Abstract
Construction waste bricks (CWB) were simply pre-treated as fillers applied in different filler layers of bioretention facility. The removal effect of runoff pollutants in CWB-modified facilities was investigated and compared with traditional facility with gravel and sand. Leaching test shows that both CWB-modified and traditional bioretention facilities leach TN, and CWB-based facilities leach Cr. CWB-based facility shows good removal effect toward TP, NH4+-N and COD, with the removal ratio of 0.90, 0.80 and 0.70, respectively. CWB-based facility also shows good removal effects toward Cu, Zn, Cd, Pb and Mn, with the removal ratio of 0.55–0.82, 0.65–0.92, nearly 1.00, above 0.90 and nearly 0.98, respectively. Generally, the removal effect of both CWB and traditional facilities on runoff pollutants is basically stable and the removal ratio is relatively high after running for 40 days. Economic calculation shows that CWB-based facilities could reduce the cost of fillers by 67.2–88.4%, which further confirmed the replacement of traditional fillers by CWB. This study could not only enhance the removal ability of runoff pollutants by bioretention facilities, but also provide a new way for the utilization of CWB.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid urbanization, the increase of impermeable areas puts pressure on urban drainage systems. Rainwater runoff merges into the water body, which increases the concentrations of pollutants, such as chemical oxygen demand (COD), total phosphorus (TP) and ammonium nitrogen (NH4+–N). The concentration of runoff pollutants sometimes exceeds the China National Standard for Environmental quality standards for surface water (GB3838-2002), indicating that the influx of runoff rainwater has aggravated the pollution of water bodies [1]. The bioretention facility is one of the main technical measures of the "sponge city". It is mainly composed of soil, sand, gravel and crushed stone. The pollutants in the runoff are mainly reduced through the interception and adsorption of the filler. The removal of pollutants, such as total nitrogen (TN), COD, NH4+-N and TP, is mainly due to the role of adsorption filtration. Studies have shown that bioretention facility has high removal efficiency on heavy metals and nutrient pollutants [2, 3]. However, there are also some studies found that bioretention facility is not effective in removing TN and TP, and the effluent concentrations are unstable [4].
Construction waste bricks (CWB) not only occupy land resources, but also bring pollution risk to the city's ecological environment. According to statistics, China produces about 1.5 billion tons of construction waste every year in urban construction [5]. However, CWB have rich porous structure, large pore size, high porosity, rough surface and large specific surface area. They are also rich in iron, aluminum elements and volcanic ash components, as shown in the following research. These characteristics make it ability to adsorb chemical substances. Many studies have used CWB as water treatment adsorbents to remove phosphates, heavy metals and other pollutants [6,7,8]. Wang et al. used CWB to remove Pb in rainwater. The results show that the average removal ratio of Pb from CWB can reach 0.99. The smaller the particle size of CWB, the greater the removal ratio of Pb [9].
In this study, CWB was used as the advanced filling materials in bioretention facilities. The removal effect of CWB on typical runoff pollutants (COD, TP, TN, NH4+-N and various heavy metals including Cu, Cr, Zn, Mn, Cd and Pb) by simulated facilities was studied. The variation rule of pollutant removal effect under long-term operation were investigated. This study could not only enhance the removal ability to runoff pollutants of bioretention facilities, but also provide a new way for the utilization of CWB.
Materials and methods
Materials
CWB were acquired from Beijing Construction Engineering Resources Recycling Investment Co., Ltd. CWB were broken into powder for characterization. CWB were also broken to small particles by sieving to achieve the particle size 2–5 mm and 5–10 mm before used. Then the particles were subjected to calcination in an oven at 105 °C for 24 h. River sand (particle size 0.5–1 mm) and gravel (particle size 5–10 mm) were also used as fillers for the bioretention facilities. Rural soil was collected from Daxing District Park of Beijing as packed soil.
Experimental set-up
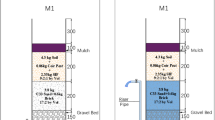
The traditional bioretention facility experimental column named “TC” was designed according to the guideline of complex bioretention facility structure in the “Sponge City Construction Technology Guide” of China. The height of the column is 1 m and the inner diameter is 10 cm, as shown in Fig. 1a. The drain pipe extends into the experimental column and is wrapped with geotextile to prevent the filler from flowing out with water. The bioretention facility is composed of surface layer, bedding layer and basement layer from top to bottom. The surface layer of the column was planting soil with the height of 300 mm. The bedding layer was filled with sand (particle size 2–5 mm) with the height of 100 mm. The basement layer was filled with gravel (particle size 5–10 mm) with the height of 250 mm. Each layer is partitioned by geotextile to prevent the filler from sinking with water. The type of vegetation used on the surface of bioretention columns was Chinese Iris. This study mainly explores the possibility of using CWB as retention layers in bioretention facilities, so the role of vegetation is not discussed. For the three CWB-modified columns, the basement layer, bedding layer and both of the two layers were replaced by CWB with the corresponding particle size, respectively. They were named of G-CWB, S-CWB and SG-CWB respectively (as shown in Fig. 1b, c and d).
