Abstract
Direct disposal of flue gas scrubbing-derived waste water with a high level (9000–10,000 mg/L) of ammonium ion (NH4 +) into aquatic systems has contributed to environmental depreciation. Here, we report a feasibility study on NH4 + recovery and conversion to struvite (NH4MgPO4·6H2O), which is a slow-release fertilizer. Such conversion also aids in compliance with the discharge limits for nitrogen-based compounds. Lab-scale experiments were performed to determine the optimum pH and molar ratio (Mg2+:NH4 +:PO4 3−) for struvite formation. A chemical equilibrium model (Visual Minteq) was also employed to corroborate the experimental results. The optimum pH for struvite precipitation was found to be pH 9 with a molar ratio of Mg2+:NH4 +:PO4³− = 1:1:1. At this pH, more than 93, 92.3 and 100% of the NH4 +, Mg2+ and PO4 3−, respectively, were removed from the scrubbing waste water Visual Minteq simulation also demonstrated optimum struvite formation at pH 9–10. Both X-ray diffraction (XRD) and scanning electron microscopy/energy dispersive spectroscopy (SEM/EDS) analysis revealed that the synthesized struvite was comparable to that of a commercial struvite. Thus, our findings confirmed the possibility of synthesizing struvite from de-NOx scrubbing wastewater utilizing the residual ammonium ions (NH4 +).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A wide variety of technologies has been developed for the reduction of NOx in flue-gas emissions. According to the stringent regulations on flue gas emission established by the IMO (International Maritime Organization), small and large ships have to have de-NOx facilities. Although the use of wet scrubbers is a popular and efficient method for removing nitrogen oxides from flue gas, this method may be associated with a characteristic drawback of eventual discharge of scrubber water into the aquatic environment. The direct disposal of wastewater containing nitrogen species has been reported to contaminate or pollute the ecosystem [1]. Since ammonium hydroxide (NH4OH) has been used as an effective scrubbing solution, it was our keen interest to recover ammonium ion (NH4 +) in the form of some useful and commercial product, achieving waste-wealth scenario. One viable approach is the recovery of NH4 + as struvite (NH4MgPO4·6H2O), which is an effective fertilizer [2]. Synthesis of struvite from scrubber wastewater is believed to ensure meeting the discharge limit for nitrogen-based compounds, set by Ministry of Agriculture, Food and Rural Affairs. Struvite precipitates as white orthorhombic crystals at a stoichiometric ratio (Mg2+:NH4 +:PO4³− = 1:1:1) according to the following chemical Eq. [3, 4]:
However, a decrease in pH accompanies the precipitation of struvite, which is attributed to the release of hydrogen ions into the solution. To account for this, instead of PO4 3−, HPO4 2− participate in the reaction according to the Eq. (2) proposed in references [5, 6]:
Struvite precipitation occurs when the product of NH4 +, Mg+ and PO4 3− concentrations exceed its solubility product [7]. The recovery of NH4 + in terms of precipitate growth has been tested in various wastewaters generated from coking wastewater [8], landfill leachate [3, 9, 10], industrial wastewater [11], poultry manure [6], and anaerobic digester effluents [2]. Studies have shown that struvite is an important source of N, Mg and P [12]. Thus, utilization of struvite has led to its identification as a slow release fertilizer, a raw material to the phosphate industry, useful for making fire resistant panels and also as a binder in cement industry [5]. Amongst the aforementioned attributes, the slow-release property, in particular, has exceptional appeal, as it prevents nutrients from being rapidly lost due to leaching and evaporation caused by intentional watering or rain [13].
The main focus of this work was to attempt struvite synthesis from scrubbing wastewater which contains a high level of NH4 + in the range of 9000–10,000 mg/L. To accomplish this purpose, the followings were attempted: (1) to precipitate NH4 +-N in the form of struvite, (2) to optimize the synthesis parameters (i.e. pH and molar ratio of Mg2+: NH4 +:PO4 3−), (3) to study the effect of impurities on crystal size (4) to examine the physical and chemical properties of the recovered products and (5) to compare the experimental results with those estimated by a Visual Minteq model.
