Abstract
Cyanide is a known toxic chemical compound that has an adverse effect on living organisms. Nonetheless, it is one of the active reagents in industries such as mining, pharmaceutical, cosmetics, and food processing companies worldwide. The beneficiation of gold and other precious metals from ore generates great amount of cyanide-bearing contaminants, which is released into the environment. The abundance of cyanide contaminants from these industries have created public health concern since the inception of metal extraction from ore. There are strict regulations on the production, transportation, utilization, and disposal of cyanide-bearing contaminants worldwide. The conventional treatment of cyanide waste is either chemical or physical process. The use of these treatment processes has certain pitfalls like operational challenges, an increase in capital cost, and generation of secondary waste. A number of microorganisms have the potential to utilize cyanide as nitrogen and carbon source and transform it into ammonia and carbon dioxide. Biodetoxification might be efficiently, economically and environmentally safe to detoxify cyanide in contaminants and attractive alternative to conventional detoxification method like chemical or physical. This paper reviews the principles and methods of biodetoxification of cyanide contaminants found in the ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing demand for precious metals in the world in recent times has brought about the advancement in technology to explore the natural habitation in search of minerals such as gold, copper, zinc, iron, and other valuable metals within the low-grade ores. Mining exploration and other industrial activities results in the production of organic and inorganic waste that affects the environment, and some of these wastes contain active chemicals used in mineral beneficiation [1]. Cyanide-bearing tailings are often abundance in these mining areas, covering several hectares of arable land, contaminating underground and surface water bodies. Tailings are considered as byproducts after needed minerals are extracted from the ore through crushing, milling, and application of varying beneficiation techniques [2]. Surprisingly, mining activities are responsible for the generation of an estimated 18 billion of cyanide-containing waste annually in the ecosystem [3]. There are global environmental challenges such as land and water bodies pollution with regards to tailings disposal through seepage from the impoundment [4]. Over millions of hectares of farmlands have been rendered useless in major mining regions such as Australia, South Africa, the US, New Zealand, and Asia-Pacific [5]. Tailings can equally be useful in other industrial settings such as the construction of roads, houses and in the form of land reclamation like backfilling. The coarse and fine tailings are potential materials for the manufacturing of cement and concrete [2]. Nonetheless, cyanide pollutant content of the tailings must be reduced to an acceptable limit, so it does not pose a health threat to the construction workers. Major lessons have been learned from mining accidents, such as dam failures and cyanide spillage that has occurred in recent times [6]. As a result, mining operation regulatory bodies around the world have set 0.2 mg/L of cyanide concentration as a limit in the tailings before disposal [4]. The conventional practice of disposing of mine tailings includes excavating and burying it in a secure landfill or impoundment dam, physical treatment (dilution, membrane, electrowinning, and hydrolysis), chemical treatment (alkaline chlorination, sulfur dioxide, hydrogen peroxide oxidation, acidification, and iron sulfide process) and biological processes. However, alkaline chlorination is the most commonly used by the mining companies worldwide since the commercialization of precious metals mining in 1889 [7]. Alkaline chlorination can be accomplished through the reaction of cyanide with chlorine gas to produce cyanogen chloride. Cyanogen chloride then hydrolyzes to release cyanate. Subsequently, excess chlorine gas is added to convert the cyanate to ammonia (see the reaction in Eq. 1 below).
There is complete detoxification of cyanide compounds within the shortest possible time in this process. However, the demand for high chemical reagents to keep the pH in alkaline medium to prevent volatilization of cyanide, the formation of chlorine and hypochlorite anions of which both are toxic compound makes the process uneconomical and environmentally unsustainable. In addition, alkaline chlorination is unable to degrade strong acid dissociations (SADs), e.g., nickel and silver [3, 8]. Over decades, much research has been conducted to find an alternative to this process, which is eco-friendly and economically viable. The biological process, which uses natural products to oxidize cyanide-bearing waste to nontoxic components, seems to gain more attention in this regard. In spite of the cyanide being deadly poisonous to almost every living creature, some microbes can tolerate its present by developing certain adaptable features. There are two main mechanisms through which bacteria resist cyanide poisoning include; (1) production of organic compounds for iron uptake, (2) the release and utilize different enzymes for varieties of biochemical reactions to oxidize cyanide contaminants to nontoxic compounds [9, 10]. Microorganisms are capable of producing organic compound known as siderophores when exposed to cyanide-bearing contaminants [11]. Siderophores have great affinity with metal-cyanide complexes, which bind the iron component and transported across the cell membrane for metabolism to occur [12]. The successful installation of biological treatment plant in Homestake Mine in Lead, South Dakota, the USA, to degrade cyanide-bearing wastewater in the early 1980s, has led to several successful studies using microorganisms to detoxify cyanide in various waste matrix [13,14,15,16,17,18,19,20]. Biodetoxification of cyanide bearing contaminants provides a strong alternative to other known methods in terms of efficiency, economically, and environmental sustainability [21,22,23]. Although it is established that microbes have the potential to remove free cyanide and other organic compounds from industrial contaminants [24], very few information is available for its efficiency to detoxify free cyanide and other cyanide complexes from contaminants. The present report elucidates the efficiency of microbes to detoxify free cyanide and other cyanide complexes in contaminants. The discussion is focused on the properties of cyanide and sources of cyanide contaminants, principle of cyanide biodetoxification, and methods of cyanide biodetoxification.
Properties of cyanide and sources of cyanide contaminants
Physiochemical properties of cyanide
Cyanide is a chemical compound composed of the carbon atom, which is bonded to nitrogen atom via triple bond (–C≡N) Jaszczak et al. [25]. Cyanide occurs as organic and inorganic compounds in a state such as solid, gaseous, and aqueous species Dzombak et al. [26]. Cyanide ions exist as hydrogen cyanide (HCN) at pKa of > 9.2 Anning et al. [27]. HCN is generated into the environment during the combustion of nitrogen compounds like protein and nitrates at a temperature of 700 °C in the absence of oxygen [28]. In aqueous solution, HCN is pale blue or colorless at room temperature, a vapour pressure (bp = 27.5 °C), and a molecular weight of 27.03 g/mol [29]. Cyanide is soluble in water at 25 °C and completely miscible in varieties of organic solvents such as alcohol. The boiling point, vapour pressure density, vapour, and liquid are estimated at 25.7 °C, 700 mmHg, 0.99 at 20 °C, and 0.68 g/mL at 25 °C, respectively [30]. At the gaseous state, cyanide is colourless, almond-like odor, and a volatility value of 1.1 × 106 mg/m3 [30]. HCN is capable of reacting with silver or gold nanoparticles at 300–650 °C in the presence of oxygen to release cyanic acid (HOCN) and cyanogen (CN)2 [31]. Furthermore, HCN reacts with oxygen to form nitrogen (N), carbon monoxide (CO), and water (H2O) with an approximate value of 723.2 kJ/mol thermal energy generation at 2780 °C [31] (see the reaction in Eq. 2).
The chemistry of cyanide makes it possible for microbial detoxification processes to occur. Most microorganisms are capable of utilizing carbon and nitrogen component of cyanide compounds by oxidizing them into ammonia and carbon dioxide. The byproduct generated is utilized for metabolic activities of the cells [18]. Cyanide has a high affinity with chelated iron [32]. Microorganisms like P. pseudoalcaligenes and Pseudomonas aeruginosa can grow in the cyanide-bearing medium as a nitrogen source by producing siderophores, which binds with chelated iron and assimilated into the cell structure [33].