Schematic diagrams of four bioretention facilities set-up. A water outlet is arranged at the bottom of the facilities, and the outlet is raised 150 mm to facilitate sampling. From top to bottom, TC (traditional column) was filled with soil, sand and gravel. G-CWB was filled with soil, sand and CWB (with size of 5–10 mm). S-CWB was filled with soil, CWB (with size of 2–5 mm) and gravel. SG-CWB was filled with soil, CWB (with size of 2–5 mm) and CWB (with size of 5–10 mm)
Test methods
CWB in powder form was used for characterization. To investigate the chemical and physical properties of CWB, its elemental composition and surface morphology were analyzed. The elemental composition was analyzed by X-ray fluorescence (XRF) techniques. The particle size of the CWB used for characterization was less than 200 mesh. The powder is fully dried and pressed at 35 t pressure for 45 s, followed by XRF test which made by Panaco in the Netherlands. The surface morphology was analyzed by scanning electron microscopy (SEM) with the condition of 3.0 kV, 10.4 mm × 5.00 k, SE(TUL) and the scale is 10.0 μm. The model of the instrument is su8020, made in Japan.
To analyze the background value of six heavy metals in the four materials (CWB, soil, gravel and sand), each material was digested with acid mixture of hydrogen nitrate perchloric acid hydrofluoric acid compound (5: 4: 5, V/V/V) by the constant temperature electric heating plate. Six heavy metals were then determined by an inductively coupled plasma mass spectrometry (ICP-MS).
The pollutants selected in this experiment were four typical runoff pollutants (COD, NH4+-N, TN and TP) and six heavy metals (Cu, Zn, Mn, Cr, Pb and Cd). The pollutants concentration of simulated runoff rainwater was prepared according to the actual runoff (Table 1). The water intake method was artificial water intake, and a water layer of 50–70 cm above the soil layer in the columns was maintained. Three stages were set in the experiments. The whole experiment was carried out at room temperature 25 °C, and the pH of influent water was adjusted to 6–7 according to the actual runoff. The first stage was the leaching test to investigate the leaching concentration of pollutants. The second stage was to evaluate the removal efficiency of the pollutants in runoff by the four experimental columns with the increase of rainfall duration. The drying period of the first two stages was set at 1 week. The third stage was to study the removal efficiency of pollutants after the long-term operation. To better simulate the influence of the four facilities on pollutant removal under working conditions, the influent water was the same as the second stage, and the drying period between each run was 4 days. All effluent pollutant concentrations in these three stages were compared with China National Standard for Environmental quality standards for surface water (GB3838-2002), as shown in Table 2 (nothing that Mn concentration is not required in the standard). The influent water volume was determined according to the design rainfall (33.6 mm) corresponding to the 10% service area ratio and the 85% runoff control rate in Beijing.
In this experiment, the effluent concentrations of typical nutrient pollutants and heavy metals were detected. Six heavy metals (Cu, Zn, Mn, Cd, Cr and Pb) were detected by ICP-MS. Four nutrient pollutants (COD, NH4+-N, TN and TP) were all measured by spectrophotometric method.
Statistical analysis
The statistical significance was analyzed by t test method. Three bioretention facilities filled with CWB were compared with traditional facility to investigate the significant differences in data. P value less than 0.05 indicates significant difference, while P values greater than 0.05 indicate no significant difference. Statistical significance in experimental data was determined using Origin2021.
Results and discussion
Characterization of construction waste brick
The elemental composition of CWB is shown in Table 3. The results showed that the most abundant elements in CWB are Si, Al, Fe and Ca, account for 52.51%, 14.68%, 10.66% and 8.90%, respectively. The surface morphology of CWB is displayed in Fig. 2. As can be seen from Fig. 2, the surface of CWB was rough, which may provide much more adsorption sites, thereby increasing the adsorption capacity toward pollutants. Table 4 shows the concentration of six heavy metals from CWB, soil, sand and gravel. It could be seen that the background value of metals in the four materials is different from each other. Yuan et al. reported the concentration of heavy metals in soils in China by reviewing of 713 articles, and the average concentrations of Cu, Zn, Cr, Cd, Pb in China soil are 25.81, 85.86, 67.37, 30.74 mg/kg [10]. The concentration of Cu, Zn, Cr, Cd and Pb in soil used in our study is slightly lower than the average concentration reported by Yuan et al. While for sand and gravel, the concentration of Cu, Cr, Zn is much lower than the average value of soil. For CWB, the concentration of Cr is larger than other materials.
