Abstract
Mercury, a highly toxic environmental pollutant with a global circulation, must be controlled worldwide. Taking the Wuda underground coal fires, one of the most severe coal fire disaster areas in China, as a typical case, this paper systematically introduces the serious environmental output and strong environmental pollution of mercury from underground coal fires. Smoke with unusually high mercury concentrations was released from surface vents and cracks, resulting in significant enrichment of mercury in the air and surface sediments. A portion of mercury (particulate and reactive gaseous mercury) was deposited near the fire zones, but the positive high mercury fluxes of the surface soils indicated that mercury would again escape from the soil–air interface. The annual gaseous mercury emissions from the underground coal fires in China were estimated to reach 4.85 tonnes. Underground coal-fired mercury can be identified as an essential part of the global mercury cycle. Although some remediation measures were implemented, the development of coal fires proved difficult to control and was destined to be accompanied by the continuous release of mercury. Given the widespread distribution of coal fire cases worldwide, mercury pollution from underground coal fires deserves attention in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Underground coal fire is a kind of environmental disaster buried in the underground coal seam that can ignite low-temperature smoldering or even high-temperature burning [1]. The burning of coal may rapidly change the climate, which may have negative consequences on the ecology [2, 3]. When the buried underground coal seams are exposed to air through rock fractures or faults, heat accumulates in the coal seams due to oxidation reactions of sulfides in coal, which then ignite as temperature rises [4, 5]. In addition, coal fires can also occur in scenarios, such as mining shafts, exposed coal seams, coal storage and transportation, and coal waste piles (gangue) [6, 7]. In geological terms, evidence from paleo-coal fires can define coal-seam fires as natural disasters. Traces of coal fires can be traced back to 4 million years ago, as evidenced by coal fires in the Powder River Basin, United States [8]. However, large-scale mining after the Industrial Revolution has dramatically exacerbated the global expansion of coal-seam fires, such as in China [9, 10], USA [1, 11], India [12], South Africa [13], and Australia [4]. The perennial combustion of underground coal fires not only causes a massive loss of coal resources, destroys the mining landscape, and subsides the bedrock surface, but also releases high concentrations of toxic gases (e.g., SO2, CO, H2S, F, Hg, and volatile organics), endangering the health of residents [14, 15].
Mercury (Hg) is a highly toxic persistent pollutant of global concern that is prone to long-range transportation. It has been recognized as a worldwide threat to human and environmental health [16, 17]. Hg emission from coal combustion is considered to be one of the most important sources of atmospheric Hg [18]. The assessment of Hg emission has received attention. Still, it tends to focus on anthropogenic impacts, such as the Hg emissions from coal-fired power plants and the scattered coal utilization. Hg emissions from underground coal fires are generally not included in emissions inventories due to a lack of understanding or even hearing of underground coal fires. Therefore, the behavior of Hg from this source should be more examined and clarified. Hg concentration in an underground coal fire in vents from Tiptop, USA, can reach 2100 μg m−3 [19], which is 40 times higher than the U.S. Occupational Safety & Health Administration (OSHA) 8-h safe exposure limits (50 μg m−3) [20], and much higher than the global atmospheric Hg background value (1.5–1.8 ng m−3) [21]. Moreover, underground coal fire Hg is emitted almost freely, because it cannot be controlled.
Coal fires mainly occurred in the vast area of northern China, from Xinjiang Uygur Autonomous Region in the west to Heilongjiang Province in the east, stretching 4800 km longitudinally [22]. More than 50 coal fields in northern China have been affected by fires, with a cumulative burning area of ~ 700 km2, direct burning of coal reserves of ~ 20 million tonnes per year, destruction of coal reserves of ~ 200 million tonnes per year due to operational difficulties, and harmful gas emissions of about 1.06 million tonnes per year [10, 23,24,25]. The Wuda underground coal fire has been burning continuously for more than 60 years and is one of the most severe coal fire disaster areas in China and even the world. Spontaneous combustion signs have been found in several upper coal seams (Nos. 1, 2, 4, 6, 7, 9, and 10), and the spread of coal fires has been a concern as the coal seams are only a few meters apart [26]. It is of urgent significance to deepening the understanding of the potential environmental impact of coal-seam fires on the ecological environment.
Hg emissions from underground coal fires have not been included in the Hg emission inventory and are a new source of emissions to the environment. As a key research topic, studies on the migration process and quantitative assessment of Hg release from underground coal fires need to be further supplemented. In this study, taking the Wuda coal fires in China as a typical case, by integrating the Hg content parameters in various environmental media, the gaseous Hg emissions from China’s underground coal fires were evaluated and the migration process of Hg from underground coal fires was clarified for the first time.
Materials and methods
Case study area
The Wuda coalfield (39° 28′ N–39° 34′ N, 106° 36′ E–106° 40′ E) is located in Wuhai City, Inner Mongolia, in northern China, with a total area of 35 km2 and an average elevation of 1150–1300 m (Fig. 1). Carboniferous–Permian coal is in the area, with coal reserves of 630 million tonnes, and the remaining recoverable coal reserves are about 190 million tonnes. The region has a typical continental arid climate with an extremely hot and dry environment. The evaporation is 3500 mm year−1, which far exceeds precipitation (170 mm year−1). The northwest wind prevails in the area, with an average annual wind speed of 4.8 m s−1. The terrain is dominated by low mountains and hills, and with the insolation duration of ~ 3000 h year−1 [15, 27].