Ecotoxicology of cyanide compounds
Cyanide is regarded as the most harmful chemical, especially the free cyanide species (HCN). Even though many microorganisms are insensitive to cyanide toxicity, they are unable to survive at an elevated concentration. Hydrogen cyanide gas was used as a chemical weapon during the past world wars. For instance, the Nazis used hydrogen cyanide gas to murder millions of people during World War II [34]. Cyanide-containing waste accumulated in the environment is detrimental to the ecosystem and human health. Cyanide can actively bind to iron ion within the biological system, thereby inactivating the metalloenzymes [35]. Presence of cyanide inhibits the cytochrome c oxidase interfering with the aerobic respiratory processes [36]. The organisms that respire through anaerobic means can also be affected by the presence of a cyanide compounds. The metalloproteinase protein found in anaerobic organisms is often inhibited when they are exposed to cyanide, making the organism sensitive to cyanide poisoning [8]. Many plants produce hydrogen cyanide through the process called cyanogenesis. There are over 2000 plant species that produce this toxic compound when there is a physical or chemical injury to their cells or when there is a fungal attack [37]. Plants like Hevea benthamiana, H. brasiliensis, Lotus corniculatus, Alliaria petiolata, Arabidopsis thaliana, Brassica kaber, B.rapa, B. napus, and Sorghum sudanense are some of the known plant species that produce some levels of hydrogen cyanide in the course of fungi infestation. However, 0.025 mg/L of HCN (hydrogen cyanide) concentration is most likely to interfere with the carbon dioxide absorption by the photosynthetic tissue and inhibit other syntheses of the plant [38, 39]. Human beings are exposed to cyanide through the lungs, gastrointestinal tract, dermal tissue, mucous membrane, and eye. Cyanide presence in the body hurt the heart, lungs, central nervous, and the endocrine system. There are endogenous mechanisms in the body that help to regulate a certain amount of hydrogen cyanide in the body. The metabolic activity of the liver by the enzyme rhodanese helps to detoxify cyanide concentration to the nontoxic component, which is excreted through urine [10]. Notwithstanding, acute exposure of cyanide salt at 200–300 mg through ingestion is very lethal and individual involved may die at the shortest possible time [40]. Long-term exposure, through the eating of cyanogenic foodstuffs like vegetables and cassava, results in diseases such as demyelination of peripheral nerves, optic neuropathy and deafness. These diseases are mostly noted among some African countries like Nigeria and Tanzania that depend heavily on cassava and other cyanogenic plants as staple foods [41]. Safeguarding the ecosystem against cyanide pollutants is very vital for humans and other life. Moreover, since industrialization is very significant in the socio-economic development of every country, there is a need to detoxify the cyanide-bearing contaminants by adapting to an environmentally acceptable method. Cyanide harms the fish when it is exposed to the marine environment. The exposure of 1–5 mg/L of NaCN (sodium cyanide) concentration within 2–3 min has the potential to damage the internal organs such as the liver, stomach, spleen, kidney, and the brain. The damage to the mucosal cells by NaCN prevents digestion and assimilation of food, which may result in sudden death [42]. Prolong exposure of hydrocyanic acid between 0.005–0.01 mg/L affects the fish eggs, growth retardation, mobility impairment, and an abnormal increase in metabolic and respiratory rate [43]. Acute toxicity that occurs between 0.1–0.3 mg/L of NaCN concentration and the fish is killed within 96 h of exposure [42]. Beside there are several reports on cyanide toxicity on avian. For an instance, black vulture died within 11 min after administering a lethal dose of 4.8 mg/L of hydrocyanic acid [44]. Davis [45] realized that a dose of 1.5 mg/L of potassium cyanide (KCN) was lethal to chicken after administering it to them through intravenous. The avian population has reduced in recent times due to some pesticides used in agricultural production. Pesticides like calcium cyanide have a direct effect on the reproductive and immune system of the birds [46]. Recently, Kadiri and Asagba [47] reported a direct effect of NaCN concentration on the kidney, liver, and a brain of the domestic chicken (Gallus domesticus L), when they were fed on feed contaminated with 3 mg/L of NaCN for four weeks. Histopathological study on the chicken revealed that there was minimal neuronal congestion in the brain; the glomerular was loosely packed in the Bowman’s capsule and mild inflammation in the kidney. In addition, there was central vein congestion, necrosis, and bile proliferation in the liver. Cyanide compounds are widely dangerous toxicants found in the ecosystem due to its poisonous nature to the living organisms.
Source of cyanide contaminants
Cyanide compounds can be detected in various environmental media such as soils, air, and water [26]. Cyanide is considered one of the polyatomic chemical compounds detected in the interspace medium [48]. Carbon and nitrogen components of HCN undergo polymerization reaction to transform into protein, which forms the basis of all living things [49]. Most microorganisms produce a certain amount of HCN as secondary metabolism for the development of α-amino derivatives like protein and lipids [33]. There are over 2000 cyanogenic plants in nature [25], plants such as Phaseolus lunatus, Manihot esculantus, Malus pumila, Prunus persica, Prunus armeniaca, and Almond produce HCNfor a defensive mechanism such as fungi and herbivore attack [50, 51]. However, the major sources of cyanide contaminants in the environment are through anthropogenic activities. The global production of cyanide compounds was estimated at an annual increase of 2%, with projected production of 1.3 million metric tons by 2022 [52]. Cyanide plays an active role in industrial activities like mining, electroplating, paint production, finishing processes, chemical production, and petroleum refining. These industries are responsible for the release of a high percentage of cyanide-bearing wastes in the environment [26]. Several accidents related to spillage of cyanide contaminants into the environment have been reported [53,54,55]. In Argentina, valve failure at Veladero mine in San Juan province led to the release of 1,072 cubic metersof cyanide-bearing solutions into the Potrerillos River, polluting the aquatic environment [54]. In Romania, the failure of a tailings pond at Baia Mare discharged at least 100,000 cubic meters of cyanide-bearing contaminants into the environment in February 2000. The contaminants made up of free cyanide and metal-cyanide complexes traveled to approximately 1200 km, polluting nearby Rivers like Sasar River, Lapus River, and Somes River in Hungary. An estimated 1,240 tons of fish were killed in the Tisza River [56]. Besides, in Guyana, an estimated 2.3 million cubic meters of cyanide-bearing wastewater was discharged into Essequibo River from collapsed walls of an earthen tailings pond in August 1995, which polluted the drinking water of neighboring communities [57]. Meanwhile, in Ghana, the distraction of pipeline carrying cyanide-containing solution by a rainstorm resulted in the spillage of cyanide-bearing wastewater into the nearby Sumang stream killing 50 fish within a 200 m, stretch of the stream. Diseases like diarrhea, abdominal pains, blurred vision, eye itchiness, skin-related infections, bloody urine, and burning sensation in the legs, were reported among the communities who eat the dead fish and used the polluted water for household activities [58]. In recent times, human efforts have contributed immensely to the rate of cyanide-related contaminants in the environment.
Principles of cyanide biodetoxification
Cyanide biodetoxification reactions
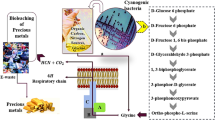
Many microorganisms have different routes of converting cyanide into nontoxic form for various environmental conditions [59, 60]. There are various biochemical reactions such as hydrolytic, oxidative, reductive, and substitution, that occur during cyanide detoxification process (Table 1). The enzymes use these media to convert cyanide to ammonia, formate, methane, carbon (IV) oxides (CO2), formic acid, and carboxylic acid [60, 61]. Discussed below are some of the biochemical reactions used by various microorganisms to degrade cyanide contaminants.
Hydrolytic reaction
Microorganisms use the hydrolytic reaction to reduce various toxic chemical components from environment. The hydrolytic reaction is a mostly used route by many bacteria and fungi to convert cyanide directly into ammonia and carboxylic acids [62]. The reaction involves hydrolysis of cyanide with the presence of enzyme to release ammonia and carbon dioxide [59]. Gupta et al. [62] reported five enzymes responsible for catalysis of cyanide into nontoxic compounds; hydratase, nitrile hydratase, thiocyanate hydrolase, and nitrilase. The enzyme hydratase is mainly from fungi origin, they mostly induced this enzyme to detoxification of cyanide compounds found in contaminants [22, 63]. Cyanide hydratase activity is mostly induced when the fungi is exposed to a low concentration of cyanide in its environment [64]. The fungi utilize this enzyme to degrade HCN release from cyanogenic plants during their attack on the plants [65]. The cyanide hydratase directly undergoes hydrolysis and disrupt the triple bond of the cyanide compound to release formamide, which is nontoxic compound [47, 66]. Nazly and Knowles [67] initially studied the efficiency of cyanide hydratase to detoxify cyanide; the cyanide hydratase of Stemphylium loti was induced by means of 32.5 mg/L of KCN. The kinetic study revealed that cyanide detoxification activity of hydratase had maximum pH range of 7.0 to 9.0 and Km of 27 mg/L and maximum Vmax of approximately 600 µmoles h−(mg protein)−. The mycelia were incubated for 16 h at 22–24 °C that brought about the total loss of the activity. Storage of 4 °C led to a 50% loss in the activity after 4 days of incubation. It was observed that cyanide hydratase was able to detoxify up to 100 mg/L of cyanide in 2 h. Cyanide hydratase has been induced from the pathogenic plant fungi such as Fusarium solani IHEM 8026, F. oxysporum, Gloeocercospora sorghi, and F. lateritium in the previous studies [68, 69]. Nitrile hydratase is induced by many bacteria and fungi and has wide industrial applications in degrading toxic chemicals found in industrial wastes. The enzyme comes in association with other enzyme like amidase, which utilizes cyanide as a nitrogen source. These microorganisms are able to tolerate cyanide by catalyzing it to amide using nitrile and, subsequently, to acid and ammonia by enzyme amidase [62].
Oxidative reaction
Oxidation reaction involves the reaction of cyanide with oxygen to degrade cyanide to ammonia and carbon dioxide. Cyanide detoxification involving this reaction requires NADPH (nicotinamide adenine dinucleotide phosphate) to disrupt the carbon–nitrogen triple bond [13]. Oxidation reaction involves three different enzymes; cyanide monooxygenase, cyanase, and cyanide dioxygenase [59]. The extra carbon source is needed with cyanide to enhance the metabolic activity of the organism using this route [47]. The microbes convert cyanide to cyanate using monoxygenase. Enzyme cyanase then catalyzes bicarbonate-dependent conversion of cyanate to ammonia and carbon dioxide [70]. Cyanase plays an important role in several bacteria, fungi, plants, and animals’ species by protecting them against cyanate poisoning. Another function of cyanase is the facilitation of ammonia assimilation and transport of carbon dioxide in plants after cyanate has been degraded [71]. Enzyme dioxygenase is utilized to convert cyanide to ammonia and carbon dioxide in the second oxidation reaction. Various microbial species use the oxidative pathway to convert cyanide to ammonia and carbon dioxide. Species such as Pseudomonas fluorscences, Bacillus pumillus, and Escherichia coli use oxidation reaction to utilize cyanide as nitrogen source for cell growth [22]. Figueria et al. [15] reported the activity of dioxygenase in Escherichia coli during the direct metabolism of cyanide to ammonia without the generation of cyanate.