Leaching of pollutants by four bioretention facilities
The leaching process of pollutants was studied at the beginning of the experiment. Figure 3 shows the leaching concentration of typical pollutants (NH4+-N, COD, TP and TN) with duration from 0–9 h. It can be found from the figure, the effluent concentration of NH4+-N, COD and TN within 9 h exceeded Class V (2.0 mg/L for NH4+-N, 10.0 mg/L for COD and 2.0 mg/L for TN) of GB 3838–2002. For NH4+-N (Fig. 3a), the leaching amount of SG-CWB was less than the other three facilities. The leaching concentration of facilities decreased with duration expect SG-CWB. After 5.5 h, the leaching concentration of NH4+-N from the four facilities was all stabilized within 4.0 mg/L. The leaching concentrations of COD and TN (Fig. 3b and d) in the four facilities had basically the same changing trends, all decreased with duration. After 9 h, the COD and TN concentrations leached from the facility dropped below 100 mg/L and 30 mg/L respectively, and both may continue to decrease. For TP (Fig. 3 c), the leaching concentration was all below 0.05 mg/L within 9 h, which could meet the Class II (0.1 mg/L) of GB3838-2002. P values deduced by t test to compare the differences between three CWB-based facilities and traditional facility are shown in Table 5. As can be seen from the table, compared with traditional facility, SG-CWB showed significant difference of removal effects on NH4+-N, COD and TN (P < 0.05). This indicated that the more traditional fillers were replaced by CWB, the less the leaching amount of those three pollutants.
Relevant research shows that the soil contains a lot of nitrogen and phosphorus nutrients [11]. To verify this statement, the physical and chemical properties of the soils (garden soil) used in this study were analyzed. The results showed that the content of organic matter was 1.38%, the content of available phosphorus was 186 mg/kg, and the content of alkali-hydrolyzable nitrogen was 123 mg/kg. Therefore, it contains high nutrient content, which is consistent with relevant studies. Therefore, a large amount of nitrogen (NH4+-N and TN) is leached from the facilities. The reason for the low phosphorus concentration in the effluent may be that after phosphorus leached from the soil, it is adsorbed and removed by the filler in the bedding and basement layer. Because soil [12], CWB [13,14,15,16] and gravel [13] all have good adsorption ability toward phosphorus. Subsequent experiments will also provide the basis for this conclusion. The leaching amount of NH4+-N from SG-CWB is less than the other three facilities. The main reason may be that CWB can adsorb and remove part of the NH4+-N leached from the soil. CWB occupies a large proportion in the filler of SG-CWB, causing the large removal amount and low leaching concentration of NH4+-N. The leaching concentration of COD and TN in the facilities filled with CWB is not significantly different from the traditional facilities, which may be due to the not obvious removal effect of CWB toward COD and TN.
Figure 4 shows the leaching process of heavy metals in the four facilities. It can be seen that the three facilities filled with CWB did not leach large amount of heavy metals expect for Cr. For the five heavy metals other than Cr, there was no obvious difference in the leaching concentration and change trend of the four facilities. The leaching concentration of the five heavy metals continued to fluctuate within 9 h, but they were always low. For Cu and Zn (Fig. 4 a and b), their leaching concentrations were always below 57 μg/L and 80 μg/L respectively, and both met the Class II (1000 μg/L for both Cu and Zn) of GB3838-2002. For Mn (Fig. 4c), the leaching concentration stabled after 4 h was always below 7 μg/L. For Cd and Pb (Fig. 4d and f), their leaching concentrations were always below 0.3 μg/L and 6 μg/L respectively, and both met the Class I (1 μg/L for Cd and 10 μg/L for Pb) of GB3838-2002. The leaching of Cr is much more in facilities with CWB than facility TC (Fig. 4e). The leaching concentration from TC was always below 10 μg/L, meeting the Class I (10 μg/L) of GB3838-2002. The leaching concentration from S-CWB decreased with duration, and stabled in 6 h, with a value below 25 μg/L. For facility G-CWB, it was always below 50 μg/L. The leaching concentration from S-CWB and G-CWB could meet the Class II (50 μg/L) of GB3838-2002. For SG-CWB, the leaching concentration was high and unstable (up to 130 μg/L), which exceeded the Class V (100 μg/L) of GB3838-2002. It can be seen from the results that the more filling amount of CWB is, the more Cr is leached. Generally, the Cr content in gravel and sand is low. To further reveal the background value of heavy metals, CWB, sand, gravel and soil were completely digested to determine the background content of heavy metals (Table 4). And the results showed that the content of Cr in these fillers was very low. Therefore, it can be concluded that CWB might contain more Cr than gravel and sand. According to Table 4, the content of Cr is slightly higher than soil and much larger than sand and gravel. Therefore, the leaching amount of Cr by CWB-based facilities is slightly higher than traditional facility without CWB. Under the action of current scouring, CWB is dissolved into the rainwater and washed out with the water. From this aspect, we should concern of the release of Cr from CWB especially in the initial stage. XRF results show that the content of Cr in CWB is slightly higher than that of other metals in this experiment (Table 3), which is consistent with the results of this leaching experiment, so it may be the reason for the leaching of Cr by CWB.