Materials and methods
Experimental method
Struvite was synthesized in a batch reactor at ambient laboratory temperature using synthetic solution and field scrubbing wastewater received from Anytech Co. Ltd. Korea. First and foremost, the conductivity of pristine wastewaters was determined using a conductivity meter (Thermo scientific Orion 3-Star benchtop pH meter, Singapore). The pre-determined test dosages of the Mg and PO3− precursors were added to the water sample, which served as an NH4 + source. The pH of the solution was adjusted by drop wise additions of 1 or 2 M NaOH solution. The test solution was then stirred with a magnetic stirrer until a stable pH was reached. The solution was left standing for 30 min to ensure complete and quantitative precipitation. The white solid precipitates formed were collected on 0.20 µm cellulose filters (Toyo Roshi Kaisha Ltd, Japan), and the concentrations of the residual ions (NH4 +, Mg2+ and PO4 3−) in the solution were determined using ion chromatography (883 Basic IC plus, Metrohm, Switzerland). The precipitate was air-dried at room temperature. To prevent further formation of undesired products, the sample was maintained at a pH below 5 by the dropwise addition of 2 M nitric acid. For particle size measurement, laser diffraction Mastersizer 2000, Malvern Instrument, UK) was used. The effect of impurities (NO3 −, SO4 2− and Ca2+) on struvite formation was assessed with concentrations of 500, 1000 and 1500 mg/L by adding KNO3 or K2SO4 or CaCl2, respectively, to synthetic waste water. In the synthetic solution, the molar ratio of Mg2+:NH4 +:PO4 3− was controlled at 1:1:1 with pH 9.
The pH of the solution was monitored throughout the experiment using a pH meter (PC 700, Eutech Instruments, Singapore). All chemicals used in the experiments were reagent grade and were procured from Daejung Chemical Co. (Seoul, Korea). The composition and properties of the synthesized struvite were compared with a commercial struvite (99% purity) purchased from Alfa Aesar (US). The morphology and elemental compositions were characterized by scanning electron microscopy/energy dispersive X-ray spectroscopic analysis (SEM/EDS; Carl Zeiss, Supra 55, Eindhoven, Netherlands). The crystal structure was analyzed by X-ray diffraction (XRD; D8 Advance, Bruker, Germany).
Chemical Equilibrium Modeling
A chemical equilibrium model freeware program (Visual Minteq version 3.1) with an extensive thermodynamic database was developed by the US EPA. This program allows calculation of the speciation, solubility and equilibrium of solid and dissolved phases of minerals in an aqueous solution [14]. Hence, it was used to simulate the struvite synthesis carried out in this work. At first, the model was run with a set of ions which incorporated all of the major ions found in a scrubbing wastewater including sodium, nitrate, sulfate, potassium and chloride at varied pH values. Similarly, in another set, only the total concentrations of magnesium, ammonium and phosphate were inserted to the model at a stoichiometric ratio of Mg2+:NH4 +:PO4 3− =1:1:1 with various pHs. There was no significant variation observed in the obtained results. Thus, the run was carried out with latter one. All possible species that could be precipitated from the solution containing Mg2+, NH4 + and PO4 3− were added to the database. However, an interference cation (Ca2+) that could be involved in a competitive precipitation by forming Ca3(PO4)2 was excluded from the model consideration due to its low concentration in the solution (for Sect. “Effect of impurities”, it was included). In Visual Minteq, there are three alternative equations for performing activity corrections, the Davies equation, the extended Debye–Huckel equation, and the SIT (specific ion interaction theory of Bronsted-Guggenheim Scatchard) [15]. In this study, the SIT method was selected as it could determine an activity coefficient for high ionic strength [16]. The ionic strength was computed by the model with the assumption that all oversaturated solids were precipitated.
Results and discussion
A synthetic fertilizer, struvite, was recovered from de-NOx scrubber wastewater as a factor of solution pH and the molar ratio of the core reactants, such as Mg2+:NH4 +:PO4 3−. pH is an important factor that determines the formation of ionic species in a solution [17], whereas, the molar ratio expresses the stoichiometric quantitation of the reacting species (here Mg2+, NH4 + and PO4 3−).
