Abstract
In this study a carbon-rich product was achieved by hydrothermal carbonization (HTC) of dead leaves at different treatment temperatures of 200–250 °C. Biomass was treated with hot deionized water for 30 min. The main objective of this study was to calculate the energy generation capability of dead leaves hydrochar by HTC process. The secondary objective was to analyze the physiochemical properties of hydrochar. There was a significant increase in the energy content and energy yield while decrease in yield of hydrochar was observed with increase in temperature. Surface area of hydrochar was maximum of 2.09 m2/g which was obtained when heated at 250 °C. Feedstock was having pore diameter of 8.26 nm which begin to increase on heating. The highest was reported at 220 °C of 21.79 with 163 % of increase. At 220 °C pore volume was also highest of 9.86 × 10−3. The highest energy content of 19.98 MJ/kg was obtained when the feedstock was heated at 240 °C which showed 21 % increase in energy content compared to that of raw biomass. Similarly, energy yield was also highest (91.67 %) at 240 °C. Therefore, it can be concluded that high-energy content hydrochar can be recovered when carbonized at 240 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrothermal carbonization is a promising thermochemical process that converts lignocellulosic feedstock into useful product [1]. Municipal solid waste components and biomass are converted into a carbon-rich, energy-dense product termed as hydrochar [2]. Feedstock containing moisture is used in the hydrothermal carbonization (HTC) process, which includes manure [3], herbaceous waste [4], municipal waste [5], algae [6, 7], wet grains [8], and others. These manifold materials usually have substantial variability of physiochemical, nutrient, and biological properties, and hence have different impact on a soil [9]. Solid carbon-rich product manufactured by HTC has several names such as biocoal, biochar, hydrochar [10] and torrefaction [11]. Friedrich Bergius discovered the HTC process in 1913, which was modified by Professor Antonietti [12]. Recently, the world is more concerned about the use of hydrochar as a fertilizer and sequestrate carbon to attenuate climate change. The advantages of hydrochar to elevate soil fertility include: rise in pH, buffer capacity, and cation exchange capacity (CEC) [13]. It also helps in increasing soil nutrients and ameliorates the physical structure of soil [14]. Relatively high carbon content in hydrochar is responsible for mitigating climate change, which slows down the release of carbon dioxide into the atmosphere [15]. The use of hydrochar depends upon its immanent properties [16]. For example, adsorption power highly depends on the surface area of hydrochar [17]. The hydrochar with high pH, CEC and water holding capacity are suitable to use as soil amendment to increase fertility [18]. During the carbonization process, the feedstock experiences a diversity of physiochemical and molecular changes, including mass loss, change in structure [19], increase in pH and alkalinity [20]. The carbonization condition, especially carbonization temperature, is an important influencing factor for hydrochar properties [21].
Converting lignocellulosic biomass into hydrochar is the paramount way to produce reasonable energy [22]. Lignocellulosic biomass is the largest organic renewable resource available on the surface of earth, which is composed of plant matter formed by photosynthesis [23]. There are three different processes to convert lignocellulosic feedstocks into biofuels. They are: (1) biochemical process, in which cellulosic and hemicellulosic sugar is converted into alcohol with the help of enzymes and microorganisms; (2) hydrothermal process, in which biomass is converted into fermented sugar and hydrogen-rich synthesis gas in the presence of super-critical water, and (3) thermochemical process, where biofuels of long carbon chain like gasoline and bio-oil are produced by pyrolysis. Carbon dioxide and hydrogen gases are also produced in this reaction [24].
The world is in search of sustainable green energy to prepare for the energy crisis in the near future. Currently, energy crisis is a serious problem related to sustainable human development. This booming requirement for biomass fuel has forced the search for other means of producing energy derived from renewable resources in order to fulfill energy demand [25]. Biomass had been used in rural areas as a leading source of energy for centuries [26]. In the past, biomass has been the fourth largest source of energy in the world with 10–14 % of total energy consumption, with coal by 12–14 %, natural gas by 14–15 %, and electricity by 14–15 % [25].
Bioenergy can be obtained from all non-fossil biological materials. As a matter of fact, bioenergy is the key to the global energy crisis. It has the ability to countervail greenhouse gases (GHG) [25].
This study emphasizes on preparing hydrochar from dead leaves under different carbonization temperature conditions. The primary objective of this study was to calculate the energy generation potential of hydrochar along with energy densification and energy yield, while the secondary objective was to analyze characteristic properties of hydrochar.
Materials and methods
Material
Dead leaves were gathered in the autumn from different places in Seoul, Republic of Korea. Leaves were crushed and passed through a series of sieves to obtain particle smaller than 2 mm as shown in Fig. 1. Biomass was dried for 24 h in an oven at 105 °C before the carbonization process [26].
Hydrothermal carbonization
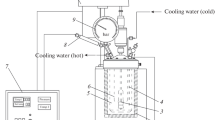
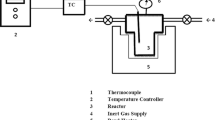
Hydrochar was produced by adding 7 g of feedstock dissolve in 35 ml of hot deionized water with a ratio of 1:5 (w/v). Hydrochar was made in a laboratory scale stainless steel reactor having approximately a working volume of 200 ml, length of 26.5 cm, inner radius of 5 cm, and outer radius of 8 cm. The gases generated during the carbonization process were collected with the help of a gas valve attached to the lid of the reactor. Preliminary feedstock was manually stirred with hot distilled water to verify proper wetting. Biomass was heated at different temperatures from 200 to 250 °C for 30 min. After carbonization at a specific temperature, the reactor was drawn out from the oven and was placed in water for quenching the reaction, and subsequent to cooling to 15 °C, the gases were released for safe opening [27]. Reactor was opened to acquire liquid and solid products. Liquid and solid products were sundered by vacuum filtration. Hydrochar was washed with acetone and was placed in oven at 105 °C for 2 h. All hydrochar samples were analyzed to their physiochemical characteristics.