As can be seen from Table 5, compared with traditional facility, SG-CWB showed significant difference of removal effects on Cu, Zn, Cr and Pb (P < 0.05). On the whole, the difference of heavy metal leaching between different facilities is consistent with the rule of typical pollutants. This also indicated that the more traditional fillers replaced by CWB, the less the leaching amount of pollutants.
Removal effect of runoff pollutants by bioretention facilities
To study the removal efficiency of the bioretention facilities with different fillers, the change rule of the effluent pollutant concentration and pollutant removal effect in different facilities with rainfall duration was investigated. Figure 5 shows the removal effect of typical pollutants (NH4+-N, COD, TP and TN) in the four facilities under the same rainfall conditions.
During the removal process of NH4+-N (Fig. 5a), the removal effect was different in each facility at the beginning. For TC and S-CWB, the effluent concentration was higher than the influent concentration in the initial stage, indicating that these two facilities will leach NH4+-N at this stage. For G-CWB and SG-CWB, the effluent concentration was always low. They all stabilized below 3.0 mg/L after 5 h of the four facilities, with the removal ratio above 0.80. However, the effluent concentration after stabilization still exceeded the GB3838-2002 (2.0 mg/L). The NH4+-N removal efficiency from G-CWB was significantly different from traditional facility with increasing of rainfall duration (P < 0.05, Table 5), which indicated the better NH4+-N removal efficiency from G-CWB. Although the facilities with CWB layer in the system did not show the best removal effect toward NH4+-N, they still maintained a high removal ratio of 0.81–0.99.
During the removal process of COD (Fig. 5 b), the facility G-CWB showed good removal effect at the beginning, and the removal ratio ranged from 0.50 to 0.75. The removal effect in the other three facilities was relatively low and similar. When the system runs for 2.5–4.5 h, the four facilities show better COD removal effects, and the removal ratio is basically stabled at more than 0.80. After 4.5 h, the removal ratio of G-CWB continued to be high, with the effluent concentration below 20 mg/L, meeting the Class III (20 mg/L) of GB3838-2002. The removal ratio of the other three facilities was reduced to 0.70 to 0.80, with the effluent concentration from 90 mg/L to 160 mg/L, exceeding GB3838-2002 (40 mg/L). The COD removal effect from G-CWB was significantly different from that of traditional facility with duration (P < 0.05, Table 5), which indicated the better COD removal effect from G-CWB. This trend was similar with the removal of NH4+-N by G-CWB.
The four facilities all showed good removal effect toward TP (Fig. 5 c). The average removal ratio of TP from facility TC, S-CWB, G-CWB and SG-CWB was 0.94, 0.93, 0.95 and 0.93, respectively. There was no obvious difference between the four facilities (P > 0.05, Table 5). The effluent concentrations of TP in the four facilities were all below 0.2 mg/L, which met the Class III (0.2 mg/L) of GB3838-2002. The removal of TP is mainly due to the rich of Ca2+ in CWB and gravel. In this study, according to the actual runoff, the influent pH was adjusted to 6–7. During the experiment, the pH of the effluent water increased to about 9 because the silicate aluminate in CWB and gravel generated OH− when it met water. In alkaline environment, Ca2+ easily combines with phosphate to form Ca3(PO4)2 as precipitation. Phosphate remains in the filler in the form of precipitation, which improves the removal ratio of TP in the effluent [2].
During the removal process of TN (Fig. 5d), the effluent concentration of the four facilities was higher than the influent concentration at the beginning, indicating that a large amount of TN had been leached. The reason may be that no stable microbial community was formed, resulting in TN still in a high concentration release stage. The effluent concentration of the four facilities decreased with duration. In the later stage of the experiment, the effluent concentration of the four facilities gradually stabilized from 15 to 20 mg/L, and there was no significant difference between the four facilities after stabilization. The effluent concentration exceeded GB3838-2002 (2.0 mg/L). The removal ratio after stabilization was only about 0.10. Generally, SG-CWB showed better efficiency on TN removal (P < 0.05, Table 5). Therefore, the effluent of the four facilities still needs further treatment to improve water quality. For example, setting up an anaerobic zone to promote the process of denitrification.