Characteristics of flue gas scrubbing waste water
The chemical composition of the raw waste water is given in Table 1. NH4 + was found in an exceedingly large quantity (9.5 g/L) in comparison to the other ionic components. Such a high concentration is attributed to the residual aqueous ammonia (NH3) as a neutralization agent of nitrogen oxides. Extra aqueous ammonia in an alkali source is dissociated to NH4 + and OH− via dissolution. The Mg+ presents at 103 mg/L (i.e. NH4 +:Mg2+ = 12: 1), could also be useful for struvite synthesis. NO2 and SO2 are the major components of a combustion flue gas from ship engines and are removed by the wet scrubber. However, probably due to low efficiency, a large amount of N- and S-containing species (as NO3 − (780 mg/L) and SO4 2− (520 mg/L), respectively) are still commonly found as residues, and could serve as inhibitors of struvite synthesis. Other potential impurities should be significantly reduced, masked or completely removed so as to ensure that NH4 is quantitatively available to achieve the synthesis of high purity struvite.
The conductivity of wastewater is a function the amount of dissolved salts. The high value of this wastewater implies a high content of various salt ions commonly found in a municipal wastes landfill leachate. In other words, such ions may form unexpected salt compounds in the solution depending on the reaction condition. Besides, the initial pH (9.8) of the wastewater may reduce the consumption of pH-controlling agents, such as sodium hydroxide.
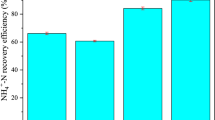
To identify the most appropriate precursors of magnesium and phosphorus for struvite synthesis, six combinations (at a stoichiometric mole ratio of 1: 1) were evaluated as summarized in Table 2. Each combination was applied to a measured aliquot of the wastewater sample. The residual ions were quantified; the lower was the amount of each ion, the larger was the amount of precipitates formed in the reaction. These precursor reagents were chosen based on their solubility in water and ease of use. Figure 1 provides the results obtained from an experiment using the different regents. Using C1, the highest percentage removal (% R) of was achieved with NH4 +, Mg2+ and PO4 3− was achieved at 93, 91 and 95%, respectively. This is in accordance with findings previously [6, 9]. In contrast, with C5 and C6 were tested, the observed residual concentrations of Mg2+ were greater than 20%, resulting in the worst % R values. Hence, further optimization experiments were performed with MgCl2·6H2O and Na2HPO4.
Synthesis of struvite
Effect of pH
To identify the most suitable pH for struvite precipitation at a stoichiometric ratio of NH4 +:Mg2+:PO4 3− = 1:1:1, optimization experiments were carried out in a pH range of 7–11. As observed as above, the pH of the initial wastewater was 9.8, but the feed of reactant precursors of Mg and P dropped down to 5.8. Thus, a little amount of alkali agent, NaOH (aq), was gradually added to meet the experimental pH values. The obtained results (Fig. 2a) showed that the % R for the ions in the synthetic sample increased with pH, reaching the optimum value at pH 9, where then they tended to plateau. A similar trend was also observed with the scrubbing wastewater sample (Fig. 2b). However, a decrease in the % R of NH4 + was seen at pH 9 to 11. At high pH, NH4 + is converted to gaseous NH3 which is released into the air [2].
On the other hand, previous studies have revealed that the solubility of struvite decreases at pH 7.5–9.0, but increased at higher pH with a minimum solubility at pH 9 [18]. Further increase in pH from 9 to 11 hinders crystallization, and also reduces the yield and purity of synthesized struvite [19]. As a result of precise observation, we found that the removal rates of NH4 +, Mg2+ and PO4 3− at pH 9 were 93.57, 98.12 and 95.39%, respectively, for a synthetic solution, whereas removal rates of 93, 92.32 and 100% were calculated for scrubbing wastewater. Hence, the optimum pH for scrubbing wastewater was identified as pH 9. This supports the deductions available in studies conducted on landfill leachate [9], poultry manure wastewater [6] and digested pig slurry [20].