Wuda coal field and surface landscape caused by underground coal fires: A Wuda coal field and coal fire areas; B, C surface smoke landscape; D, E smoking vents and surface cracks, and surrounding sulfur and mirabilite; F collection scene of surface soil; G, H coal fire sponge; I boreholes used to monitor underground coal fires
The outbreak of the Wuda coal fire can be traced back to 1961, when the coal seams of No. 9 and No. 10 in the Suhaitu minefield took the lead in the spontaneous combustion of coal seams. Since then, the development of coal fires has intensified, from 6 surface fire areas in 1978 to 26 surface fire areas in 2004, with a total area of about 4 km2 [28, 29]. In 2009, Shenhua Remote Sensing Exploration Co., Ltd. re-investigated the area covered by the Wuda fire, which covered an area of 4.86 km2. Since 2009, the local government has vigorously curbed the development of the Wuda coal-seam fire and extinguished the visible surface flames. However, despite the staged governance results achieved in the distribution of the Wuda fire area from discrete to concentrated, the development trend that the fire area rebound is a cause for concern. Surface vents and fissures, with or without smoke, are scattered throughout the coal fire zone, and heavy smog in the near-surface air can be observed year-round.
Collection of index parameters in boreholes
To monitor the development of coal fire in a specific coal seam between the main roadway (80–120 m underground) of the Suhaitu minefield and the ground, and to ensure the safety of underground transportation, the borehole monitoring project was implemented. The function of setting the boreholes was to collect the gas in the borehole for component analysis, and then evaluate the underground coal fire and its development and corresponding countermeasures. The drilling depth is 13–42 m, the diameter is 10 cm, and there are 44 drilling holes in total. Most of the boreholes were set in the area where the coal seam had not been mined (the depth is 1 m from the coal-seam floor rock), and several drilling holes were set in the Goaf. These boreholes were equipped with steel pipes with air inlets on the pipe walls to allow the inflow of gas released from the coal seam, while the near-surface outlet ends of the steel pipes were sealed with steel caps to isolate air exchange [30]. Gas acquisition and on-site tests were performed after opening the steel caps.
Results and discussion
Hg release from Wuda underground coal seams
The Hg content in the raw coal of the Wuda coalfield has been evaluated many times. For example, reports from Wang [31], Hong [30], and Li [32] et al. indicated that the average contents were 261 ng g−1 (112–450 ng g−1, n = 4), 227 ng g−1 (48–589 ng g−1, n = 30), and 317 ng g−1 (273–346 ng g−1, n = 7), respectively. Thus, the average Hg content in the raw coal can be estimated to be 246 ng g−1, which is lower than the average Hg concentration of Chinese coal (290 ng g−1) [33]. In addition, Wang et al. [31] tested the average Hg content of burned coals in the Wuda underground coal seam to be 14 ng g−1 (9–24 ng g−1). These data are obtained by comparing the Hg content of raw coal—about 94% of the Hg was released due to coal fires.
Due to coal fires, the buried coal seams provided a continuous Hg source, and gas composition analysis data from the borehole monitoring project can reveal this hidden behavior. The average Hg concentration was 637 ng m−3 (n = 144) at the drill holes in the #2 fire zone, 3694 ng m−3 (n = 432) in the #3 fire zone, 1095 ng m−3 (n = 144) in the #4 fire zone, 4481 ng m−3 (n = 396) in #5 fire zone, and 6364 ng m−3 (n = 468) in #9 fire zone, respectively. The overall mean Hg concentration was 4165 ng m−3 (34–62,513 ng m−3, n = 1584), which was 45 times higher than the concentration measured in the near-surface atmosphere around the boreholes, indicative of high Hg released from the boreholes [30]. Nine boreholes in the #10 fire zone were used to monitor the dynamics of the No. 7 coal-seam goaf. Because most of the coal has been mined, gaseous Hg concentrations were relatively low, ranging from 5.69 to 158.19 ng m−3, with an overall average of 49 ng m−3 (detected in July, September, and October 2018) [25]. The high Hg concentrations in the boreholes can indicate the smoldering of underlying coal seams.
Hg pollution caused by Wuda underground coal fire
Atmospheric Hg pollution
Gaseous mercury
The most direct and significant Hg emission channels for coal fire areas are smoking vents and surface cracks. These channels transport underground coal-fired Hg into the air, diffusing and advecting into the surrounding area. The Hg concentration in the fumes from smoking vents and surface cracks at 30 spots in the central area is 200–1350 ng m−3, with an average Hg of 464 ng m−3 [34]. The temperature of smoking vents and cracks’ surface were mostly 150–280 °C, with several exceptions exceeding 300 °C. The extremely high concentration values and high-temperature data obtained from smoking vents and surface cracks undoubtedly demonstrate the vigorous development of underground coal fires, accompanied by uncontrolled Hg emissions. In addition, coal gangue piles stacked on the surface were also burning, and the average Hg concentration in the vents reached 5908 ng m−3 (1022–31,750 ng m−3) [35]. The resulting consequences are doomed to high levels of Hg in surface air. The Hg content in near-surface air in the coal fire central area was 257 ng m−3 (211–375 ng m−3), and that in the peripheral area was 89 ng m−3 (23–211 ng m−3) [34]. This result reveals that underground coal fires can lead to severe Hg pollution, as evidenced by Hg monitoring data from coal fire area vents in the Wyoming coal fires, Powder River Basin, USA (12,100 ng m−3) [36], Kentucky coal-seam fires, USA (7000–610,000 ng m−3) [37], and Witbank and Sasolburg coal fires, South Africa [38]. Furthermore, atmospheric Hg concentration in the downwind urban area reached 33 ng m−3, which was much higher than previously reported data in other cities or regions, e.g., Guiyang (9.72 ng m−3) [39], Guangzhou (5.4 ng m−3) [40], and Changchun (18.4 ng m−3) [41] in China, as well as Kagoshima City in Japan (3.5 ng m−3) [42].
