds to the stretching vibration of O–H in the H2O molecules[37], and the strong peak at 1621 cm−1 can be assigned to the vibration of carboxylic group C=O. The two medium strong peaks at 1363 and 1317 cm−1 are attributed to the vibrations of C–O and O–H groups, respectively [38]. The two peaks below 1000 cm−1 are due to the presence of Fe–O group [39]. The results indicate that the recovered sample may be FeC2O4·xH2O.
Figure 5e shows TG-DTG curves of the FeC2O4·xH2O sample heating from room temperature to 800 °C in air. A weight loss of 21.7 wt% was detected from room temperature to 208 °C, which is resulted from the loss of crystallization water. Since 1 mol FeC2O4·2H2O contains 20.0 wt% crystallization water, it can be calculated that the sample is FeC2O4·2H2O. The 1.65 wt% more than theoretical value may be due to the trace decomposition of FeC2O4. In the temperature range of 204 to 260 °C, the mass loss (about 31.41 wt%) likely corresponds to FeO, CO and CO2, which comes from the decomposition of FeC2O4. The sample mass has a slight increase (about 2.9 wt%) when the temperature is higher than 500 °C. This is resulted from the reaction between FeO and O2.
Therefore, the Fe(C2O4)33− in diluted leachate is first reduced to Fe(C2O4)22− during the irradiation, and then precipitates FeC2O4·2H2O is formed. The reaction may be represented as follows [40]:
In order to obtain a high recovery efficiency of lithium, the filtrate was evaporated at 80 °C in a beaker to remove most of water after the removal of iron. Subsequently, the Li+ and PO43− in the solution were recovered as the form of Li3PO4 by adjusting the pH to 7 with sodium hydroxide solution. After collected and dried for 8 h at 80 °C, the XRD pattern of the recycled Li3PO4 is shown in Fig. 5f, and it agrees well with the standard pattern peaks.
Conclusions
In summary, DES based on C5H14CINO/H2C2O4 was used to recycle the spent LiFePO4 cathodes for spent lithium-ion batteries. The RSM was used to investigate the interactions of temperature, S/L ratio and reaction time on the leaching efficiencies. Under the optimal experimental conditions of temperature of 106 °C, S/L ratio of 0.02 and reaction time of 110 min, the leaching efficiencies of Li and Fe is 95.3% and 85.2%, respectively. XRD, UV–VIS, FT-IR, and TG-DTG measurement results show that FeC2O4·2H2O is obtained by irradiating the leachate using an ultraviolet lamp, which is the precursor of LiFePO4 cathode materials. This work developed an environmentally friendly and feasible process for spent LiFePO4 recycling.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sattar R, Ilyas S, Bhatti HN, Ghaffar A (2019) Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep Purif Technol 209:725–733
Xin C, Gao J, Luo R, Zhou W (2022) Prelithiation reagents and strategies on high energy lithium-ion batteries. Chemistry Eur J 28(23):e202104282
Nayak PK, Yang L, Brehm W, Adelhelm P (2018) From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises. Angew Chem Int Ed 57(1):102–120
Yu W, Guo Y, Shang Z, Zhang Y, Xu S (2022) A review on comprehensive recycling of spent power lithium-ion battery in China. Etransportation 11:100155
Harper G, Sommerville R, Kendrick E, Driscoll L, Slater P, Stolkin R, Walton A, Christensen P, Heidrich O, Lambert S, Abbott A, Ryder K, Gaines L, Anderson P (2019) Recycling lithium-ion batteries from electric vehicles. Nature 575(7781):75–86
Natarajan S, Aravindan V (2018) Burgeoning prospects of spent lithium-ion batteries in multifarious applications. Adv Energy Mater 8(33):1802303