Effect of molar ratio
Theoretically, struvite was formed under conditions of equal molar concentrations of magnesium, ammonium and phosphate (Eq. 1). To investigate the recovery rate of NH4 + which might be present in various forms, several different Mg2+ and PO4 3− ratio ranges were investigated. In general, since Mg2+ has been found to be a limiting factor in this reaction, some researchers have attempted to add excess Mg2+ sources with the goal of achieving a high yield of struvite [3]. However, it has been reported that increasing the Mg2+ concentration leads to a reduction in the purity of precipitated struvite, thereby resulting in increased levels of by-product formation [17]. This work focused on determining the optimum molar ratio so as to achieve the maximum removal of ions from scrubbing wastewater.
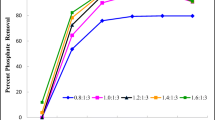
Based on the above results, the reaction pH was adjusted to 9.0 when applying the scrubbing wastewater solution. The test molar ratios of MgCl2·6H2O and Na2HPO4 given in Table 3 were examined by focusing on the utilization of reactant ions present in the solution. A comparison with respect to the extent of removal of these constituent ions between the various molar ratios is shown in Fig. 3. This result indicates the dependency of synthesis yield on the co-existence of constituent ions.
It was found that at a molar ratio of 1:1:1.5, excess PO4 3− enhanced the % R of NH4 + and Mg2+. However, the residual PO4 3− in the supernatant and hence the effluent could cause nitrification issues in the water system. The purifying cost of such nutrients including phosphorus and magnesium compounds cannot be ignored in wastewater treatment plants. Similar to previous studies that attempted to use minimum doses, our work did not intend some by-products, such as Mg3(PO4)2·8H2O and Mg(OH)2, to remain in the precipitate. When the molar ratio of PO4 3− relative to the others was as less than 1, its concentration did not increase as much as the decrease of Mg2+. This indicated that despite the overdosing of either Mg2+ or PO4 3−, no further improvement in the % R of NH4 +-N could be achieved. In addition, a remarkable decrease in the % R of NH4 + was found when a smaller dosage of the reagent was used. Thus, it could be confirmed that the molar ratio of NH4 +:Mg2+:PO4 3 = 1:1:1 was sufficient to effectively remove NH4 +, Mg2+ and PO4 3− ions from flue gas scrubbing wastewater. The amount of reagents added depends on the characteristics of the wastewater and the operating conditions of the reaction processes.
Effect of impurities
Any substance other than the material being crystallized can be considered as ‘impurity’, so even the solvent from which the crystals are grown is in the strictest sense an impurity. Calcium, sulfate and nitrate are present in the flue gas scrubbing wastewater in the range of 500–800 mg/L. These impurities can influence NH4 + recovery and in the purity of struvite by co-precipitating with the struvite crystal. It might also affect the crystal growth rate by inhibiting the increase in crystal size.
The experiment for this part was carried out in synthetic solution as mentioned in Sect. “Experimental method”. When NO3 −, SO4 2− and Ca2+ were added as impurity ranging from 500 to 1500 mg/L in the synthetic solution, the removal efficiency of NH4 + and PO4 3− did not show considerable change as presented in Table 4. The effect of impurities on crystal size was also studied as shown in Fig. 4. With the increase in (NO3 −, SO4 2− and Ca2+) ions concentration from 500 to 1500 mg/L, there was a negligible change in the median crystal size from 36.16 µm (without impurities) to 34.53 µm which is clearly visible in the Fig. 4. Chemical equilibrium model Visual Minteq predicted the formation of different calcium phosphate species due to the presence of Ca2+ and showed no effect when NO3 − and SO4 2− were added as an impurity. Although the formation of calcium phosphate species is thermodynamically suitable, its kinetics is very slow which is not accounted by the model [14]. Hence, it was concluded in scrubbing waste water that struvite precipitation was not affected by the presence of NO3 −, SO4 2− and Ca2+ ions up to 1500 mg/L.