Reductive reaction
Biodetoxification of cyanide via reductive reaction results in electron transfer. Enzyme nitrogenase involves the reductive reaction by utilizing cyanide as a nitrogen source to produce methane and ammonia [22]. The use of the reductive reaction is not common among the cyanide-degrading microbes. Klebsiella oxytoca has been shown to utilize cyanide as a sole nitrogen source when it was grown in cyanide contaminants. Klebsiella oxytoca converts cyanide to ammonia and methane via the activity of nitrogenase, which may have proceeded utilizing the reductive reactions [72]. The nitrogenase catalyzes the reduction of dinitrogen, N2, through the interaction with magnesium ATP (Adenosine Triphosphate) to generate two moles of ammonia. The enzyme nitrogenase is induced by nitrogen-fixing bacteria to utilize cyanide ion (CN−) as the substrate to release ammonia into the soil [65]. Stam et al. [65] reported a reduction in initial CN− concentration of 343 mg/L by Rhizobium ORS 571 in nitrogen-fixing culture. Although the respiratory chain of the cells was inhibited due to the presence of 343 mg/L of cyanide, an increase in growth yield was observed in the bacteria after the agitation time. The researchers attributed the detoxification of the initial cyanide concentration to metabolic activities of enzyme nitrogenase, which occurred via reductive reaction.
Substitution reaction
Microorganisms such as Escherichia coli, Acremonium strictum, Klebsiella pneumonia, and Rhodospirillum palustria are some of the known species that use this reaction for degradation of cyanide to less toxic compounds [73]. The known enzymes involved in the substitution reaction include; 3-mercaptopyruvate sulfurtransferases, pyridoxal phosphate, and rhodanese. Cyanogenic microorganism utilizes pyridoxal phosphate enzyme to convert cyanide to nitrile derivatives of α-amino acids via the substitution route [29]. Enzyme rhodanese plays an active role by preventing the activation of cytochrome c oxidase when the mammalian body is exposed to cyanide. Bacteria like Chromobacterium violaceum are capable of inducing rhodanase after cyanide exposure [6]. Atkinson [74] reported cyanide detoxification of Bacillus stearothermophilus utilizing enzyme rhodanase. The nucleophilic and thiophilic property of cyanide enables it to undergo substitution reaction using 3-mercaptopyruvate sulfurtransferase enzyme to produce thiocyanate. The thiocyanate is then detoxified via carbonyl or cyanate reaction [29]. Biodetoxification of cyanide via carbonyl route utilizes an enzyme hydrolase to release carbonyl sulfide (COS), while the cyanate reaction involves the use of enzyme cyanase to produce sulfate and carbon dioxide; however, ammonia is produced as a byproduct in both reaction [29]. Kelly and Baker [75] in their study identified carbonyl sulfide (COS) or cyanate routes as synonymous with the hydrolytic reaction. They indicated two stages that occur during the carbonyl sulfide; the thiocyanate hydrolase enzyme hydrolyzes the triple bond, which directly cleavage to generate ammonia and carbonyl sulfide. The second stage is the hydrolysis of carbonyl sulfide to carbon, and sulfide; subsequently, the sulfide is oxidized to sulfate. In contrast, the recent investigation by Berben et al. [76] has revealed that the thiocyanate detoxification reaction is an oxidation reaction, which is catalyzed by the thiocyanate dehydrogenase.
Factors that influence biodetoxification of cyanide
Microbial detoxification efficiency of cyanide contaminants is mostly influenced by certain inherent factors. The physiological and metabolic potency of the microbes is a significant factor to determine the rate of biodetoxification of cyanide in the contaminants [3]. The high expenses incurred in designing and testing of the process is one of the economic challenges that need to be considered when utilizing microbes for detoxification of cyanide contaminants [59]. Many other abiotic factors such as temperature, initial cyanide concentration, pH, oxygen level, agitation time, and nutrient availability have an impact on the microbial metabolism of cyanide-containing waste [60] (Table 2). The above-stated parameters have a direct effect on the percentage of cyanide concentration that can be biodetoxified in the industrial contaminants [77]. A few of these factors are discussed below.
Effects of temperature
The coldness or the hotness of the medium has an impact on the microbial activity. Many microorganisms have capabilities to thrive in hot environmental conditions while others are not. Microbial detoxification of cyanide contaminants is dependent on the growth of the microbes present in the medium. The growth of microbes is enhanced when there is a balance between cyanide complexes and the temperature of the medium [78]. The rate of cyanide biodetoxification increases in certain microbes at a lower temperature, while in other microbes, it increases at elevated temperature [79]. Khamar et al. [80] reported 25 °C as optimum temperature for detoxification of cyanide in gold mine tailings by genus Halomonas. Meanwhile, Adjei and Ohta [81] found 30 °C as a desirable temperature for utilization of cyanide as a nitrogen source by Burkholderia cepacia strain C-3 in minimal liquid media. The optimum temperature for biodetoxification of cyanide is reported at the range of ~ 4 °C to > 30 °C, however, Mirizadeh et al. [82] showed tolerance of 34.2 °C in 500 mg/L of a cyanide-bearing medium by strain C3 isolated from a wastewater treatment plant from coke–oven–gas condensate. In a related development, Dwivedi et al. [83] reported an optimum temperature of 35 °C by Bacillus cereus in a batch reactor. The growth of P. pudia was inhibited when the temperature of the medium exceeded 40 °C [78].
Effects of initial cyanide concentration
Cyanide concentration in medium plays an important role in the biodetoxification of cyanide in the industrial waste since many microbes are poisoned at a high level of initial cyanide concentration. The growth of Rhodococcus UKMP-5 M in a cyanide-bearing medium was reported between 60–80 mg/L [84]. Kunz et al. [85] reported excess cyanide concentration of 1627.9 mg/L as an inhibitory factor to the growth and degradation efficiency of Pseudomonas fluorescens NCIMB 11,764. Cyanide detoxification efficiency at a certain concentrations is related to the inherent characters of microbial species. For example, Cabuk et al. [9] showed degradation of 130 mg/L KCN by Trametes versicolor at 30 °C, pH 10.5 over 42 h. Similarly, Pseudomonas pseudoalcaligenes was able to detoxify 810 mg/L of HCN and other cyano-metal complexes under alkali conditions [33]. Recently, Moradkhani et al. [86] reported 93.5% detoxification efficiency by Pseudomonas parafulva at an initial cyanide concentration of 200 mg/L. They pointed out that an increased in cyanide concentration beyond 500 mg/L had a negative influence on the detoxification efficiency of the bacteria. Karamba et al. [87] reported the effect of cyanide concentration on bacteria growth and degradation efficiency of Serratia marcescens strain AQ07 isolated from the soil. They observed that cyanide concentration of 200 mg/L resulted in lower growth of 16.1 log cfu/mL and 85% detoxification. Meanwhile, at a lower concentration of 50 mg/L, there was an increase in bacteria growth of 16.39 log cfu/mL and detoxification rate of 89.6%. They asserted that the reduction in bacteria growth rate and its associated detoxification could be cyanide poisoning, which occurred when there was an increased concentration.
Effects of pH on biodetoxification
The pH of the medium is an important parameter to consider for biodetoxification of cyanide in contaminants. The pH influences the growth of microbes and biodetoxification of cyanide concentration in the cyanide-bearing waste [7]. Dash and Balomajumder [22] reported an optimum pH range of microbial detoxification of cyanide contaminants being between 6 and 9. Nonetheless, there are several reported biodetoxification investigations with a pH lower than 6 and higher than 9 [17, 19]. The pH of 6.3 was noted as the optimum pH to detoxify 6.5 mg/L KCN by Rhodococcus UKMP-5 M within 24 h [19]. Luque-Almagro et al. [33] reported that, Pseudomonas pseudoalcaligenes CECT5422 could tolerate an initial pH of 11.5 and capable of assimilating 1470 mg/L of NaCN in a minimal mineral medium. The bacterial strain Bacillus sp. CN-22 isolated from a cyanide contaminated electroplating sludge was capable of tolerating a pH of 10.3 and detoxified 96.69% of 700 mg/L of cyanide at 30 °C and 193 rpm [88]. Biodetoxification of cyanide contaminants can occur under a wide range of pH [7]. Barclay et al. [89] reported metal-cyanide biodetoxification of mixed and pure cultures of fungi at a pH range of 4 and 7 when they isolated Fusarium oxysporum, F. solani, Trichoderma polysporum, Scytalidium thermophilum, and Panicillium miczynski from acidic gas works soil. Many bacteria such as P. putida, Pseudomonas paucimobilis and P. fluorescens that are capable of detoxifying metal-cyanide complexes at a neutral pH have been studied [90]. Pseudomonas fluorescens utilized ferrocyanide as a sole nitrogen source in a batch reactor fermenter, which degraded 79% of cyanide in the contaminants at the optimum pH of 5 [77]. The pH of the contaminated materials has direct effect on the metabolic activities of the indigenous microorganisms, which influence their ability to utilizes cyanide as sole nitrogen source for their growth.