Zhang J, Lei Y, Lin Z, Xie P, Lu H, Xu J (2022) A novel approach to recovery of lithium element and production of holey graphene based on the lithiated graphite of spent lithium ion batteries. Chem Eng J 436:135011
Lei Y, Zhang J, Chen X, Min W, Wang R, Yan M, Xu J (2022) From spent lithium-ion batteries to high performance sodium-ion batteries: a case study. Mater Today Energy 26:100997
Liu K, Tan Q, Liu L, Li J (2019) Acid-free and selective extraction of lithium from spent lithium iron phosphate batteries via a mechanochemically induced isomorphic substitution. Environ Sci Technol 53(16):9781–9788
Medić D, Sokić M, Nujkić M, Đorđievski S, Milić S, Alagić S, Antonijević M (2023) Cobalt extraction from spent lithium-ion battery cathode material using a sulfuric acid solution containing SO2. J Mater Cycles Waste Manag 25:1008–1018
Sasai R, Fujimura T, Uesugi R, Aketo T, Komatsu K (2023) Eco-friendly recovery process of lithium from reduction roasting residue powder based on hydrothermal extraction. J Mater Cycles Waste Manage 25(1):389–395
Wang WY, Yen CH, Lin JL, Xu RB (2019) Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J Mater Cycles Waste Manage 21(2):300–307
Zhang P, Yokoyama T, Itabashi O, Wakui Y, Inoue K (1998) Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 50(1):61–75
Chen L, Tang X, Zhang Y, Li L, Zeng Z, Zhang Y (2011) Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 108(1–2):80–86
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sources 195(4):939–954
Fan E, Li L, Zhang X, Bian Y, Xue Q, Wu J, Wu F, Chen R (2018) Selective recovery of Li and Fe from spent lithium-ion batteries by an environmentally friendly mechanochemical approach. ACS Sustain Chem Eng 6(8):11029–11035
Chen JP, Li QW, Song JS, Song DW, Zhang LQ, Shi XX (2016) Environmentally friendly recycling and effective repairing of cathode powders from spent LiFePO4 batteries. Green Chem 18(8):2500–2506
Qiu XJ, Zhang BC, Xu YL, Hu JG, Deng WT, Zou GQ, Hou HS, Yang Y, Sun W, Hu YH, Cao XY, Ji XB (2022) Enabling the sustainable recycling of LiFePO4 from spent lithium-ion batteries. Green Chem 24(6):2506–2515
Liu P, Fei Z, Zhang Y, Dong P, Meng Q, Yang X (2022) Efficient oxidation approach for selective recovery of lithium from cathode materials of spent LiFePO4 batteries. Jom 74(5):1934–1944
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114(21):11060–11082
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126(29):9142–9147
Abbott AP, Capper G, Davies DL, McKenzie KJ, Obi SU (2006) Solubility of metal oxides in deep eutectic solvents based on choline chloride. J Chem Eng Data 51:1280–1282
Zürner P, Frisch G (2019) Leaching and selective extraction of indium and tin from zinc flue dust using an oxalic acid-based deep eutectic solvent. ACS Sustain Chem Eng 7(5):5300–5308
Tang S, Zhang M, Guo M (2022) A novel deep-eutectic solvent with strong coordination ability and low viscosity for efficient extraction of valuable metals from spent lithium-ion batteries. ACS Sustain Chem Eng 10:975–985
Wang H, Li M, Garg S, Wu Y, Nazmi Idros M, Hocking R, Duan H, Gao S, Yago AJ, Zhuang L, Rufford TE (2021) Cobalt electrochemical recovery from lithium cobalt oxides in deep eutectic choline chloride+urea solvents. Chemsuschem 14(14):2972–2983
Chen Y, Lu Y, Liu Z, Zhou L, Li Z, Jiang J, Wei L, Ren P, Mu T (2020) Efficient dissolution of lithium-ion batteries cathode LiCoO2 by polyethylene glycol-based deep eutectic solvents at mild temperature. ACS Sustain Chem Eng 8(31):11713–11720
Tran MK, Rodrigues M-TF, Kato K, Babu G, Ajayan PM (2019) Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat Energy 4:339–345