Characterization of synthesized struvite
The formation of struvite was first ascertained through the XRD and SEM observation. Figure 5 shows the shape and crystal structure of the precipitated crystals. The micrograph of the precipitate obtained at pH 9 from both synthetic and scrubbing wastewater (Fig. 5b, c) indicated that the crystals had a distinctive orthorhombic structure bearing a close similarity to the commercial sample (Fig. 5a). Since only the necessary precursors were present in the synthetic solutions, very systematic crystal growth with regular shapes was obtained. By contrast, various clumps of varied morphology and broken crystals were seen among the crystals derived from the wastewater; this likely because many impurities hindered the reaction and remained in the precipitates. The shape of the crystals from the field waste water at pH 10 was much more unclear than at pH 9. The sizes of the wastewater-derived crystals ranged from 31 to 201 µm in length. The grain size of struvite could be affected by the influent concentrations of PO4 3− and NH4 + as well as particle retention time in the reactor.
Furthermore, the XRD analysis determined that the position and intensity of the struvite signatory peak matched that of the commercial product. In addition, the JCPDS files (15–0762) indicated that synthesized crystals using either synthetic or scrubbing wastewater were undoubtedly struvite (Fig. 5b, c, d). Thus, the experimental pretreatment carried out prior to the synthesis reaction was appropriate for wastewater, and the achieved purity level of the synthesized struvite was acceptable. However, at pH 10, the XRD results showed noisy patterns with a reduction in peak size and definition.
The elemental composition (in weight percentage) of the struvite except hydrogen was comparatively analyzed by EDS as summarized in Table 5. The EDS peaks showed high composition levels for O, P and Mg with the lowest amount of N identified. Sodium seems to have originated from the increased level of impurities present in the field waste solution or from the ionic constituent of intrinsic P precursor, Na2HPO4. The elemental weight percentages of the commercial struvite revealed the high content of P. The results showed that the synthesis of struvite was more strongly affected by pH than by the source wastewater. After assessing the potential of struvite as a vegetable fertilizer, the synthesized forms of struvite performed similarly to commercial fertilizers. In other words, the struvites synthesized from both synthetic and scrubbing wastewater at pH 9 were closer in composition to commercial struvite than those prepared at pH 10.
These results indicate that XRD method cannot be used as the sole method for determining struvite content in the precipitate. However, when supported by spectroscopic elemental analysis, a more confirmatory result about the purity of the struvite was attained. In practice, the identical composition based on the molecular formula was somewhat different from those of the present samples. The precipitates prepared at pH 10 (Fig. 5d) indicate clearly struvite, but may include some amorphous impurities, such as NaCl. As over pH 9, the possibility of formation of impurities or by-products including Mg3(PO4)2 also increases because the availability of NH4 + decreases.
Computational investigation of synthesis
To substantiate the findings from the experiments on the optimum composition of the reactants, an equilibrium computer model (Visual Minteq 3.1) was used and the results were compared with the experimental results. Initially, the optimized relative molar ratio (1:1:1) of NH4 +, Mg2+ and PO4 3− was calculated. By virtue of the modeling results, different aqueous species, such as Mg2+, MgHPO4 (aq), MgOH−, MgPO4 −, HPO4 2−, H2PO4 −, H3PO4, PO4 3−, NH4 + and NH3 (aq) could possibly be formed depending on the pH in the aqueous system as shown in Fig. 6. Here, various dependencies of ion concentration on the solution pH were seen. With an increase in pH, the availability of NH4 + in the solution decreased, whereas NH3 rapidly increased at pH above 9. This is supported by the finding that the availability of OH− ions, which donate their electron pairs to NH4 +, increases at higher pH, leading to the formation of aqueous NH3. Moreover, the concentration of Mg2+ also decreased with increasing pH. This can be explained as follows: Mg2+ at higher pH becomes available as MgPO4 − or remains as MgHPO4. The HPO4 2− concentration, which is shown at the base of the graph (Fig. 6), is a limiting agent of the reaction. Therefore, its availability determines the extent of the struvite formation. Since the availability of HPO4 2− is maximal at pH 9–10, it is inferred that this is as the optimum pH range for struvite synthesis.