At the same time, surface Hg fluxes were potentially and continuously occurring in an invisible form. A dynamic flux chamber method [43] was used at the Wuda coalfield to measure Hg flux in the surface soils of the fire zone. The measurement principle is to determine whether Hg is emitted or deposited and the corresponding flux, according to the difference between the outlet Hg concentration and the inlet Hg concentration (the Hg concentration in the ambient air) of the flux chamber within the delineated surface soil area. The Hg fluxes in the #3 fire zone were 76–174 ng m−2 h−1, with an average value of 99 ng m−2 h−1. The Hg fluxes in the #6 fire zone were 80–318 ng m−2 h−1, with an average value of 177 ng m−2 h−1. The no-fire area, located upwind of #3 and #6 fire zones, had lower values of 4–29 ng m−2 h−1 and 14–62 ng m−2 h−1, respectively, with an average of 19 and 32 ng m−2 h−1 [44]. These Hg flux values were positive, indicating that the soil was transporting more Hg to the air than it was deposited. In addition, the significant differences in flux values across the different zones indicated the inhomogeneity of Hg fluxes and potential effects from underground coal fires. Based on data available for comparison, the exchange flux of Hg between soil and air in the Wuda fire area was much higher than in many sites, such as forest areas (− 2.5 to 27.2 ng m−2 h−1) [45], wetlands (~ 3.5 ng m−2 h−1) [46], landfills (~ 20.0 ng m−2 h−1) [47], Hg mines and volcanic areas (17.1 ng m−2 h−1) [48], bare soil (6.5 ± 0.2 ng m−2 h−1) [49], urban areas (7.8 ± 7.1 ng m−2 h−1) [50], forests (~ 2.2 ng m−2 h−1) [51], and grasslands (1.0 ± 0.7 ng m−2 h−1) [52].
Particulate mercury
Generally, gaseous elemental mercury (GEM) is the primary form of atmospheric mercury, and atmospheric particulate mercury (PHg) often contributes less than 10% to the total atmospheric mercury [53, 54]. The regional sedimentation and water solubility of PHg are stronger than that of GEM [55], and it can enter and permanently damage the human body through inhalation, dietary consumption, and skin exposure [56]. By collecting TSP samples from the Wuda fire area, the particulate mercury (PHg) content in the near-surface air was determined to be 25–45 ng m−3, with an average content of 33 ng m−3 [57]. This value was also rare and much higher than previously reported data such as Beijing (1.18 ng m−3) [58], Shanghai (0.43 ng m−3) [59], and Changchun (0.02–1.98 ng m−3) [41]. PHg in Wuda coal fire area may exist as inorganic forms, such as HgCl2, HgS, HgO, and Hg(NO3)2·H2O, and predominantly adheres to fine particulate matter (≤ 2.5 μm) [57]. Hg0 is known to convert to PHg under certain oxidative conditions [60, 61], but the formation process of PHg in coal fire zones has not been elucidated.
Hg contamination of surface sediment
In the Wuda coalfield, the Hg concentration was 289 (11–765) ng g−1 (n = 11) in the dustfall (upper ~ 1.5 mm of the ground surface) and 216 (15–970) ng g−1 in the surface soil (upper ~ 20 cm of the ground surface) [32]. Concentrations of Hg in topsoil and dust are lower than those of some mercury-contaminated metal mines, such as the Zarshuran gold mine in Iran (24,200 ng g−1) [62], Almaden Hg mine, Spain (6000–8,889,000 ng g−1) [63], Phichit gold mine, Thailand (210–20,960,000 ng g−1) [64]. On the vertical profile of 0–30 cm, the soil Hg content decreased with the increase in depth, and was most enriched in the top layer [31]. This suggests that Hg deposits originating from surface flue gas vents and fissures are the source of Hg in the surface soil of the coal fire area. Meanwhile, the strong correlation between Hg and total carbon content observed in the soil also suggests that organic matter released from underground coal fires is pooled to the surface along with Hg. Soils in the downwind direction had higher levels of Hg, with concentrations around ten times higher than those in the windward direction.
Sulfur distributed near cracks and vents can have Hg levels of 900–3000 ng g−1. Coal Fire Sponge (CFS) [65], a "cow dung-like" raised coal fire derivative distributed on the surface of the coal fire area, with strong acidity and strong corrosiveness. It appears to be present on the surface of coal fire zones in different countries. Under each sponge is a vent hole of an underground coal fire [4, 66], but it looks like a raised lump of soft clay. The CFS in the Wuda fire area was enriched in acid, sulfur, and F, with the average parameters of pH, HF, and SO42− were 2.06 (0.61–3.84), 16.65 ppb (6–38 ppb), 188 mg g−1 (112–387 mg g−1), respectively [67]. CFS samples from the #6 and the #8 fire zones were tested (n = 73), and the Hg content in CFS ranged from 2653 to 38,470 ng g−1, with an average value of 13,967 ng g−1 [68]. The composition of CFS is not clear, but it exhibits a strong trapping and adsorption effect on mercury.