Effects of nutrient availability
The physical modification of the contaminated environment promotes the optimization of biodetoxification processes [91]. Nutrient availability in the cyanide contaminants is very significant since it actives immobilized cells to increase the efficiency of the biodetoxification and environmental control. Many bacteria species with potential to detoxify cyanide in industrial contaminants are heterotrophic and demand nutrients like carbon and nitrogen source for cell growth [92]. Notwithstanding, Dash et al. [83] noted that having carbon present in industrial polluted waste is a challenge to the rate of biodetoxification. Microorganism utilizes the cyanide component present in waste matrix as a nitrogen source and converts it to ammonia, hence, its reduction in the contaminated media [93]. Perhaps, it is necessary to supplement the medium with readily metabolized carbon sources like glucose, acetate, fructose, mannose, galactose, and agricultural extracts to promote cell viability and biodetoxification [92]. On the contrary, excessive supply of nutrients may hinder the metabolic activity of the microbes [94]. Some microorganisms such as P. fluorescense P70 and Bhurkholderia cepacia strain C3 are unable to grow in the cyanide contaminated medium without supplementary carbon source [81]. Mirizadeh et al. [82] reported glucose and fructose as a readily supported carbon source that facilitated the biodetoxification of free cyanide concentration up to 500 mg/L by strain C2. One of the parameters of their investigation was to identify the effectiveness of these carbon sources; sucrose, sodium acetate, fructose, and glucose. They had biodetoxification efficiency of 57%, 72%, 82%, and 85% respectively. Research conducted by Hope and Knowles [95] contradict the above assertion. They pointed out that the biodetoxification of cyanide in contaminants in the presence of reducing sugar was not due to metabolic activities of the microbes but the reaction between cyanide and sugar, which generate ammonia. They cited the growth of Klebsiella planticola in their investigation as classical example. They stressed that, the growth of the bacteria was due to the consumption of ammonia generated from the reaction process, but not from the product of cyanide metabolism in the contaminants. Barany [96] indicated that, carbonyl group (ketone and aldehyde) react with cyanide in presence of metal iron to generate a stable cyanide species under natural conditions (40 °C and neutral pH) which is inaccessible for microbial biodetoxification. Luque-Almagro et al. [33] suggested the use of other carbon sources instead of glucose since it has the potential to react with cyanide (the Kiliani reaction), which can influence the biodetoxification capabilities of the microbes. They proposed the use of acetate and D, L-malate as a suitable carbon source for microbial utilization of cyanide. This was confirmed by Khamar et al. [80] who reported on co-culture strains (BN1 and DNB) ability to utilize acetate as a sole carbon source to detoxify 75% of initial 50 mg/L of cyanide concentration after 96 h-cultivation. The above discussion indicated that, the kind of nutrients available for cell viability has a greater influence on the efficiency of microbial biodetoxification of cyanide contaminants.
Limitation of biodetoxification of cyanide contaminants
Although biodetoxification can be applied in diverse ways to treat harmful chemicals in the environment, there are certain limiting factors that impede its application. There are some harmful substances like stable cyanide species (metallocyanide) and other metal pollutants since they are not substrate for the growth of microbes. Such contaminants are very difficult to treat via biological means utilizing indigenous microorganisms. However, the development of engineered microorganisms in recent times has provided new pathway for degradation of these non-biodegradable cyanide contaminants [97]. Admassu and Korus [92] identified the absence of cyanide contaminated material characteristics, microbial physiology, complicated design, and operational processes as some basic drawbacks in biodetoxification technology. Physical properties of the toxicants such as water solubility, water partition coefficient (Kow) (concentration ratio of chemical between two media in equilibrium), vapor pressure, and Henry’s Law tend to limit microbial detoxification cyanide-containing contaminants. For example, hydrophobic compounds with high water coefficient are mostly not biodegradable [92]. More so, Alexander [12] pointed out certain unreported factors like transport effects, resistance of microbes to the toxicants, inhibition and cometabolism as limiting factors that alter the rate of cyanide biodetoxification. The presences of the large and active population of protozoan in many cyanide-bearing wastewaters affect the rate of biodetoxification. The protozoans feed on the indigenous bacteria, which reduces its density and prolong the acclimatization of the bacteria in contaminants [12]. The principal objective of biodetoxification is to identify the above-stated limitations, control and optimize them for complete biodegradation of cyanide contaminants emanate from industrial operations.
Methods of cyanide biodetoxification
Microbial biodetoxification techniques
Various known available strategies can be employed to control and optimize microorganisms to facilitate their effectiveness to detoxify contaminants in the environment. There are two main microbial biodetoxification techniques; in-situ (includes biosparging, bioventing, bioaugmentation, and phytoremediation) and ex-situ (includes windrow, bioreactor, biopiling, and land farming) (Fig. 1) [98, 99].
In-situ biodetoxification
According to Azubuike et al. [98], in-situ biodetoxification can be explained as the use of the biological procedures to detoxify environmental contaminants, which take place in the contaminated site. This biological process involves the scientific and multi-discipline approach to optimize and control the indigenous microorganism population to influence their ability to convert toxicants into nontoxic waste component [97].
Biosparging
This is one of the in-situ techniques, which utilizes microbes to degrade contaminants within the saturated zone and mostly takes place in groundwater [100]. Small-diameter air injectors are placed below the soil sediments and the air is pumped into it under high pressure to improve oxygen concentration. The aeration of the contaminated environment activates the naturally occurring microbes to degrade the pollutants. This approach is inexpensive and requires less technology to construct air injectors [101]. Factors like soil permeability and pollutant biodegradability determine the effectiveness of this technique to remediate a contaminated environment [102]. The method could be very useful to detoxify cyanide-contaminated soil from industrial activities. The cyanide-contaminated soil below certain depth can be remediated through this approach. However, the technique is associated with certain demerit such as inability to predict the flow of air in the saturated region [98]. More so, the technique can be effective during natural detoxification process in the mine tailings dams.
Bioventing
This technique is similar to biosparging but this involves injecting low airflow rate to the unsaturated area to increase the oxygen availability in the soil to serve as an electron acceptor, while the indigenous microorganisms utilize the carbon source present in the waste matrix for their cell growth [101]. The major objective of this approach is to facilitate the completion of the biodetoxification of cyanide contaminants underground, to prevent the concentration from escaping to the earth’s surface through vaporization [97]. In bioventing, microbes are stimulated by supplying them with nutrients (gaseous ammonia vapors) and moisture to promote cell viability to enhance biodegradation of the contaminants to the nontoxic component [102]. This approach may be efficient to remediate the cyanide and related complexes, which contaminates the groundwater through seepage from the tailings dam.
Bioaugmentation
Bioaugmentation involves the introduction of microbial consortia or genetically engineered microbes into the contaminated region to augment the biodetoxification capabilities of the indigenous microbes [103]. The conditions such as low population, the initial concentration of the contaminants, and the stress to indigenous microbes because of exposure to high cyanide concentration may necessitate addition of exogenous microbes to the contaminants. The microbes must have environmental adaptability to survive in the new habitation [98]. Park et al. [104] reported detoxification of 14 mg/L of cyanide from coke wastewater by bioaugmentation through the utilization of yeast (Cryptococcus humicolus). The yeast and unidentified cyanide-degrading microbial consortia were inoculated into fluidized-bed type process (Bio-SAC) (1280 m3) supplemented with glucose and other nutrients for 2 months. The authors noticed that the yeast inoculation remarkably detoxified the KCN (potassium cyanide) concentration to 0.1 mg/L. However, the researchers recorded poor removal efficiency of cyanide in the contaminants from the bioprocess when operated it in full-scale because of lack of organic carbon and slow rate of degradation. They reported a lack of organic carbon and a slow rate of biodegradation as a factor for the inefficiency. Supplementing the contaminated media with nutrients enhance the biodetoxification efficiency of cyanide contaminants in the bioaugmentation process. Two fungi species, F. solani and F. oxysporum isolated from formal gasworks soil were capable of detoxifying 50% of 16.3 mg/L metallocyanide complex contaminant utilizing glucose as carbon and energy sources [21]. The researcher stated that, the detoxification of the cyanide from the contaminants was as a result of the strains utilizing the nitrogen source as grow substrate.
Phytoremediation
Many plants species have been reported as having the potential to resist cyanide toxicity (Table 3) [105]. Most plant and associated rhizosphere microorganisms are reported as potentially useful to remediate the cyanide-contaminated environment. For instance, Hong et al. [106] reported 85% detoxification of iron cyanide contaminated soil from industrial activities after cultivating two cyanogenic plant species (Sorghum bicolor and Linum usitassium) for 200-day phytoremediation study. Cyanide-detoxifying plants have various adaptations to degrade cyanide contaminants in the soil and finally assimilate it into their tissues [36]. Chemical reactions that occur between free cyanide and sulfur in the plant materials after nutrient uptake results in the generation of thiocyanate, which is comparatively nontoxic byproduct [40]. Cultivation of plants on the cyanide-contaminated land can serve as an alternative to chemical measures of controlling cyanide poisoning in mined areas [59]. The cyanide-degrading enzymes in plants (ß-cyanoalanine synthase) connect the HCNand cysteine to cyanoalanine. Cyanide is metabolized via ß-cyanoalanine pathway to release asparagine, aspartate, and ammonia [107]. Cyanide degradation has been reported in wheat (Triticum aestivum L.) and sorghum (Sorghum bicolor L.) [108]. In a related studies, Trapp et al. [83] demonstrated the cyanide degradation efficiency of Sorghum bicolor L. Phytoremediation studies conducted in 60 days revealed that Sorghum bicolor L was able to degrade up to 125 mg/L cyanide concentration from the cyanide contaminated soil via their roots and leaves. The production of asparagine, aspartate, and ammonia by the enzyme provides supplementary ammonia source to the plants [108]. Hidayati et al. [109] proposed the use of indigenous plants capable of resisting cyanide toxicants as a green technology to remediate cyanide contaminated rivers and paddy fields through small scale and large-scale industrial mining activities. Their argument emanated from a phytoremediation study they conducted utilizing Paspalum conjugate and Cyprus kyllingia. They observed complete detoxification of 16.52 mg/L cyanide by Paspalum conjugate and 33.16 mg/L by Cyprus kyllingia from the contaminated environment. The cultivation of cyanogenic plants in the mined environment is the most economical and eco-friendly technology of detoxifying cyanide-contaminated land for agricultural activities. Cultivation of cyanide detoxifying plants not only removes the cyanide components, but can also enrich the top soil through addition organic nutrients.