Furthermore, the saturation index (SI) which can be used to establish the stability order of precipitation or dissolution of solid in contact with its mother solution was estimated from the following equation [21]:
where, IAP is the ion activity product of magnesium, ammonium and phosphate, the value of which was 7.08 × 10− 14 here [18], and Ksp is the solubility product of struvite. Figure 7 depicts SI as a function of pH and shows the potential solid phases that could be formed from the solution. When IAP is greater than Ksp, the solution becomes supersaturated, and; struvite nucleate; and grows up [22]. As shown in Fig. 7, struvite precipitation could be formed over a wide range of pH at the stoichiometric ratio. However, other solid compounds, such as Mg(OH)2, MgHPO4 and Mg3(PO4)2, were also generated. At pH higher than 9.5, the formation of Mg(OH)2 increased steeply which might have affected the amount of struvite synthesized. The formation of MgHPO4 maintained a nearly constant SI of 2, which then declined as the pH rose above 9.5.
On the other hand, the saturation index of Mg3(PO4)2 was found to be highest at pH 9.5–11. Due to a lower solubility product (Ksp = 10−25) than struvite (Ksp = 10−14), Mg3(PO4)2 is easily formed and apt to be the most likely interfering salt during struvite formation even though its precipitation rate is so low [23]. In particular, Mg3(PO4)2 tends to be formed at higher pH values as PO4 3− ions occur at pH > 9 according to the following equilibrium reaction:
Since struvite is synthesized from the different Mg2+ and PO4 3− complexes in the aqueous source solution, slight change in pH can result in various forms of chemical compounds. The results estimated by Visual Minteq were found to be close to the experimental findings, despite a slight but expected deviation. Although the model predicted that the optimum pH would be higher than 9, our findings proved that struvite synthesis was efficiently achieved at pH 9. Such observed variation in optimum pH could be attributed to the difference in ionic strength and unaccounted inhibitory effects among ions between the two research approaches [24]. It should also be noted that Visual Minteq does not account for kinetics or struvite production during the short reactor residence time, which serves as other potential contributors to the discrepancy [14]. Considering the precursors Mg(OH)2 and Mg3(PO4)2, the desirable pH for struvite formation is 9. The SEM/EDS and XRD analyses (Fig. 5; Table 5) performed at pH 9 and 10 also provided a rationale for the optimum pH. Finally, despite the prediction of various by-products, such as Mg(OH)2, MgHPO4 and Mg3(PO4)2, by the model, our experimental synthesis resulted in pure struvite, thereby demonstrating the validity of the model.
Conclusions
This work attempted to utilize residual ammonium ions in wastewater discharged from de-NOx scrubber of ship engines using aqueous ammonia. The fabrication of a synthetic struvite fertilizer containing a high amount of nitrogen was attempted using de-NOx scrubber-based wastewater as starting medium. In the reaction to recover NH4 + with the addition of various sources of magnesium and phosphorous, the impacts of solution pH and the molar ratio of the reactants were investigated while focusing on the utilization of constituent ion species in the solution. From the various initial molar ratios of reactants tested, the highest utilization of NH4 + was achieved with the stoichiometric ratio of NH4 +: Mg2+:PO4 3 = 1:1:1. It was at the optimum pH of 9 that the highest removal of ion species (93 % of NH4 +, 92.3 % Mg2+ and 100 % PO4 3−) was achieved for the struvite synthesis. It was observed that the presence of NO3 −, SO4 2− and Ca2+ ions did not affected the struvite precipitation in this experiment up to 1500 mg/L.
Particle morphology observed on SEM images revealed the orthorhombic structure of the struvite which the XRD analysis identified a match of its chemical structure with that of the commercial one. The theoretical chemical equilibrium model derived using Visual Minteq estimated the desirable pH range to be pH 9–11, which was similar to that derived from our experimental findings. The model enabled estimation of the potential influence of by-products in the progress of the reaction.