Migration of Hg from underground coal
Once it has entered the environment, Hg cycles between the air, land, and water, until it is eventually sequestered from the system through the processes, such as burial in deep ocean sediments or lake sediments [69]. Calculating the release of Hg from coal fires is a topic that needs to be explored, which can then be incorporated into an assessment system for the global Hg cycle. Therefore, it is necessary to understand the whereabouts of coal fire mercury.
Hg in coal-fired flue gas exists mainly in elemental (Hg0) and particulate (PHg) forms, and may also exist in small amounts in reactive gaseous mercury (RGM) [39, 70]. Among them, RGM is easily dissolved and transformed, and can be quickly removed from the atmosphere through wet and dry deposition processes. On the contrary. Hg0 is insoluble and stable, and its atmospheric lifetime can reach 1 year [71]. The existence lifetime of PHg in the atmosphere is longer than that of RGM, but much lower than that of Hg0. Due to the extremely low rainfall in the Wuda region, there are few opportunities for Hg to be wet deposited, and dry deposition is usually the main driving force for Hg accumulation in the soil (Fig. 2). Hg from underground coal fires, although CFS fixes a portion of it (probably PHg and RGM) in the form of HgO and HgSO4 [67], is much lower than that released into the air. Subsequently, RGM and PHg are readily deposited in the mining area and surrounding areas under dry deposition. However, their migration did not end there. Based on the positive high Hg fluxes exhibited by the surface soils of the coal fire areas, it is suggested that the Hg deposited on the surface is re-emitted to the atmosphere. This process is carried out with the participation of photoreduction, which realizes the gasification of Hg in the soil in the form of Hg0 [72]. Hg is released from the Wuda coal fires to surrounding areas by diffusion and advection, ultimately participating in the global Hg cycle.
It is worth mentioning that the urban area of Wuda is located 5 km downwind from the coal fire area. Therefore, this means that Hg from the coal fire area will be preferentially transported to the urban area and potentially threaten residents' health. Further speculation is that the Wuda urban area, with a population of ~ 130,000, may have suffered from coal fire Hg contamination for decades. The relationship between the Wuda coal fire and the adjacent urban area is just one case, and more extensive cases are distributed worldwide.
As China's most deeply researched coal fire area, the data indicators obtained from the Wuda coalfield can provide a reference standard for evaluating the Hg emissions from underground coal fires in China. Due to the more vital migration ability of gaseous Hg, atmospheric Hg output from underground coal fires was primarily estimated based on gaseous Hg releases (formula 1). The values of M, Ca, Cb, C0, Pa, and Pb were determined to be 2 × 107 [10, 25], 246, 14 [31], 290 [33], 257 [34], 33 [57], respectively. The results show that the annual Hg emission from underground coal fires in China is 4.85 tonnes, accounting for 2.4% of the conventional coal combustion emissions (202.3 tonnes) [73], which exceeds the annual Hg emissions from fuel combustion in Australia, New Zealand & Oceania Emissions (3.57 tonnes) [74].
where Q is the annual emission of gaseous Hg (t); M is the annual burning loss of raw coal (t); Ca is the Hg content in the raw coal (mg kg−1); Cb is the Hg content in the burned coal (mg kg−1); C0 is the Hg content in Chinese coal (mg kg−1); Pa is the gaseous Hg concentration in the near-surface (ng m−3); Pb is the PHg concentration in the near surface (ng m−3).
Uncontrolled Hg release from underground coal fires
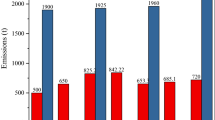
From 2006 to 2008, a surface block excavation method was adopted in the Wuda fire area to eliminate the coal fires. The original idea was to dig out the burning coal, backfill it with soil, and lay the surface with a hardener. However, in hindsight, it turned out to be an extremely unsuccessful project. This action led to the accelerated spread of coal fires and the destruction of almost all surface vegetation in the mining area, due to the failure to properly assess the difficulty of coal fire control [70]. Recently, governance measures have changed. The liquid nitrogen ejection project for the underground coal formation has been carried out several times in the Wuda coal fire area in an attempt to curb the further development of the coal fires. Figure 3 shows the variations of index parameters in boreholes 17-7-9 (Fig. 3a) and 17-7-9 (Fig. 3b) from the #10 fire zone before and after the liquid nitrogen ejection, including CO, Hg, and temperature (T). The execution dates of liquid nitrogen ejections were September 22 and September 30, 2018, and both injections reached 11.3 tonnes. It was observed that after the liquid nitrogen injection was performed on September 22, the CO concentration and T were significantly lower (based on the data on September 23 and 25) than those before ejection (the data on September 18), where the T even reached < 0 °C. However, after the ejection was performed again on September 30, although the changing trend of CO concentration in different boreholes diverged, the Hg concentration and T showed a rebounding trend. Judging from the evolution of temperature, implementing the liquid nitrogen ejection project can only briefly suppress the development of underground coal fires, but the rebound trend also followed. Since Hg vaporization is inherently highly controlled by temperature rather than atmospheric conditions, Hg release can proceed normally, even in an oxygen-free atmosphere. Therefore, even if no high-frequency and continuous Hg concentration monitoring data were acquired during the project implementation, the temperature-based rebound trend suggests that the coal-fired Hg release will continue.

Data were compiled from Shan et al. [26]
Variations in borehole temperatures, CO, and Hg concentrations before and after the ejection of liquid nitrogen.