In general, bioretention facilities filled with CWB have no significant improvement in the removal efficiency of typical pollutants compared with traditional facility. However, the removal ratio of NH4+-N, COD and TP can be stabilized above 0.80, 0.70 and 0.90, respectively. The bioretention facility shows good removal effect of these three pollutants. However, for TN, the leaching of pollutants at the early stage of rainfall needs to be considered, and the final removal ratio is only about 0.20. Therefore, the removal effect of TN by bioretention facilities needs to be improved. Using CWB as biological retention filler can effectively reduce the project cost on the premise of ensuring the effluent quality of typical pollutants.
The removal effects of the four facilities on different heavy metals are quite different. Facility showed good removal effect on Cd and Pb (above 99% for Cd and 71%–95% for Pb), and general removal effect on Cu and Zn (48%–82% for Cu and 71%–94% for Zn). However, the facilities had not any of the removal effect on Cr and Mn, and a large amount of them will be leached. Figure 6 showed the removal effect of six heavy metals with duration in various facilities.
For the removal of Cu and Zn (Fig. 6a and b), there was no obvious difference between the four facilities. The removal ratio of both of them was always fluctuating, ranged from 0.55 to 0.82 for Cu and 0.65–0.92 for Zn. The effluent concentrations were below 50 μg/L for Cu and 160 μg/L for Zn, meeting the Class II (1000 μg/L for both of them) of GB3838-2002. Generally, S-CWB showed better Cu removal effect, and G-CWB showed better Zn removal effect (P < 0.05, Table 5). The removal of the two metals in facilities mainly because the effluent is alkaline, which causes the metal to be precipitated. Compared with the removal of Cu, Zn showed a larger removal ratio due to the higher influent concentration.
For the removal of Cd and Pb (Fig. 6e and f), the four facilities all showed relatively excellent removal effects. Especially for the removal of Cd, the removal ratio is close to 1.00, with the effluent concentration below 0.5 μg/L. The remove rates for Pb were about 0.90, with the effluent concentration below 10 μg/L. The effluent concentration of both of them met the Class I (1 μg/L for Cd and 10 μg/L for Pb) of GB3838-2002. Generally, G-CWB showed better Cd removal effect, and SG-CWB showed better Pb removal effect (P < 0.05, Table 5).
For the removal of Cr (Fig. 6c), the facilities with CWB show high risk for Cr release. The effluent concentration of G-CWB and SG-CWB can reach 0.25 mg/L at the beginning. The release amount in the facilities with CWB gradually decreases with time, and stabled after 5 h. After stabilization, facility S-CWB was still in the release state, and the removal effect of G-CWB and SG-CWB was not obvious. Due to the low Cr content in runoff, even if the facility leaches, the effluent concentration is still below 50 μg/L, meeting the Class II (50 μg/L) of GB3838-2002. The facility of TC showed certain removal effect on Cr with an average removal ratio of 0.81 and effluent concentration below 10 μg/L, meeting the Class I (10 μg/L) of GB3838-2002. The effluent Cr concentration of the three CWB filled facilities was significantly different from that of the traditional facility (P < 0.05, Table 5).
For the removal of Mn (Fig. 6d), the effects of the four facilities were different. The facility TC and G-CWB released a large amount of Mn. The effluent Mn concentration ranged from 300 to 1200 μg/L of TC, and 800 μg/L to 1200 μg/L of G-CWB. In Table 5, the P value of G-CWB was less than 0.05, that is, there was a significant difference compared with traditional facilities, but there was still Mn leaching in this facility. The facility S-CWB and SG-CWB showed a significant removal effect for Mn (P < 0.05, Table 5), with the removal ratio of 0.98 and 0.98, respectively. The stabilized effluent concentration of the two facilities was below 15 μg/L. The main reason for this phenomenon may be that sand contains a large amount of Mn, which is easily washed away by water. Thus, the facilities with sand layer (TC and G-CWB) released Mn, and facilities without sand layer (S-CWB and SG-CWB) could remove Mn. Therefore, replacing sand with CWB will show a better environmental effect on Mn. In response to this phenomenon, the physical and chemical properties of sand were studied in this research. It was found that the content of total manganese (Mn) in sand could reach 0.072 g/kg. The higher content of Mn in sand can provide theoretical basis for the above inference.