References
Lee SI, Weon SY, Lee CW, Koopman B (2002) Removal of nitrogen and phosphate from wastewater by the addition of bittern. Chemosphere 51:265–271
Celen I, Turker M (2001) Recovery of ammonia as struvite from digester effluents. Environ Technol 22(11):1263–1272
Zhang T, Ding L, Ren H (2009) Pretreatment of ammonium removal from landfill leachate by chemical precipitation. J Hazard mater 166:911–915
Ali MI (2005) Struvite crystallization from nutrient rich waste water, PhD Thesis, School of Engineering. James Cook University, Townsville
Stratful I, Scrimshaw MD, Lester JN (2001) Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res 35(17):4191–4199
Yetilmezsoy K, Zengin ZS (2009) Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure waste water by MAP precipitation as a slow release fertilizer. J Hazard Mater 166:260–269
Doyle JD, Parsons SA (2002) Struvite formation, control and recovery. Water Res 36:3925–3940
Zhang T, Ding L, Ren H, Xiong X (2009) Ammonium nitrogen removal from coking wastewater by chemical precipitation recycle technology. Water Res 43:5209–5215
Li XZ, Zhao QL, Hao XD (1999) Ammonium removal from landfill leachate by chemical precipitation. Waste Manag 19:409–415
Di Iaconi C, Pagano M, Ramadori R, Lopez A (2010) Nitrogen recovery from a stabilized municipal landfill leachate. Biores Technol 101:1732–1736
Diwani GE, Rafie SE, Ibiari NE, El-Aila HI (2007) Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer. Desalination 214:200–214
Li XZ, Zhao QL (2003) Recovery of ammonium-nitrogen from landfill leachate as a multi-nutrient fertilizer. Ecol Eng 20:171–181
Shu L, Schneider P, Jegatheesan V, Johnson J (2006) An economic evaluation of phosphorus recovery as struvite from digester supernatant. Biores Technol 97:2211–2216.
Celen I, Buchanan JR, Burns RT, Robinson RB, Raman DR (2007) Using an equilibrium model to predict amendments required to precipitate phosphorus as struvite in liquid swine manure. Water Res 41:1689–1696
Ali MI, Schneider PA (2008) An approach of estimating struvite growth kinetics incorporating thermodynamics and solution chemistry, kinetics and process description. Chem Eng Sci 63:3514–3525
Gustafsson JP, (2013) Visual Minteq ver. 3.0/3.1. KTH Royal institute of Technology, Sweden. http://vminteq.lwr.kth.se/visual-minteq-ver-3-0/
Gadekar S, Pullammanappallil P (2010) Validation and applications of a chemical equilibrium model for struvite precipitation. Environ Model Assess 15:201–209
Nelson NO, Mikkelsen RL, Hesterberg DL (2003) Struvite precipitation in anaerobic swine lagoon liquid: effect of pH and Mg:P ratio and determination of rate constant. Biores Technol 89:229–236
Hao XD, Wang CC, Lan L, Van Loosdrecht MCM (2008) Struvite formation, analytical methods and effects of pH and Ca++. Water Sci Technol 58(8):1687–1692
Cerrillo M, Palatsi J, Comas J, Vicens J, Bonmati A (2015) Struvite precipitation as a technology to be integrated into a manure anaerobic digestion treatment plant-removal efficiency, crystal characterization and agricultural assessment. J Chem Technol Biotechnol 90:1135–1143
Wu Y, Zhou S (2012) Improving the prediction of ammonium nitrogen removal through struvite precipitation. Environ Sci Pollut Res 19:347–360
Rahman MM, Salleh MAM, Rashid U, Ahsan A, Hossain MM, Ra CS (2014) Production of slow release crystal fertilizer from wastewaters through struvite crystallization–a review. Arab J chem 7:139–155
Mamais D, Pitt PA, Cheng YW, Loiacono J, Jenkins D (1994) Determination of ferric chloride dose to control struvite precipitation in anaerobic sludge digesters. Wat. Environ Res 66:912–918
Lin AY (2012) Precipitation of phosphate minerals from the effluent of anaerobically digested manure. Masters Thesis, Department of Civil Engineering, University of South Florida, USA
Acknowledgements
This work was financially supported by the Korean Ministry of Environment (No. 201400110019), and the authors are grateful for the instrument support by Kyung Hee University (KHU-20152127).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thapa, S., Ha, T.Y., Lee, H. et al. Recovery of ammonium ion as struvite from flue gas scrubbing wastewater. J Mater Cycles Waste Manag 20, 293–301 (2018). https://doi.org/10.1007/s10163-016-0579-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-016-0579-8