Ex-situ biodetoxification
The treatment of cyanide concentration in gold mine tailings can be off-site. Some of the off-site techniques of cyanide biodetoxification are discussed below.
Bioreactor
The bioreactor is a medium in which biological reaction occurs to generate new products. Bioreactors for ex-situ biodetoxification of cyanide contaminants are classified as slurry or aqueous reactors. Reactors are engineered structures designed to process solid or slurry cyanide contaminants utilizing inoculum (microorganisms) to biotransform the contaminants into nontoxic components [101]. The conditions (oxygen, nutrient, pH, and temperature) in the bioreactor are optimized to mimic natural environmental conditions of the microbes to enhance their growth and biodetoxification of the contaminants [98]. Mekuto et al. [110] observed biodetoxification of free cyanide (CN−) concentration of 250 mg/L and 450 mg/L, utilizing consortia of Bacillus genus in continuous mode. The researchers noticed that, the bacteria could detoxify up 80% and 32% from the initial cyanide concentration after 200 h incubation. Using bioreactor in detoxifying cyanide-containing waste has advantages such ability to adjust the various parameters necessary for the biological reaction to stimulate growth of microbes and enhance detoxification of contaminants in the reactor [98].
Biopiling
Biopile bioremediation techniques involve the transfer of contaminants from the on-site and treat it off-site with soil amendments, inoculum (microbes), and supported by aeration. The bioremediation parameters (nutrients, oxygen, and pH) are optimized to ensure an efficient metabolic process by the microbes [101]. There is no up to date information on application of biopile in remediating cyanide contaminants but very popular in controlling hydrocarbon pollutants. For an instance, Dias et al. [111] observed 71% efficiency of hydrocarbon biodegradation in fresh hydrocarbon contaminated Antarctic soil using biopile technology. Several applications of biopile techniques to remediate the polluted environment are well established [98, 112]. This technique has a wider application in treating polluted environment and may be very useful for cyanide biodetoxification in waste matrix. Treating cyanide-containing waste close to water bodies and human habitation could be very detrimental due to structural failure, which may results in spillage. This technique becomes necessary if there a need to treat the cyanide contaminants off-site.
Landfarming
This is biological technique use in treating hazardous contaminated waste. The contaminated waste is transported into a pit lined with high-density synthetic clay, water and nutrients are supplied via a delivery tube on sub-surface. The medium is supplied with oxygen through the porous space beneath the medium. Pipes are laid between the contaminated soil and the layer of the sand to collect the runoff, which are recycled to prevent underground water pollution. Landfarming biological approach to treat cyanide contaminated is cost-effective and environmentally friendly due to unforeseen accidents that may arise from detoxification process [92]. This treatment process will curtail a situation of cyanide-bearing wastewater spillage from collapsed dams and valve failures, which are common accidents in treatment of cyanide contaminants. Land farming, has been widely used in remediating hydrocarbon-polluted environment [113, 114], however, there is no up to date report on its application in treating cyanide-bearing contaminants.
Biodetoxification of cyanide-bearing contaminants
Many microorganisms found in nature are capable of utilizing cyanide contaminants as a source of nutrients for cell viability through the enzymatic attack. The use of microorganisms to remediate the environment from contaminants can be generally referred to as bioremediation or otherwise biodetoxification [97]. Biological detoxification of cyanide contaminants has been demonstrated as most effective when compared to the physical or chemical processes of detoxifying cyanide contaminants for the past decades [115]. Biodetoxification technology employs organisms to remove cyanide concentration from the contaminants, these techniques ensure total clean up and can be useful to degradation of other organic and inorganic contaminants in environment (Table 4) [116]. Biodetoxification of cyanide contaminants can be achieved using plants (phytoremediation) or microorganisms. Plants phytoremediation of cyanide-bearing waste has recently been established as a feasible approach. Plants like Salix babalonica L, S. alba L, S. eriocephala L are some of the known plants species that can assimilate cyanide concentration from cyanide-polluted soil [117, 118]. The presence of oxidase in the mitochondrial electron transport chain and endogenous cyanide-degrading enzyme-like a cyanase make plants tolerate a certain level of cyanide concentration [119]. Microorganisms are capable of surviving in extreme environmental conditions by developing certain adaptation measures such as varying their growth rate, converting basic DNA to produce protein, or relating with other organisms in the contaminated media to exist [120]. Microorganisms can strive in both aerobic and anaerobic environmental conditions. Haghighi-Podeh and Siyahati-Ardakani [34] identified Osculatoria, Philodina, Carchesium, Pseudomonas, and Bacillus as bacteria capable of surviving in the aerobic medium condition in their study. Bacillus pumilus strain is gram-positive, aerobic; endospores forming bacteria that can tolerate up to 100 mg/L of KCN cyanide [121]. Khamar et al. [80] reported cyanide detoxification of 75% when they inoculated Halomonas daqingensis into minimal salt medium supplemented with 50 mg/L cyanide concentration for 5-days under aerobic conditions. Furthermore, Moradkhani et al. [86] cultivated Pseudomonas parafulva NBRC in basal salt solution using cyanide as sole nitrogen source. After 13 days of incubation, they observed a 93.5% reduction in the 500 mg/L of the initial cyanide concentration with bacteria growth from 1.00 × 107 to 9.00 × 107 CFU/mL. Anaerobic bacteria are capable of degrading cyanide concentration in various contaminated media [22]. The use of anaerobic condition to grow bacteria was first studied in the late 1980s [122], and had since become an interesting approach to culture bacteria for various biodetoxification exercises [60]. Several studies have been conducted in recent times using anaerobic bacteria to detoxify cyanide in a batch culture medium [123,124,125]. Another economic advantage of using anaerobic bacteria is the generation of biogas (methane) and hydrogen gas for sustainable energy supply [126, 127]. Many bacteria that are capable of reducing sulfate do so through an anaerobic process. The sulfate-reducing bacteria can equally be used effectively to detoxify free cyanide from the contaminated environment [128]. There are two categories of microorganisms that are mostly utilized to detoxify cyanide-containing waste; bacteria and fungus [83]. There are several reports on the efficiency of bacteria to detoxify cyanide in the waste matrix over the past decades [15, 129, 130]. Akcil et al. [7] investigated the potential of using bacteria to treat cyanide-bearing effluents after the gold ‘cyanidation’ process. They observed that Pseudomonas sp. was able to tolerate up to 100–400 mg/L of cyanide under laboratory conditions. Authors concluded that, the bacteria strain exhibited cyanide detoxification potential in the contaminated media. Similarly, Shin et al. [67] reported successful detoxification of 50 mg/L of free cyanide concentration in synthetic wastewater within 21 days using phylum Proteobacterium in stirred-tank bioreactors. The bacteria were able to reduce the initial concentration of 50–2.8 mg/L with viable cells growth of 2.5 × 107 CFU/mL at a pH of 8.4. Luque-Almagro et al. [33] reported 1470 mg/L of free cyanide detoxification by Pseudomonas pseudoalcaligenes in alkaline medium using ammonium, nitrate, cyanate, cyanoacetamide, nitroferricyanide, and many other cyanide-metal complexes as a nitrogen source. Dwivedi et al. [83] conducted a study on the removal of cyanide using Bacillus cereus and supported with the almond shell as a carbon source. The bacterium was cultivated in a 250 mL conical flask containing 100 mL of sterilized culture media with 100 mg/L of pure sterilized KCN as a nitrogen source. They achieved biodetoxification efficiency of 84.7% within 15 min and the highest efficiency of 95.87% at the end of 60 h. Many fungi can infect cyanogenic plants with pathogens despite the toxic nature of the plant. This may be due to the presence of cyanide degrading enzymes [68]. Enzyme hydratases found in most plant pathogenic fungi such as Fusarium solani, Gloeocercospora sorghi, Fusarium lateritium, and Leptosphaeria maculans can convert cyanide to ammonia and formate [68, 131]. Hydratases are enzymes capable of converting toxic chemicals like cyanide into nontoxic components via hydration reaction [60]. Many reports are available for the effectiveness of fungi to detoxify cyanide-bearing waste [89]. Recently, Akinpelu et al. [93] reported Fusarium oxysporum EKTO1/02 from the rhizosphere of Zea mays that has been exposed to cyanide-containing pesticides to degrade cyanide. They inoculated the isolated microbes in a medium containing 100 mg/L and incubated it in a rotary shaker for 120 h; they had 77.6% of free cyanide being detoxified. The biodetoxification process of removing cyanide from contaminants is simple to operate and does not involve the use of toxic chemical reagents. This method is the most economical and environmentally friendly when compared to the chemical process [38]. It is unambiguous from the above discussion that biodetoxification techniques are diverse and have proven efficient in remediating cyanide-containing industrial contaminants from the environment.
Conclusion
The anthropogenic activity such as mining, electroplating, and agriculture have significantly contributed to the abundance of harmful chemical like cyanide in the ecosystem, which harm humans, plants, and aquatic life. Biodetoxification technology utilizes living organisms, usually bacteria, fungi, and plants to clean up the excess cyanide concentration from the environment. This approach, which is economically viable, eco-friendly, and less complex to operate, is a perfect alternative to traditional remediation techniques such as chemical, physical, and natural attenuation. The cyanide biodetoxification requires in-depth understanding of microbial metabolism and their physiology. Though microbial acclimatization in cyanide-bearing contaminants take longer period, its effect on detoxifying free cyanide and other strong acid dissociation complexes is very efficient when compared to chemical (oxidation) detoxification process. Recently, much attention has been given to microbial cyanide detoxification contaminants because of its environmental safety. Further studies must consider factors like chemical reaction of cyanide with the available nutrients, improvement in microbial acclimatization, and removal of microscopic prey like protozoan for effective detoxification process. Selecting an appropriate living organism for detoxification of cyanide can improve the process significantly and produce positive outcome.