In general, for bioretention facilities filled with CWB, the removal ratio of Cu, Zn and Pb can be stable at 0.70, 0.80 and 0.90, respectively. The removal ratio of Cd and Mn can reach nearly 1. But facilities filled with sand will leach Mn, and facilities filled with CWB will leach Cr. The reasons why CWB can leach Cr have been analyzed in the previous. Therefore, the effluent water from facilities filled with CWB needs to be further treated for Cr to ensure effluent safety. The processing method can be to add filler layer which has removal effect on Cr.
The effect of bioretention facilities on pollutant removal under long-term operation
To investigate the effect of the four facilities on the removal of runoff pollutants under long-term operation, facilities were tested for long-term operation and operated every 4 days. Figure 7 showed the removal effect of typical pollutants in various facilities under long-term operation. As can be seen from Fig. 7a, the late removal ratio of NH4+-N from TC was more than 0.80. The facility with CWB shows better removal effect, with the late removal ratio of more than 0.90. But the difference was not significant compared with traditional facilities. S-CWB and SG-CWB showed better removal ratio of NH4+-N, so fine brick plays an important role in the removal process of NH4+-N. For the removal of COD (Fig. 7 b), the four facilities all showed good removal effect toward COD. The removal ratio of COD from the four facilities was all above 0.60. After the seventh water inflow experiment, the removal ratio tended to be stable, all above 0.80. There was no significant difference in removal effect among different facilities. For the removal of TP (Fig. 7c), it can be found in the figure that the four facilities showed better removal effect on TP, with the removal ratio ranging from 0.90 to 0.99. The removal ratio of the facility SG-CWB is the highest and most stable, and the removal ratio is always above 0.98. TP removal from SG-CWB was significantly different from that of traditional facility (P < 0.05, Table 5). The removal of TN (Fig. 7d) from the four facilities can be divided into two stages. The first stage is the process of the initial operation (the first three inflow experiments), the removal ratio increased rapidly, from 0.08 to 0.98. In the second stage (the last seven inflow experiments), the removal ratio rose slowly in fluctuations, then stabilized above 0.80 after the eighth inflow experiment. The increase of the removal ratio may be due to the continuous growth of microorganisms. As the microorganisms grow, the nitrification and denitrification of nitrogen by the microorganisms increase, thereby reducing the amount of TN in the effluent.
Figure 8 shows the removal effect of the four facilities on the removal of six heavy metals (Cu, Zn, Cr, Mn, Cd and Pb) in long-term operation. There was no obvious difference in the removal of Cu, Zn, Cd and Pb between the four facilities, and the effect is relatively stable. They showed good removal effect on Cd and Pb, and general removal effect on Cu and Zn. Compared with TC, facilities with CWB showed unstable removal effect on Cd, but the removal effect on Mn has been significantly improved. This result is basically consistent with the result of the second experimental stage.
For the removal of Cu (Fig. 8a), the removal ratio ranged from 0.46 to 0.90. There was no significant difference in removal effect among different facilities (P > 0.05, Table 5), but the removal ratio in SG-CWB is slightly more unstable than the other three facilities. The removal of Cu is mainly due to precipitation. The pH of the effluent is above 7.5, Cu will combine with OH− to form a precipitate and be trapped by the filler. For the removal of Zn (Fig. 8b), the removal ratio ranged from 0.74 to 0.97. There was no significant difference in removal effect among different facilities (P > 0.05, Table 5). The removal of Zn is similar to that of Cu and mainly depends on precipitation. The influent concentration of Zn is larger, which leads to a higher removal ratio. In the later stage of the experiment, the removal ratio of the facilities filled with fine bricks decreased, indicating that the removal effect of fine bricks on Zn was worse than that of sand. For the removal of Cd (Fig. 8e), the removal ratio ranged from 0.98 to 0.99 of all of the four facilities, which is relatively high and stable. There was also no significant difference in removal effect among different facilities (P > 0.05, Table 5). For the removal of Pb (Fig. 8f), the removal ratio ranged from 0.80 to 0.96 of all of the four facilities. The removal ratio of the eighth simulation experiment of facility TC and the ninth simulation experiment of facility SG-CWB suddenly decreased, which may be caused by the Pb trapped in the filler being washed out during the water influent process. The removal effect from S-CWB and G-CWB was significantly different from that of traditional facility (P < 0.05, Table 5).
For the removal of Cr (Fig. 8c), different facilities showed different Cr removal effects, and the difference was significant (P < 0.05, Table 5). The removal effect of CWB-based facilities is worse than TC, and facility G-CWB will leach Cr, resulting in a negative removal ratio. The average removal ratio for the four facilities are 0.68 for TC, 0.40 for S-CWB, 0.19 for G-CWB and 0.41 for SG-CWB. Therefore, it is not conducive to fill CWB into bioretention facilities for the removal of Cr in runoff.