Hg: a potential index gas of underground coal fires
Dynamic monitoring of underground coal fires is important in coal fire prevention and control. As a conventional high-precision geochemical detection method, indicator gas measurement plays an essential role in underground coal fire monitoring and early predicting potential coal fire disasters. According to the generation cause, coal fire indicator gases can be divided into three categories, including oxidizing gases (e.g., CO, CO2), alkene gases (e.g., C2H2, C2H4, C2H6, C3H8), and special gases (radon) [24, 75,76,77,78]. In practice, CO is usually used as the first choice indicator gas due to its early generation. A large amount of production, fast production speed, and the indicator are economical and effective [79, 80].
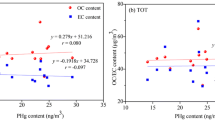
Some potential coal fire indicator gases, such as C2H4, C2H2, C3H6, and C2H6, cannot be applied in monitoring the Wuda underground coal fires. Because C2H4, C2H2, and C3H6 were not detected in the borehole gas, C2H6 was detected in only half of the boreholes and at lower levels (Table S1). The monitoring data of gas emitted in boreholes from areas with strong underground coal fire development (#2, #3, #4, #5, and #9 fire zones) show that the detected concentrations of Hg and CO were 34–62,513 ng m−3 and 0.006–0.594% [30]. The ratio of the highest detected concentration to the lowest detected concentration was 1838 times and 99 times, respectively, which indicates that the resolution of Hg is much greater than that of CO. Interestingly, field monitoring data from a wide zone range (#2, #3, #4, #5, #9, and #10 fire zones) showed that Hg release content from underground coal fires was significantly correlated with CO release content, with the R2 of 0.78 (Fig. 4a). Similarly, the correlation between Hg and CO was reproduced in monitoring data from the Wyoming coal fires, USA (Fig. 4a). Furthermore, Hg release was more responsive to temperature than CO, with a higher correlation coefficient of R2 = 0.74 (Fig. 4b). This is due to the fact that the Hg release process is highly controlled by its speciations and temperature. The Hg in Wuda coal mainly occurs in the speciation of HgS [81]. The central temperature of thermal decomposition for HgS is 310 °C [82]; that is, before reaching this temperature, the release concentration of Hg keeps rising. Even in an oxygen-free atmosphere (e.g., nitrogen and argon), Hg release can still occur. Due to the volatile nature of Hg, it is also applied in some important geological fields, such as earthquake prediction, detecting oil and gas, and searching for geothermal resources. In conclusion, Hg, as a common gas released from coal fires, has the potential to act as an indicator gas to monitor the development dynamics of underground coal fires due to its temperature sensitivity and high resolution.
Conclusions
This study aims to analyze the environmental impact, migration process, and release amount of Hg from underground coal fires, based on the integrated analysis of the observed data. Taking the Wuda underground coal fire area as an example, smoke with abnormally high Hg concentrations was released from surface vents and cracks, resulting in significant enrichment of Hg in the air and surface sediments. Particulate and reactive gaseous Hg tended to deposit near the fire zones. However, Hg would again escape from the soil-air interface, as indicated by the positive high Hg fluxes of the surface soils. Estimated 4.85 tonnes of gaseous Hg per year originate from underground coal fires in China and attempt to participate in the global Hg cycle. In addition, Hg, a common gas released by coal fires, has the potential as an indicator gas for monitoring the development and dynamics of underground coal fires due to its temperature sensitivity and high resolution.
Drawing on the governance experience of underground coal fires in Wuda, it is not easy to achieve the ideal effect by adopting the methods of isolating oxygen and cooling the coal seams intermittently. This is plagued by factors such as the complexity of the geological structure, the temperature rebound of the coal seams caused by the high-temperature surrounding rock, and the expensive engineering treatment cost. In the future, the control of underground coal fires should be regarded as one of the environmental protection causes that the world should jointly deal with. Given the widespread distribution of coal fire cases worldwide, Hg emissions from underground coal fires can be considered the essential strongholds for global Hg warehouses. Further assessment of global Hg emissions from underground coal fires is recommended.
Data availability
The data used in this article are available from the corresponding author upon reasonable request.