References
Smith A (1988) Cyanide degradation and detoxification in a heap leach. In: van Zyl JA, Ian H, Kiel J. (eds) Introduction to evaluation, design and operation of precious metal heap leaching projects, 1st edn. Society for Mining Metallurgy, pp 293–305
Nazly N, Knowles CJ (1981) Cyanide degradation by immobilised fungi. Biotechnol Lett 3(7):363–368
Botz MM (2001) Overview of cyanide treatment methods. In: Mudder T (ed) Mining environmental management. Mining Journal Books Ltd., London, pp 28–30
Williamson A, Johnson MS (1981) Reclamation of metalliferous mine wastes. In: Lepp NW (eds) Effect of heavy metal pollution on plants. Pollution Monitoring Series, vol 2. Springer, Dordrecht. pp. 185–212. https://doi.org/10.1007/978-94-009-8099-0_6.
Sinha R, Valani D, Sinha S et al (2010) Bioremediation of contaminated sites: a low-cost nature’s biotechnology for environmental clean up by versatile microbes, plants & earthworms. In: Faerber T, Herzog J (eds) Solid waste management and environmental remediation. Nova Science, USA
Rodger PB (1981) Cyanide degradation by Chromobacterium violaceum. In: Vennesland B, Conn EE, Knowles CJ, Westley J, Wissing F (eds) Cyanide in biology. Academic Press, New York, pp 301–310
Akcil A, Karahan A, Ciftci H et al (2003) Biological treatment of cyanide by natural isolated bacteria (Pseudomonas sp). J Miner Eng 16(7):643–649. https://doi.org/10.1016/S0892-6875(03)00101-8
Young C, Jordan T (1995) Cyanide remediation: current and past technologies. In: Proceedings of the 10th Annual Conference on Hazardous Waste Research. May 23–24, 1995, Kansas State Univ., Manhattan, Kansas, USA
Cabuk A, Unal AT, Kolankaya N (2006) Biodegradation of cyanide by a white rot fungus Trametes versicolor. Biotechnol Lett 28(16):1313–1317. https://doi.org/10.1007/s10529-006-9090-y
Nelson L (2006) Acute cyanide toxicity: mechanisms and manifestations. J Emerg Nurs 32(4):8–11. https://doi.org/10.1016/j.jen.2006.05.012
Andrews SC, Robinson AK, Rodríguez-Quiñones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27(2–3):215–237. https://doi.org/10.1016/S0168-6445(03)00055-X
Alexander M (1994) Biodegradation and bioremediation, 2nd edn. Academic Press, New York
Ebbs S (2004) Biological degradation of cyanide compounds. Curr Opin Biotechnol 15(3):231–236. https://doi.org/10.1016/j.copbio.2004.03.006
Ezzi MI, Lynch JM (2005) Biodegradation of cyanide by Trichoderma spp. and Fusarium spp. Enzyme Microbial Technol 36(7):849–854
Figueria M, Ciminelli V, De Andrade M et al (1996) Cyanide degradation by an Escherichia coli strain. Can J Microbiol 42(5):519–523. https://doi.org/10.1139/m96-070
Goncalves M, Pinto A, Granato M (1998) Biodegradation of free cyanide, thiocyanate and metal complexed cyanides in solutions with different compositions. J Environ Technol 19(2):133–142. https://doi.org/10.1080/0959333190861665
Igeño MI, Orovengua E, Guijo MI et al (2007) Biodegradation of cyanide-containing wastes by Pseudomonas pseudoalcaligenes CECT5344. Commun Curr Res Educ Topics Trends Appl Microbiol 1:100–107
Kuyucak N, Akcil A (2013) Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner Eng 50:13–29
Maniyam MN, Sjahrir F, Ibrahim A et al (2013) Biodegradation of cyanide by Rhodococcus UKMP-5M. Biologia 68(2):177–185. https://doi.org/10.2478/s11756-013-0158-6
Watts MP, Moreau JW (2018) Thiocyanate biodegradation: harnessing microbial metabolism for mine remediation. Microbiol Aust 39(3):157–161
Baxter J, Cummings S (2006) The impact of bioaugmentation on metal cyanide degradation and soil bacteria community structure. J Biodegrad 17(3):207–217. https://doi.org/10.1007/s10532-005-4219-6
Dash RR, Gaur A, Balomajumder C (2009) Cyanide in industrial wastewaters and its removal: a review on biotreatment. J Hazard Mater 163(1):1–11. https://doi.org/10.1016/j.jhazmat.2008.06.051
Lu Z, Cai M (2012) Disposal methods on solid wastes from mines in transition from open-pit to underground mining. Proc Environ Sci 16:715–721
Akcil A (2002a) Cyanide control in tailings pond: ovacik gold mine, Turkey. In: Proceedings of Seventh International Symposium on Environmental Issues and Waste Management in Energy and Mineral Production (SWEMP), 7–10 october, 2002, Cagliar, sardinia, Italy, 437–441. Kluwer Academic Publishers. https://doi.org/10.1023/A:1022608213814
Jaszczak E, Polkowska Ż, Narkowicz S, Namieśnik J (2017) Cyanides in the environment, analysis, problems and challenges. Environ Sci Pollut Res 24(19):15929–15948
Dzombak DA, Ghosh RS, Young TC (2005) Physical–chemical properties and reactivity of cyanide in water and soil. CRC Press, Boca Raton, pp 69–104
Anning C, Wang J, Chen P, Batmunkh I et al (2019) Determination and detoxification of cyanide in gold mine tailings: a review. Waste Manag Res 37(11):1117–1126
Borgerding M, Klus H (2005) Analysis of complex mixtures–cigarette smoke. Exp Toxicol Pathol 57:43–73
Raybuck SA (1992) Microbes and microbial enzymes for cyanide degradation. J Biodegrad 3(1):3–18
Baskin SI, Kelly JB, Maliner BI, Rockwood GA et al (2008) Cyanide poisoning. Med Asp Chem Warf 11:372–410
Gail E, Gos S, Kulzer R, Lorösch J, Rubo A et al (2000) Cyano compounds, inorganic. Ullmann's Encycl Ind Chem 5:171–188. https://doi.org/10.1002/14356007.a08_159.pub3
Luque-Almagro VM, Moreno-Vivián C, Roldán MD (2016) Biodegradation of cyanide wastes from mining and jewellery industries. Curr Opin Biotechnol 38:9–13
Luque-Almagro VM, Huertas MJ, Martínez-Luque M et al (2005) Bacterial degradation of cyanide and its metal complexes under alkaline conditions. Appl Environ Microbiol 71(2):940–947. https://doi.org/10.1128/AEM.71.2.940-947.2005
Haghighi-Podeh MR, Siyahati-Ardakani G (2000) Fate and toxic effect of cyanide on aerobic treatment system. Water Sci Technol 4(3–4):125–129. https://doi.org/10.2166/wst.2000.0368
Solomonson LP (1981) Cyanide as a metabolic inhibitor. In: Vannesland B, Conn EE, Knowles CJ, Westly J, Wissing F (eds) Cyanide in biology. Academic press, New York, pp 11–28
Yu X-Z (2015) Uptake, assimilation and toxicity of cyanogenic compounds in plants: facts and fiction. Int J Environ Sci Technol 12(2):763–774. https://doi.org/10.1007/s13762-014-0571-6
Gleadow RM, Woodrow IE (2002) Mini-review: constraints on effectiveness of cyanogenic glycosides in herbivore defense. J Chem Ecol 28(7):1301–1313. https://doi.org/10.1023/A:1016298100201
Botz MM, Mudder TI, Akcil A (2005) Cyanide treatment: physical, chemical and biological process. In: Adams M (ed) Advances in gold ore processing. Elsevier Inc., Amsterdam, pp 672–700
Lieberei R, Biehl B, Giesemann A et al (1989) Cyanogenesis inhibits active defense reactions in plants. Plant Physiol 90(1):33–36. https://doi.org/10.1104/pp90.1.33
Kjeldsen P (1999) Behaviour of cyanides in soil and groundwater: a review. Water Air Soil Pollut 115:279–308
Kulig KW, Ballantyne B (1991) Cyanide toxicity. In: Case studies in environmental medicine. Agency for toxic substance and diseases Registry (ATSDR). 15: 5–7
Rubec PJ, Soundararajan R (1990) Chronic toxic effects of cyanide on tropical marine fish. In: Proceedings of the Seventeenth Annual Toxicity Workshop: November 5–7, 1990, Vancouver, BC, Canada.