Jin H, Zhang J, Wang D, Jing Q, Chen Y, Wang C (2022) Facile and efficient recovery of lithium from spent LiFePO4 batteries via air oxidation–water leaching at room temperature. Green Chem 24(1):152–162
Liu X, Li T, Wu S, Ma H, Yin Y (2020) Structural characterization and comparison of enzymatic and deep eutectic solvents isolated lignin from various green processes: toward lignin valorization. Biores Technol 310:123460
Jha MK, Kumari A, Jha AK, Kumar V, Hait J, Pandey BD (2013) Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manage 33(9):1890–1897
Wang M, Tan Q, Liu L, Li J (2019) A low-toxicity and high-efficiency deep eutectic solvent for the separation of aluminum foil and cathode materials from spent lithium-ion batteries. J Hazard Mater 380:120846
Heydarian A, Mousavi SM, Vakilchap F, Baniasadi M (2018) Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. J Power Sources 378:19–30
Pozdnyakov IP, Kel OV, Plyusnin VF, Grivin VP, Bazhin NM (2008) New insight into photochemistry of ferrioxalate. J Phys Chem A 112(36):8316–8322
Taxiarchou M, Panias D, Douni I, Paspaliaris I, Kontopoulos A (1997) Removal of iron from silica sand by leaching with oxalic acid. Hydrometallurgy 46(1):215–228
Taxiarchou M, Panias D, Douni I, Paspaliaris I, Kontopoulos A (1997) Dissolution of hematite in acidic oxalate solutions. Hydrometallurgy 44(3):287–299
Nazrul Islam SMK, Asw K, Gulshan F (2013) Photocatalytic efficiency of mill scale for the degradation of textile dye by photo fenton and photo-ferrioxalate system under UV and sunlight. Environ Ecol Res 1:129–134
Zheng R, Zhao L, Wang W, Liu Y, Ma Q, Mu D, Li R, Dai C (2016) Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Adv 6(49):43613–43625
Yu Z, Shi Z, Chen Y, Niu Y, Wang Y, Wan P (2012) Red-mud treatment using oxalic acid by UV irradiation assistance. Trans Nonferrous Metals Soc China 22(2):456–460
Lahiri A (2010) Influence of ascorbate and oxalic acid for the removal of iron and alkali from alkali roasted ilmenite to produce synthetic rutile. Ind Eng Chem Res 49(18):8847–8851
Chen J, Zhang H, Tomov IV, Wolfsberg M, Ding X, Rentzepis PM (2007) Transient structures and kinetics of the ferrioxalate redox reaction studied by time-resolved EXAFS, optical spectroscopy, and DFT. J Phys Chem A 111(38):9326–9335
Acknowledgements
The work was supported by the Central Guidance on Local Science and Technology Development Fund of Henan Province (Z20221343028), Independent Innovation Fund Project of Wuhan University of Technology (2019III123CG), 111 Project (B17034) and Innovative Research Team Development Program of Ministry of Education of China (IRT_17R83). XRD, UV-VIS and FT-IR examinations were assisted by the Center of Material Research and Analysis of Wuhan University of Technology. Thanks to Wuhan GEM Co., Ltd., China for its strong support.
Author information
Authors and Affiliations
Contributions
CW: Methodology, conceptualization, writing—review and editing, supervision, project administration. HY: Formal analysis, investigation, data curation, writing—original draft. CY: Validation, supervision, project administration, funding acquisition. YL: Conceptualization, methodology, resources, investigation, writing- review and editing, supervision, project administration, funding acquisition. LB: Project administration, funding acquisition. SY: Supervision, project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Yang, H., Yang, C. et al. A novel recycling process of LiFePO4 cathodes for spent lithium-ion batteries by deep eutectic solvents. J Mater Cycles Waste Manag 25, 2077–2086 (2023). https://doi.org/10.1007/s10163-023-01654-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01654-3