References
Stracher GB, Taylor TP (2004) Coal fires burning out of control around the world: thermodynamic recipe for environmental catastrophe. Int J Coal Geol 59(1–2):7–17
Gambhir A, Tavoni M (2019) Direct air carbon capture and sequestration: how it works and how it could contribute to climate-change mitigation. One Earth 1(4):405–409
Elahi E, Khalid Z, Tauni MZ, Zhang H, Lirong X (2022) Extreme weather events risk to crop-production and the adaptation of innovative management strategies to mitigate the risk: a retrospective survey of rural Punjab, Pakistan. Technovation 117:102255
Kuenzer C, Stracher GB (2012) Geomorphology of coal seam fires. Geomorphology 138(1):209–222
Sehn JL, de Leão FB, da Boit K, Oliveira ML, Hidalgo GE, Sampaio CH, Silva LF (2016) Nanomineralogy in the real world: a perspective on nanoparticles in the environmental impacts of coal fire. Chemosphere 147:439–443
Querol X, Izquierdo M, Monfort E, Álvarez E, Font O, Moreno T, Wang Y (2008) Environmental characterization of burnt coal gangue banks at Yangquan, Shanxi Province, China. Int J Coal Geol 75(2):93–104
Kataka MO, Matiane AR, Odhiambo BDO (2018) Chemical and mineralogical characterization of highly and less reactive coal from Northern Natal and Venda-Pafuri coalfields in South Africa. J Afr Earth Sci 137:278–285
Heffern EL, Coates DA (2004) Geologic history of natural coal-bed fires, Powder River basin, USA. Int J Coal Geol 59(1–2):25–47
Zhang X, Kroonenberg SB, De Boer CB (2004) Dating of coal fires in Xinjiang, northwest China. Terra Nova 16(2):68–74
Zeng Q, Dong J, Zhao L (2018) Investigation of the potential risk of coal fire to local environment: a case study of Daquanhu coal fire, Xinjiang region, China. Sci Total Environ 640:1478–1488
Silva LF, Oliveira ML, Neace ER, O’Keefe JM, Henke KR, Hower JC (2011) Nanominerals and ultrafine particles in sublimates from the Ruth Mullins coal fire, Perry County, Eastern Kentucky, USA. Int J Coal Geol 85(2):237–245
Syed TH, Riyas MJ, Kuenzer C (2018) Remote sensing of coal fires in India: a review. Earth Sci Rev 187:338–355
Onifade M, Genc B, Said KO, Fourie M, Akinseye PO (2022) Overview of mine rescue approaches for underground coal fires: a South African perspective. J S Afr Inst Min Metall 122(5):213–226
Tan B, Zhang F, Zhang Q, Wei H, Shao Z (2019) Firefighting of subsurface coal fires with comprehensive techniques for detection and control: a case study of the Fukang coal fire in the Xinjiang region of China. Environ Sci Pollut Res 26(29):29570–29584
Hong X, Liang H, Chen Y, Liu Y, Shi Y (2018) Distribution of fluorine in the surface dust of Wuda coal base, Inner Mongolia of Northern China. J Geochem Explor 188:390–397
Anna M, Andrey F, Eugenia V (2022) Comparison of the performance of different methods to stabilize mercury-containing waste. J Mater Cycles Waste Manag 24(3):1134–1139
Choi Y, Rhee SW (2022) Comprehensive analysis on mercury stream of cold cathode fluorescent lamps (CCFLs) in Korea (Republic of). J Mater Cycles Waste Manag 24(6):2375–2384
Pirrone N, Cinnirella S, Feng X, Finkelman RB, Friedli HR, Leaner J, Telmer K (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10(13):5951–5964
Hower JC, Henke K, O’Keefe JM, Engle MA, Blake DR, Stracher GB (2009) The Tiptop coal-mine fire, Kentucky: preliminary investigation of the measurement of mercury and other hazardous gases from coal-fire gas vents. Int J Coal Geol 80(1):63–67
U.S Department of Labor Occupational Safety Health Administration (2004) Safety and health topics: mercury (vapor) (as Hg). http://www.oshs.gov/dts/chemicalsampling/data/CH_250510.html. Accessed 18 Jan 2009
Jaffe D, Prestbo E, Swartzendruber P, Weiss-Penzias P, Kato S, Takami A, Kajii Y (2005) Export of atmospheric mercury from Asia. Atmos Environ 39(17):3029–3038
Li C, Sun J, Shi J, Liang H, Cao Q, Li Z, Gao Y (2020) Mercury sources in a subterranean spontaneous combustion area. Ecotoxicol Environ Saf 201:110863
Kong B, Li Z, Yang Y, Liu Z, Yan D (2017) A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ Sci Pollut Res 24(30):23453–23470
Zhang J, Guan HY, Cao DY (2008) Underground coal fires in China: origin, detection, fire-fighting, and prevention. China Coal Industry Publishing House, Beijing (in Chinese)
Voigt S, Rüter H (2006) The Sino German coal fire research initiative—research concepts and general aspects of coal seam fire mitigation. In: Buhrow C, Schächter HN, Schmidt R (eds) Kolloquium Ressourcen und Umwelt 2006—Kohle und China, 30–31 März 2006, pp 247–253 (Freiberg, Germany)
Shan B, Wang G, Cao F, Wu D, Liang W, Sun R (2019) Mercury emission from underground coal fires in the mining goaf of the Wuda Coalfield, China. Ecotoxicol Environ Saf 182:109409
Hong X, Yang K, Liang H (2021) Characterization of acidity and sulfate in dust obtained from the Wuda coal base, northern China: spatial distribution and pollution assessment. Environ Sci Pollut Res 28(25):33219–33230
Song Z, Kuenzer C (2017) Spectral reflectance (400–2500 nm) properties of coals, adjacent sediments, metamorphic and pyrometamorphic rocks in coal-fire areas: a case study of Wuda coalfield and its surrounding areas, northern China. Int J Coal Geol 171:142–152