Leduc G (1984) Cyanides in water: toxicology significance. In: Weber LJ (ed) Aquatic toxicology. Raven Press, New York, pp 153–224
Wiemeyer SN, Hill EF, Carpenter JW et al (1986) Acute oral toxicity of sodium cyanide in birds. J Wildl Dis 22(4):538–546. https://doi.org/10.7589/0090-3558-22.4.538
Davis RH (1981) Cyanide detoxification in domestic fowl. In: Vannesland B, Conn EE, Knowles CJ, Westly J, Wissing F (eds) Cyanide in biology. Academic press, New York, NY, pp 57–60
Arya AK, Singh A, Bhatt D (2019) Pesticide applications in agriculture and their effects on birds: an overview. In: Contaminants in agriculture and environment: health risks and remediation 5:10
Kadiri H, Asagba SO (2019) The chronic effects of cyanide on oxidative stress indices in the domestic chicken (Gallus domesticus L.). J Basic Appl Zool 80(1):30. https://doi.org/10.1186/s41936-019-0098-y
Oró J (1972) Extraterrestrial organic analysis. Sp Life Sci 3(4):507–550
Jones DA (1962) Selective eating of the acyanogenic form of the plant Lotus corniculatus L. by various animals. Nature 193(4820):1109–1110
Memariani Z, Farzaei MH, Ali A, Momtaz S (2020) Nutritional and bioactive characterization of unexplored food rich in phytonutrients. Elsevier, Amsterdam, pp 157–175
Süntar I, Yakıncı ÖF (2020) Potential risks of phytonutrients associated with high-dose or long-term use Phytonutrients in food. Elsevier, Amsterdam, pp 137–155
Reisch MS (2017) Cyanide glitters for some. In: Use of the deadly chemical is on the rise in the gold mining industry. Chem Eng News 95(39):18–19
Cunningham SA (2005) Incident, accident, catastrophe: cyanide on the Danube. Disasters 29(2):99–128
Helwege A (2015) Challenges with resolving mining conflicts in Latin America. Extr Ind Soc 2(1):73–84
Macklin MG, Brewer PA, Hudson-Edwards KA, Bird G et al (2006) A geomorphological approach to the management of rivers contaminated by metal mining. Geomorphology 79(3–4):423–447
Ani E-C, Cristea VM, Agachi PS (2012) Mathematical models to support pollution counteraction in case of accidents. Environ Eng Manag J (EEMJ) 11(1):7–13
Ramraj R (2001) The Omai disaster in Guyana. Geogr Bull Gamma Theta Upsilon 43(2):83–90
Amegbey NA, Adimado AA (2003) Incidents of cyanide spillage in Ghana. Miner Process Extr Metall 112(2):126–130
Kumar R, Saha S, Dhaka S et al (2017) Remediation of cyanide-contaminated environments through microbes and plants: a review of current knowledge and future perspectives. Geosyst Eng 20(1):28–40
Luque-Almagro VM, Cabello P, Sáez LP et al (2018) Exploring anaerobic environments for cyanide and cyano-derivatives microbial degradation. Appl Microbiol Biotechnol 102(3):1067–1074. https://doi.org/10.1007/s00253-017-8678-6
Baxter J, Cummings SP, Antonie VL (2006) The current and future applications of microorganism in the bioremediation of cyanide contamination. Springer Neth 90(1):1–17. https://doi.org/10.1007/s10482-006-9057-y
Gupta N, Balomajumder C, Agarwal V (2010) Enzymatic mechanism and biochemistry for cyanide degradation: a review. J Hazard Mater 176(1–3):1–13. https://doi.org/10.1016/j.jhazmat.2009.11.0388
Fry W, Evans P (1977) Association of formamide hydro-lyase with fungal pathogenicity to cyanogenic plants. Phytopathology 67:1001–1006. https://doi.org/10.1094/Phyto-67-1001
Desai J, Ramakrishna C (1998) Microbial degradation of cyanides and its commercial application. J Sci Ind Res 57:441–453. https://doi.org/10.1002/chin.199904253
Stam H, Stouthamer AH, van Verseveld HW (1985) Cyanide assimilation in Rhizobium ORS 571: influence of the nitrogenase catalyzed hydrogen production on the efficiency of growth. Arch Microbiol 143(2):196–202
Kunz DA, Nagappan O, Silva-Avalos J et al (1992) Utilization of cyanide as nitrogenous substrate by Pseudomonas fluorescens NCIMB 11764: evidence for multiple pathways of metabolic conversion. Appl Environ Microbiol 58(6):2022–2029
Shin D, Park J, Park H et al (2019) Key microbes and metabolic potentials contributing to cyanide biodegradation in stirred-tank bioreactors treating gold mining effluent. Miner Process Extr Metall Rev 41:1–11
Cluness MJ, Turner PD, Clements E et al (1993) Purification and properties of cyanide hydratase from Fusarium lateritium and analysis of the corresponding chy1 gene. J Microbiol 139(8):1807–1815. https://doi.org/10.1099/00221287-139-8-1807
Siedow JN, Umbach AL (1995) Plant mitochondrial electron transfer and molecular biology. Plant Cell 7(7):821–831. https://doi.org/10.1105/tpc.7.7821
Ganesan K, Raza S, Vijayaraghavan R (2010) Chemical warfare agents. J Pharm Bioallied Sci 2(3):166. https://doi.org/10.4103/0975-7406.68498
Guilloton M, Espie G, Anderson P (2002) What is the role of cyanase in plants. Rev Plant Biochem J Biotechnol 1:57–79
Kao C, Liu J, Lou H et al (2003) Biotransformation of cyanide to methane and ammonia by Klebsiella oxytoca. Chemosphere 50(8):1055–1061
Westley J, Adler H, Westley L et al (1983) The sulfurtransferases. Fundam Appl Toxicol 3(5):377–382. https://doi.org/10.1093/toxsci/3.5.377
Atkinson A (1975) Bacteria cyanide detoxification. J Biotechnol Bioeng 17:457–460
Kelly DP, Baker SC (1990) The organosulphur cycle: aerobic and anaerobic processes leading to turnover of C1-sulphur compounds. FEMS Microbiol Rev 7(3–4):241–246. https://doi.org/10.1111/j.1574-6968.1990.tb04919.x
Berben T, Overmars L, Sorokin DY et al (2017) Comparative genome analysis of three thiocyanate oxidizing Thioalkalivibrio species isolated from soda lakes. Front Microbiol 8:254. https://doi.org/10.3389/fmicb.2017.00254
Dursun A, Çalık A, Aksu Z (1999) Degradation of ferrous (II) cyanide complex ions by Pseudomonas fluorescens. Process Biochem 34(9):901–908
Dash RR, Balomajumdar C (2014) Treatment of cyanide bearing effluents by adsorption, biodegradation and combined processes: effect of process parameters. J Desalination Water Treat 52(16–18):3355–3366. https://doi.org/10.1080/1944.2013.800330
Adams D, Komen J, Pickett T (2001) Biological cyanide degradation. In: Young CA (ed) Cyanide: social, industrial and economic aspects. The Mineral Metals & Material Society, Warrendale, pp 203–213
Khamar Z, Makhdoumi-Kakhki A, Gharaie MM (2015) Remediation of cyanide from the gold mine tailing pond by a novel bacterial co-culture. Int Biodeterior Biodegrad 99:123–128. https://doi.org/10.1106/j.ibiod.2015.01.009
Adjei MD, Ohta Y (2000) Factors affecting the biodegradation of cyanide by Burkholderia cepacia strain C-3. J Biosci Bioeng 89(3):274–277. https://doi.org/10.1016/s1389-1723(00)88833-7
Mirizadeh S, Yaghmaei S, Nejad ZG (2014) Biodegradation of cyanide by a new isolated strain under alkaline conditions and optimization by response surface methodology (RSM). J Environ Health Sci Eng 12(1):85
Dwivedi N, Balomajumder C, Mondal P (2016) Comparative evaluation of cyanide removal by adsorption, biodegradation, and simultaneous adsorption and biodegradation (SAB) process using Bacillus cereus and almond shell. J Environ Biol 37(4):551–556
Trapp S, Larsen M, Pirandello A et al (2003) Feasibility of cyanide elimination using plants. Eur J Miner Process Environ Protect 3(1):128–137
Razanamahandry LC, Andrianisa HA, Karoui H et al (2016) Biodegradation of free cyanide by bacterial species isolated from cyanide-contaminated artisanal gold mining catchment area in Burkina Faso. Chemosphere 157:71–78
Moradkhani M, Yaghmaei S, Nejad ZG (2017) Biodegradation of cyanide under alkaline conditions by a strain of Pseudomonas putida isolated from gold mine soil and optimization of process variables through response surface methodology (RSM). Period Polytech Chem Eng 62(3):265–273. https://doi.org/10.3311/ppch.10860
Karamba KI, Ahmad SA, Zulkharnain A et al (2016) Optimisation of biodegradation conditions for cyanide removal by Serratia marcescens strain AQ07 using one-factor-at-a-time technique and response surface methodology. Rend Fis Acc Lincei 27(3):533–545. https://doi.org/10.1007/s12210-016-0516-8
Wu CF, Xu XM, Zhu Q et al (2014) An effective method for the detoxification of cyanide-rich wastewater by Bacillus sp CN-22. Appl Microbiol Biotechnol 98(8):3801–3807. https://doi.org/10.1007/s00253-013-5433-5
Barclay M, Hart A, Knowles CJ et al (1998) Biodegradation of metal cyanides by mixed and pure cultures of fungi. Enzyme Microbial Technol 22(4):223–231
Patil Y, Paknikar K (2000) Development of a process for biodetoxification of metal cyanides from waste waters. Process Biochem 35(10):1139–1151. https://doi.org/10.1016/S0032-9592(00)00150-3
Mueller JG, Cerniglia CE, Pritchard PH (2005) Bioremediation of environments contaminated. In: Ronald L, Crawford Crawford LD (eds) Bioremediation: principles and applications. Cambridge University Press, New York, p 125. https://doi.org/10.1017/CBO9780511608414
Admassu W, Korus RA (1996) Engineering of bioremediation processes: needs and limitations. Biotechnol Res Ser 6:13–34