For the removal of Mn (Fig. 8d), the removal effect in facilities filled with CWB is significantly improved compared to facility TC (P < 0.05, Table 5). TC leached a large amount of Mn, resulting in a negative removal ratio and unstable removal effect. The removal effect of facility G-CWB is better than that of TC, but it is unstable and sometimes leaching occurs. The facilities S-CWB and SG-CWB showed better removal effect on Mn, with removal ratio ranging from 0.87 to 0.99. This showed that fine bricks have a better removal effect on Mn. The removal effect of the four facilities on runoff pollutants under long-term operation is basically stable and the removal ratio is relatively high, indicating that it is feasible to use CWB as fillers in the bioretention facility.
In general, bioretention facilities filled with CWB have a relatively stable removal effect on pollutants under long-term operation. The removal of TN also tends to be stable after running twice. For Cr, no leaching occurs after two runs. This indicates that Cr in CWB has leaching risk in the early stage of facility construction, but no leaching after repeated operation. Therefore, it can be inferred that if the CWB is pre-treated, such as rinsing, the risk of contamination can be greatly reduced.
Environmental implications
At present, CWB in the process of treatment will cause dust pollution, in the process of stacking will produce secondary pollution. This makes them difficult to recycle. Their use is limited to road materials and cement products. CWB have rich pore structure, low price, rich surface mineral elements and adjustable particle size. Applying them to the field of environmental pollution control, it can solve many problems in the application of natural aggregates, such as sand and gravel, which are difficult to solve (For example, few adsorption sites, poor synergistic pollution control efficiency of a variety of pollutants, high price, and strong geographical restrictions on supply, etc.). Although CWB have potential applications in the field of water treatment, most of the studies are only laboratory batch experiments. This study gives full play to the characteristics of CWB and develops key technologies for the preparation and application of environmental pollution remediation materials based on CWB, and provides scientific measures for technical support for benign resource utilization of CWB. Aiming at the prominent environmental problems in the rapid development of urbanization, the application technology of CWB-based bioretention facilities is systematically researched and developed.
As solid wastes, using CWB as the basement filler of facilities will reduce costs. In terms of transportation costs, CWB are usually produced in cities, while traditional gravel fillers are usually produced in mountainous areas. Therefore, in the construction of urban infrastructure, the transportation cost of CWB is much lower than gravel and sand. For the filler itself, the price of CWB (about 4.5 dollars/ton) is much lower than gravel (about 48.8 dollars/ton) and sand (about 13.7 dollars/ton). Therefore, if the gravel and sand in the traditional bioretention facility are both replaced with CWB, the cost of fillers will be reduced by about 67.2%–88.4%. Although, CWB-based facilities in most cases show similar removal efficiency toward pollutants compared with traditional bioretention facility. On the basis of ensuring the effluent quality of the facility and economic reasons, CWB can be used as a kind of cheap bioretention facility filler to replace the traditional filler. On the other hand, it is worth noting that the leaching phenomenon should be concerned before CWB application. Therefore, the measures should be further investigated to reduce the leaching risk.
Conclusion
In this study, CWB were used as fillers in bioretention facility to investigate the removal effect of runoff pollutants with different combinations of soil, sand and gravel. The results show that it is feasible to use CWB as fillers in bioretention facilities. The conclusions could be drawn as follows:
-
(i)
Both CWB-based and traditional bioretention facilities will leach a large amount of COD and TN, and the leaching amount decreased with duration, but they will not leach TP. CWB-based facilities leach less NH4+-N compared with traditional facility. The leaching amount of heavy metals is limited except for Cr from CWB-based facilities.
-
(ii)
Both CWB-based and traditional bioretention facilities show good removal effect on TP, NH4+-N and COD, the removal ratio can reach 0.90, 0.80 and 0.70 respectively. TN will be leached from the facilities at the beginning of rainfall, and the removal ratio after stabilization in the later stage is only about 0.10. Therefore, the removal effect of TN needs to be improved.
-
(iii)
Both CWB-based and traditional bioretention facilities show good removal effects on Cu, Zn, Cd and Pb, with the removal ratio from 0.55–0.82, 0.65–0.92, nearly 1.00 and above 0.90, respectively. CWB-based facilities will leach a large amount of Cr in the initial stage, while the removal ratio of Cr by traditional facility can reach 0.80. The removal ratio of Mn by facilities without sand can reach about 0.98, while facilities with sand will leach a large amount of Mn.