Cao QY, Qian YH, Liang HD, Wang Z (2019) The species and spatial distribution characteristics of atmospheric particulate mercury in Wuda-Wusitai Industrial Park, China. China Environ Sci 39(12):4989–4998
Hong X, Liang H, Lv S, Jia Y, Zhao T, Liang W (2017) Mercury emissions from dynamic monitoring holes of underground coal fires in the Wuda Coalfield, Inner Mongolia, China. Int J Coal Geol 181:78–86
Wang G, Cao F, Shan B, Meng M, Wang W, Sun R (2019) Sources and assessment of mercury and other heavy metal contamination in soils surrounding the Wuda underground coal fire area, Inner Mongolia, China. Bull Environ Contam Toxicol 103(6):828–833
Li C, Liang H, Chen Y, Bai J, Cui Y (2018) Distribution of surface soil mercury of Wuda old mining area, Inner Mongolia, China. Hum Ecol Risk Assess Int J 24(5):1421–1439
Cao QY, Yang L, Ren WY, Song YL, Huang SY, Wang YT, Wang ZY (2021) Spatial distribution of harmful trace elements in Chinese coalfields: an application of WebGIS technology. Sci Total Environ 755:142527
Liang Y, Liang H, Zhu S (2014) Mercury emission from coal seam fire at Wuda Inner Mongolia, China. Atmos Environ 83:176–184
Liang Y, Liang H, Zhu S (2016) Mercury emission from spontaneously ignited coal gangue hill in Wuda coalfield, Inner Mongolia, China. Fuel 182:525–530
Engle MA, Radke LF, Heffern EL, O’Keefe JM, Hower JC, Smeltzer CD, ter Schure A (2012) Gas emissions, minerals, and tars associated with three coal fires, Powder River Basin, USA. Sci Total Environ 420:146–159
O’Keefe JM, Henke KR, Hower JC, Engle MA, Stracher GB, Stucker JD, Lemley EW (2010) CO2 CO, and Hg emissions from the Truman Shepherd and Ruth Mullins coal fires, eastern Kentucky, USA. Sci Total Environ 408(7):1628–1633
Pone JDN, Hein KA, Stracher GB, Annegarn HJ, Finkleman RB, Blake DR, Schroeder P (2007) The spontaneous combustion of coal and its by-products in the Witbank and Sasolburg coalfields of South Africa. Int J Coal Geol 72(2):124–140
Fu X, Feng X, Sommar J, Wang S (2012) A review of studies on atmospheric mercury in China. Sci Total Environ 421:73–81
Wang Z, Chen Z, Duan N, Zhang X (2007) Gaseous elemental mercury concentration in atmosphere at urban and remote sites in China. J Environ Sci 19(2):176–180
Fang F, Wang Q, Li J (2001) Atmospheric particulate mercury concentration and its dry deposition flux in Changchun City, China. Sci Total Environ 281:229–236
Kono Y, Tomiyasu T (2014) Variations in atmospheric mercury concentration in Kagoshima City during 2010–2012. Bunseki Kagaku 63(1):17–21
Huang HC, Lee CL, Lai CH, Fang MD, Lai IC (2012) Transboundary movement of polycyclic aromatic hydrocarbons (PAHs) in the Kuroshio Sphere of the western Pacific Ocean. Atmos Environ 54:470–479
Li C, Liang H, Liang M, Chen Y, Zhou Y (2018) Soil surface Hg emission flux in coalfield in Wuda, Inner Mongolia, China. Environ Sci Pollut Res 25(17):16652–16663
Choi HD, Holsen TM (2009) Gaseous mercury fluxes from the forest floor of the Adirondacks. Environ Pollut 157(2):592–600
Kyllönen K, Hakola H, Hellén H, Korhonen M, Verta M (2012) Atmospheric mercury fluxes in a southern boreal forest and wetland. Water Air Soil Pollut 223(3):1171–1182
Lindberg SE, Zhang H, Gustin M, Vette A, Marsik F, Owens J, Xiao Z (1999) Increases in mercury emissions from desert soils in response to rainfall and irrigation. J Geophys Res Atmos 104(D17):21879–21888
Engle MA, Gustin MS, Zhang H (2001) Quantifying natural source mercury emissions from the Ivanhoe Mining District, north-central Nevada, USA. Atmos Environ 35(23):3987–3997
Gabriel MC, Williamson DG, Zhang H, Brooks S, Lindberg S (2006) Diurnal and seasonal trends in total gaseous mercury flux from three urban ground surfaces. Atmos Environ 40(23):4269–4284
Liu F, Cheng H, Yang K, Zhao C, Liu Y, Peng M, Li K (2014) Characteristics and influencing factors of mercury exchange flux between soil and air in Guangzhou City. J Geochem Explor 139:115–121
Schroeder WH, Beauchamp S, Edwards G, Poissant L, Rasmussen P, Tordon R, Banic CM (2005) Gaseous mercury emissions from natural sources in Canadian landscapes. J Geophys Res Atmos 110:D18302
Ericksen JA, Gustin MS, Xin M, Weisberg PJ, Femandez GCJ (2006) Air–soil exchange of mercury from background soils in the United States. Sci Total Environ 366:851–863
Miller MB, Howard DA, Pierce AM, Cook KR, Keywood M, Powell J, Gustin MS, Edwards GC (2021) Atmospheric reactive mercury concentrations in coastal Australia and the Southern Ocean. Sci Total Environ 751:141681
Fu XW, Feng X, Dong ZQ, Yin RS, Wang JX, Yang ZR, Zhang H (2010) Atmospheric gaseous elemental mercury (GEM) concentrations and mercury depositions at a high-altitude mountain peak in south China. Atmos Chem Phys 10:2425–2437
Sun G, Feng X, Yang C, Zhang L, Yin R, Li Z, Wu Y (2020) Levels, sources, isotope signatures, and health risks of mercury in street dust across China. J Hazard Mater 392:122276
Trasande L, Landrigan PJ, Schechter C (2005) Public health and economic consequences of methyl mercury toxicity to the developing brain. Environ Health Perspect 113(5):590–596