Akinpelu EA, Adetunji AT, Ntwampe SKO et al (2018) Performance of Fusarium oxysporum EKT01/02 isolate in cyanide biodegradation system. Environ Eng Res 23(2):223–227. https://doi.org/10.4491/eer.2017.154
Wang X, Wang Q, Wang S et al (2012) Effect of biostimulation on community level physiological profiles of microorganisms in field-scale biopiles composed of aged oil sludge. Biores Technol 111:308–315. https://doi.org/10.1016/j.biortech.2012.01.158
Hope KM, Knowles CJ (1991) The anaerobic utilisation of cyanide in the presence of sugars by microbial cultures can involve an abiotic process. FEMS Microbiol Lett 8:217–220. https://doi.org/10.1016/0378-1097(91)90598-5
Barany S (ed) (2004) Role of interfaces in environmental protection, vol 4. Springer, Dordrecht, p 1564. https://doi.org/10.1007/978-94-010-0183-0
Crawford RL, Crawford DL (2005) Bioremediation: principles and applications. Cambridge University Press, New York
Azubuike CC, Chikere CB, Okpokwasili GC (2016) Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol 32(11):180. https://doi.org/10.1007/s11274-016-2137-x
Das S (ed) (2014) Microbial biodegradation and bioremediation, 1st edn. Elsevier Inc., Amsterdam, pp 23–54. https://doi.org/10.1016/C2013-0-13533-7
Brar SK, Verma M, Surampall R et al (2006) Bioremediation of hazardous wastes: a review. Pract Period Hazard Toxic Radioact Waste Manag 10(2):59–72. https://doi.org/10.1061/(ASCE)1090-025X(2006)10:2(59)
Sharma S (2012) Bioremediation: features, strategies and applications. Asian J Pharm Life Sci 2(2):2231–4423
Atlas RM, Philp J (2005) Bioremediation: applied aquifers. Applied microbial solutions for real-world environmental cleanup. American Society for Microbiology press, Washington, pp 139–236
Adams GO, Fufeyin PT, Okoro SE et al (2015) Bioremediation, biostimulation and bioaugmention: a review. Int J Environ Bioremediat Biodegrad 3(1):28–39. https://doi.org/10.12691/ijebb-3-1-5
Park D, Lee DS, Kim YM et al (2008) Bioaugmentation of cyanide-degrading microorganisms in a full-scale cokes wastewater treatment facility. Biores Technol 99(6):2092–2096. https://doi.org/10.1016/j.biortech.2007.03.027
Bushey JT, Small MJ, Dzombak DA et al (2006) Parameter estimation of a plant uptake model for cyanide: application to hydroponic data. Int J Phytorem 8(1):45–62. https://doi.org/10.1080/15226510500507052
Hong L, Banks M, Schwab A (2008) Removal of cyanide contaminants from rhizosphere soil. J Bioremediat 12(4):210–215
Manning K (1988) Detoxification of cyanide by plants and hormone action. Cyanide compounds in biology. John Wiley and Sons, Chichester, pp 92–110. https://doi.org/10.1002/9780470513712.ch7
Ebbs S, Kosma DK, Nielson EH et al (2010) Nitrogen supply and cyanide concentration influence the enrichment of nitrogen from cyanide in wheat (Triticum aestivum L.) and sorghum (Sorghum bicolor L.). Plant Cell Environ 33(7):1152–1160. https://doi.org/10.1111/j.1365-3040.2010.02136.x
Hidayati N, Juhaet T, Syarif F (2009) Mercury and cyanide contaminations in gold mine environment and possible solution of cleaning up by using phytoextraction. HAYATI J Biosci 16(3):88–94. https://doi.org/10.4308/hjb.16.3.88
Mekuto L, Ntwampe SKO, Jackson VA (2015) Biodegradation of free cyanide and subsequent utilisation of biodegradation by-products by Bacillus consortia: optimisation using response surface methodology. Environ Sci Pollut Res 22(14):10434–10443. https://doi.org/10.1007/s11356-015-4221-4
Dias RL, Ruberto L, Calabró A et al (2015) Hydrocarbon removal and bacterial community structure in on-site biostimulated biopile systems designed for bioremediation of diesel-contaminated Antarctic soil. Polar Biol 38(5):677–687. https://doi.org/10.1007/s00300-014-1630-7
Gomez F, Sartaj M (2014) Optimization of field scale biopiles for bioremediation of petroleum hydrocarbon contaminated soil at low temperature conditions by response surface methodology (RSM). Int Biodeterior Biodegrad 89:103–109. https://doi.org/10.1016/j.ibiod.2014.01.010
Raymond RL, Hudson JO, Jamison VW (1976) Oil degradation in soil. Appl Environ Microbiol 31(4):522–535
Song HG, Bertha R (1990) Effects of jet fuel spills on the microbial community in soil. Appl Environ Microbiol 56(3):646–651
Akcil A (2003) Destruction of cyanide in gold mill effluents: biological versus chemical treatments. Biotechnol Adv 21(6):501–511. https://doi.org/10.1016/s0734-9750(03)00099-5
Jaysankar D, Ramaiah N, Bhosle NB et al (2007) Potential of mercury-resistant marine bacteria for detoxification of chemicals of environmental concern. Microbes Environ 22(4):336–345. https://doi.org/10.1264/jsme2.22.336
Ashraf M, Ahmad MSA, Ozturk M (2010) Plant adaptation and phytoremediation. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9370-7
Yu X-Z, Zhou PH, Yang YM (2006) The potential for phytoremediation of iron cyanide complex by willows. Ecotoxicology 15(5):461–467. https://doi.org/10.1007/s10646-006-0081-5
Aichi M, Nishida I, Omata T (1998) Molecular cloning and characterization of a cDNA encoding cyanase from Arabidopsis thaliana. Plant Cell Physiol 39:135–135
Das S, Dash HR (2014) Microbial bioremediation: a potential tool for restoration of contaminated areas. In: Das S (ed) Microbial biodegradation and bioremediation. Elsevier Inc., Amsterdam, pp 1–21
Meyers PR, Gokool P, Rawlings DE et al (1991) An efficient cyanide-degrading Bacillus pumilus strain. J Microbiol 137(6):1397–1400
Fedorak PM, Hrudey SE (1989) Cyanide transformation in anaerobic phenol-degradation methanogenic culture. Water Sci Technol 21:67–76
Chakrabortis S, Veeramani H (2006) Effects of HRT and recycle ratio on removal of cyanide, phenol, thiocyanate, and ammonia in an anaerobic–anoxic–aerobic continous system. Process Biochem 4(1):96–105. https://doi.org/10.1016/j.procbio.2005.03.067
Novak D, Franke-Whittle IH, Pirc ET, Jernan V et al (2013) Biotic and Abiotic process contribute to success anearobic degradation of cyanide by UASB rector biomass treating brewery. Water Res 47(11):3644–3653. https://doi.org/10.1016/j.watres.2013.04.027
Nwokoro O, Dibua MEU (2014) Degradation of soil cyanide by single and mixed cultures of Pseudomonas stutzeri and Bacillus subtilis. Arch Ind Hyg Toxicol 65(1):113–119. https://doi.org/10.2478/1004-1254-65-2014-2449
Liu G, Shen J (2004) Effects of culture and medium conditions on hydrogen production from starch using anaerobic bacteria. J Biosci Bioeng 98(4):251–256. https://doi.org/10.1016/S1389-1723(04)00277-4
Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85(4):849–860. https://doi.org/10.1007/s00253-009-2246-7
Song Y-C, Piak B-C, Shin H-S et al (1998) Influence of electron donor and toxic materials on the activity of sulfate reducing bacteria for the treatment of electroplating wastewater. Water Sci Technol 38(4–5):187–194. https://doi.org/10.1016/S0273-1223(98)00527-7
Dubey S, Holmes D (1995) Biological cyanide destruction mediated by microorganisms. World J Microbiol Biotechnol 11(3):257–265. https://doi.org/10.1007/BF00367095
Kandasamy S, Dananjeyan B, Krishnamurthy K et al (2015) Aerobic cyanide degradation by bacterial isolates from cassava factory wastewater. Braz J Microbiol 46(3):659–666. https://doi.org/10.1590/S1517-838246320130516
Wang P, VanEtten HD (1992) Cloning and properties of a cyanide hydratase gene from the phytopathogenic fungus Gloeocercospora sorghi. Biochem Biophys Res Commun 187(2):1048–1054
Akopyan TN, Braunstein AE, Goryachenkova EV (1975) Beta-cyanoalanine synthase: purification and characterization. Proc Natl Acad Sci 72(4):1617–1621. https://doi.org/10.1073/pnas.72.4.1617
Gurbuz F, Ciftci H, Akcil A (2009) Biodegradation of cyanide containing effluents by Scenedesmus obliquus. J Hazard Mater 162(1):74–79
Ebel M, Evangelou MW, Schaeffer A (2007) Cyanide phytoremediation by water hyacinths (Eichhornia crassipes). Chemosphere 66(5):816–823
Sankaranarayanan A, Gowthami M (2015) Cyanide degradation by consortium of bacterial species isolated from sago industry effluent. J Environ Treat Tech 3(1):41–46
Tiong B, Bahari ZM, Lee N et al (2015) Cyanide degradation by Pseudomonas pseudoalcaligenes strain W2 isolated from mining effluent. Sains Malays 44(2):233–238
Acknowledgements
The National Natural Science Foundation of China (No. 51674161) and the Major Program of the Shandong Province Natural Science Foundation (No. ZR2019BEE075) supported this project.
Funding
The author(s) received no financial support for the research, authorship, and publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest concerning the research, authorship, and publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cosmos, A., Erdenekhuyag, BO., Yao, G. et al. Principles and methods of bio detoxification of cyanide contaminants. J Mater Cycles Waste Manag 22, 939–954 (2020). https://doi.org/10.1007/s10163-020-01013-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01013-6