-
(iv)
The removal effect of both CWB-based and traditional bioretention facilities on runoff pollutants under long-term operation is basically stable and the removal ratio is relatively high. The removal ratio can reach 0.98 for Cd, 0.95 for TP, 0.90 for NH4+-N, COD and TN, 0.80 for Zn and Pb, and 0.60 for Cu. Compared with traditional facility, CWB-based facilities are more conducive to the removal of Mn, but not conducive to the removal of Cr.
-
(v)
CWB-based bioretention facilities can not only reduce the cost of fillers by 67.2%–88.4%, but also save a lot of transportation costs.
References
Han YL, Liu ZZ (2015) Research on characteristics of the receiving water body polluted by rainwater runoff. Res Soil Water Conserv 22:65–71
You ZY, Zhang L, Pan SY, Chiang PC, Pei SL, Zhang SJ (2019) Performance evaluation of modified bioretention systems with alkaline solid wastes for enhanced nutrient removal from stormwater runoff. Water Res 161:61–73. https://doi.org/10.1016/j.watres.2019.05.105
Zuo XJ, Guo ZY, Wu X, Yu JH (2019) Diversity and metabolism effects of microorganisms in bioretention systems with sand, soil and fly ash. Sci Total Environ 676:447–454. https://doi.org/10.1016/j.scitotenv.2019.04.340
Chen XL, Peltier E, Sturm BSM, Young CB (2013) Nitrogen removal and nitrifying and denitrifying bacteria quantification in a stormwater bioretention system. Water Res 47:1691–1700. https://doi.org/10.1016/j.watres.2012.12.033
Li ZD, Wu JY, Yue XJ, Qiu FX, Yang DY, Zhang T (2020) Study on the application of waste bricks in emulsified oil-water separation. J Clean Prod 251:119609. https://doi.org/10.1016/j.jclepro.2019.119609
Gu DG, Zhu XZ, Vongsay T, Huang MS, He Y (2013) Phosphorus and nitrogen removal using novel porous bricks incorporated with wastes and minerals. Pol J Environ Stud 22:1349–1356
Hussain S, Gul S, Khan S, Rehman HU, Ishaq M, Khan A, Jan FA, Din ZU (2015) Removal of Cr(VI) from aqueous solution using brick kiln chimney waste as adsorbent. Desalin Water Treat 53:373–381
Arsenovic M, Radojevic Z, Stankovic S (2012) Removal of toxic metals from industrial sludge by fixing in brick structure. Constr Build Mater 37:7–14. https://doi.org/10.1016/j.conbuildmat.2012.07.002
Yang LQ, Wang JL, Zhang XR, Che W (2014) Adsorption characteristics of Pb from urban stormwater runoff by construction wastes. J Southeast Univ Engl Edit 30:212–219
Yuan X, Xue N, Han Z (2021) A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J Environ Sci 101:217–226
Gong JR, Zhu CC, Yang LL, Yang B, Wang B, Baoyin TT, Liu M, Zhang ZH, Shi JY (2020) Effects of nitrogen addition on above-and belowground litter decomposition and nutrient dynamics in the litter-soil continuum in the temperate steppe of Inner Mongoli, China. J Arid Environ 5:5. https://doi.org/10.1016/j.jaridenv.2019.104036
Wang JJ, Li T, Zhang Y (2014) Water treatment residual as a bioretention media amendment for phosphorus removal. Environ Sci 35:4642–4647
Singh RP, Jiang YH, Wen XP (2010) Phosphorus removal efficiency of different media and macrophyte in constructed wetland. Adv Mater Res 113–116:2368–2372
Boujelben N, Bouhamed F, Elouear Z, Bouzid J, Feki M (2014) Removal of phosphorus ions from aqueous solutions using manganese-oxide-coated sand and brick. Desalin Water Treat 52:2282–2292
Jia CR, Dai YR, Chang JJ, Wu CY, Wu ZB, Liang W (2013) Adsorption characteristics of used brick for phosphorus removal from phosphate solution. Desalin Water Treat 51:5886–5891
Zhou D, Bi CJ, Chen ZL, Deng HG (2013) Laboratory study of the removal efficiencies for ammonium and total phosphorus from runoff using layered filtration systems. Adv Mater Res 726–731:2198–2205
Acknowledgements
This research was supported by Construction of High Level Teaching Teams in Universities of Beijing–the Youth Top–Notch Talent Cultivation Program (CIT&TCD201804051); National Natural Science Foundation of the China (51508017); The Youth Beijing Scholars program (NO.024); The Fundamental Research Funds of Beijing Vocational College of Agriculture (XY-YF-17-13).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, X., Tian, Y., Guo, S. et al. Removal of runoff pollutants by construction waste bricks-based bioretention facilities. J Mater Cycles Waste Manag 24, 1444–1459 (2022). https://doi.org/10.1007/s10163-022-01394-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01394-w