Qian Y, Liang Y, Cao Q, Wang Z, Shi Y, Liang H (2022) Concentration and speciation of mercury in atmospheric particulates in the Wuda coal fire area, Inner Mongolia, China. Environ Sci Pollut Res 29(3):3879–3887
Liu S, Nadim F, Perkins C, Carley RJ, Hoag GE, Lin Y, Chen L (2002) Atmospheric mercury monitoring survey in Beijing, China. Chemosphere 48(1):97–107
Xiu GL, Shi SY, Zhang DN (2003) Preliminary study on characteristics of particulate mercury in fine particles in ambient air. Shanghai Environ Sci 22(5):310–316 (in Chinese)
Ebinghaus R, Kock HH, Temme C, Einax JW, Löwe AG, Richter A et al (2002) Antarctic springtime depletion of atmospheric mercury. Environ Sci Technol 36:1238–1244
Weiss-Penzias P, Amos H, Selin N, Gustin M, Jaffe D, Obrist D et al (2015) Use of a global model to understand speciated atmospheric mercury observations at five high-elevation sites. Atmos Chem Phys 15:1161–1173
Karbassi A, Nasrabadi T, Rezai M et al (2014) Pollution with metals (As, Sb, Hg, Zn) in agricultural soil located close to Zarshuran gold mine, Iran. Environ Eng Manag J 13:155–220
Higueras P, Oyarzun R, Biester H et al (2003) A first insight into mercury distribution and speciation in soils from the Almaden district, Spain. J Geochem Explor 80:95–104
Pataranawat P, Parkpian P, Polprasert C et al (2007) Mercury emission and distribution: potential environmental risks at a small-scale gold mining operation, Phichit Province, Thailand. J Environ Sci Health Part A 42(8):1081–1093
Hower JC, O’Keefe JM, Henke KR, Wagner NJ, Copley G, Blake DR, Silva LF (2013) Gaseous emissions and sublimates from the Truman Shepherd coal fire, Floyd County, Kentucky: a re-investigation following attempted mitigation of the fire. Int J Coal Geol 116:63–74
Querol X, Zhuang X, Font O, Izquierdo M, Alastuey A, Castro I, López-Soler A (2011) Influence of soil cover on reducing the environmental impact of spontaneous coal combustion in coal waste gobs: a review and new experimental data. Int J Coal Geol 85(1):2–22
Li C, Shi J, Cao Q, Luo Y, Liang H, Du C, Shi J (2021) Role of H+, HF, SO42− and kaolin in fixing Hg of coal fire sponge. Sci Total Environ 772:14551
Liang Y, Zhu S, Liang H (2018) Mercury enrichment in coal fire sponge in Wuda coalfield, Inner Mongolia of China. Int J Coal Geol 192:51–55
Rumayor M, Gallego JR, Rodríguez-Valdés E, Díaz-Somoano M (2017) An assessment of the environmental fate of mercury species in highly polluted brownfields by means of thermal desorption. J Hazard Mater 325:1–7
Kuenzer C, Zhang J, Sun Y, Jia Y, Dech S (2012) Coal fires revisited: The Wuda coal field in the aftermath of extensive coal fire research and accelerating extinguishing activities. Int J Coal Geol 102:75–86
Zhou J, Feng X, Liu H, Zhang H, Fu X, Bao Z, Zhang Y (2013) Examination of total mercury inputs by precipitation and litterfall in a remote upland forest of Southwestern China. Atmos Environ 81:364–372
Bergquist BA, Blum JD (2007) Mass-dependent and-independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 318(5849):417–420
Wu ZJ, Ye HF, Shan YL, Chen B, Li JS (2020) A city-level inventory for atmospheric mercury emissions from coal combustion in China. Atmos Environ 223:117245
United Nations Environment Programme (2019) Global Mercury Assessment 2018
Liang Y, Zhang J, Wang L, Luo H, Ren T (2019) Forecasting spontaneous combustion of coal in underground coal mines by index gases: a review. J Loss Prev Process Ind 57:208–222
Deng J, Ge S, Qi H, Zhou F, Shi B (2021) Underground coal fire emission of spontaneous combustion, Sandaoba coalfield in Xinjiang, China: investigation and analysis. Sci Total Environ 777:146080
Engle MA, Olea RA, O’Keefe JM, Hower JC, Geboy NJ (2013) Direct estimation of diffuse gaseous emissions from coal fires: current methods and future directions. Int J Coal Geol 112:164–172
Tian FC, Liang YT, Zhu HQ, Chen MY, Wang JC (2022) Application of a novel detection approach based on non-dispersive infrared theory to the in-situ analysis on indicator gases from underground coal fire. J Cent South Univ 29:1840–1855
O’Keefe JM, Neace ER, Lemley EW, Hower JC, Henke KR, Copley G, Blake DR (2011) Old Smokey coal fire, Floyd County, Kentucky: estimates of gaseous emission rates. Int J Coal Geol 87(2):150–156
Hou X, Guo L, Wang F, Xu Y, Dong X, Gao D, Sun Y (2019) Research on sources appointment of abnormal co in underground mine. Feb-Fresenius Environ Bull 28(4):2897–2907
Cao QY, Yang L, Qian YH, Liang HD (2020) Study on mercury species in coal and pyrolysis-based mercury removal before utilization. ACS Omega 5(32):20215–20223
Rumayor M, Diaz-Somoano M, Lopez-Anton MA, Martinez-Tarazona MR (2013) Mercury compounds characterization by thermal desorption. Talanta 114:318–322
Acknowledgements
This research is supported by the National Natural Science Foundation of China (41772157 and 41371449) and the State Key Laboratory of Coal Resources and Safe Mining (SKLCRSM17ZZ01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, Q., Cheng, Y., Kusakabe, T. et al. Mercury emission from underground coal fires: a typical case in China. J Mater Cycles Waste Manag 25, 2706–2715 (2023). https://doi.org/10.1007/s10163-023-01616-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01616-9