Analysis
All the hydrochar samples were analyzed for yield, ash content, pH, electronic conductivity (EC), elementary analysis, cation exchange capacity (CEC), Brunauer–Emmett–Teller (BET) surface area, energy yield, energy content, and energy densification ratio.
The ash content of all hydrochar was deliberated by weight loss after placing them in the furnace at 600 °C for 3 h. Samples were drawn out and weighed after cooling [28]. The ash content was drawn out and was expressed as percentage of total hydrochar weight.
The pH and EC were determined at the same time. 1 g of hydrochar was mixed with 20 ml of distilled water. Solution was oscillated and equilibrated for 1.5 h before analysis. Upon completion, pH and EC were measured with a digital meter.
The yield of hydrochar was derived from the amount of biomass.
Elementary analyzer (ThermoElectron Flash EA 1112) was used to measure C, H, N, S and O present in hydrochar. All samples were weighed in a tin solid capsule and then dropped into a reactor where oxidation/reduction reaction took place at a temperature between 900 and 1000 °C. Oxygen was calculated by difference.
The CEC of hydrochar was determined by using ammonium acetate, sodium chloride and ethanol replacement methods [29]. 1 g of hydrochar was centrifuged with 20 ml of ammonium acetate for 10 min. Solid and liquid fractions were separated by vacuum filtration. Solid hydrochar was placed in a 20 ml solution of sodium chloride and was re-filtrated. The solution was kept for analyses. Liquid Kjeldahl was used for measuring CEC.
BET surface areas of all hydrochar samples were analyzed by nitrogen BET method [30]. Surface area was derived by nitrogen adsorption. Surface area of hydrochar was calculated using the BET equation [31]. Pore volume and average pore diameter were also calculated.
The advance bomb calorimeter CAL2K was used to measure the energy content of dried hydrochar. Benzoic acid was used as a standard. 1 g of hydrochar was inserted into the bomb and was ignited in the presence of oxygen [32].
The equation for energy densification ratio and energy yield is given as:
Results and discussion
Physiochemical properties of hydrochar
All the physicochemical properties of hydrochar are shown in Table 1. Analyses were performed to determine the effect of high temperature on the characteristics of hydrochar along with the energy yield of hydrochar derived from dry leaves. Temperature has a larger effect on hydrochar in contrast to retention time [10], therefore 30 min of retention time and temperature from 200 to 250 °C was selected. Each experiment was conducted in triplicate. By increasing temperature, the yield of a hydrochar will decrease while the ash content will increase [33–35]. The ash content gradually started to increase as the temperature was raised, ranging from 11.08 % to almost 21 %. The highest ash content of 21.04 % was observed at 220 °C with 89.89 % increase. At 200 °C, the maximum yield of 70.98 % was achieved, which started to decrease with further increase in temperature. The lowest yield of 57.39 % was analyzed at 250 °C, which shows 13.59 % of decrease in yield of hydrochar after heating to 250 °C. The relation of hydrochar yield and ash content is shown in Fig. 2. High carbonization temperature can increase the pH value of hydrochar [20, 21]. The pH of feedstock was 4.34, which increased to 5.45 when heated at 250 °C. The high pH hydrochar helps to offset soil acidity [14], which shows greater use of hydrochar in soil. As the temperature increased, EC decreased markedly from 1893 to 747 µs/cm. CEC also decreased with increase in temperature. The greater value of CEC has a positive effect on soil improvement which helps reduce leaching from the soil of subtropical regions [20]. Gaskin et al. [29] reported the reduction of CEC value with increase in carbonization temperature, which was due to loss of acidic functional groups. The surface area of hydrochar showed great variation. Surface area of the feedstock was 1.37 m2/g and after treatment at 250 °C, it increased to 2.09 m2/g, with 52 % of increase in surface area of hydrochar. The increase in surface area is due to volatilization and depletion of organic compounds during carbonization, which makes a vacuum in the hydrochar matrix [21]. Carbonization produce porosity in hydrochar samples, which is the main physical feature when hydrochar is applied in soil processes. There are 3 categories of pores depending upon their internal diameter, named as micropores (less than 2 nm), mesopores (From 2 to 50 nm), and macropores (larger than 50 nm) [36]. Pore diameters of all the hydrochar samples are given in Table 1, which clearly indicate that all are mesopores in nature. The mesopores on hydrochar helps to increase water holding capacity and preserve the moisture content in soil [37]. Feedstock was having pore diameter of 8.26 nm which begin to increase on heating. The highest was reported at 220 °C of 21.79 with 163 % of increase. At 220 °C pore volume was also highest of 9.86 × 10−3. Scanning electron microscope (SEM) of hydrochar sample is given in Fig. 3.
Elementary analysis
Elementary analysis of all hydrochar products is given in Table 2. Carbonization process of biomass involves condensation, decarboxylation and dehydration reaction, which effect in the reduction of oxygen, hydrogen and carbon. The loss of oxygen is of great significance and much useful, since it is responsible for increase in energy content [38]. Carbon content started to increase gradually when the temperature was raised while slight drop in oxygen content was observed. The highest carbon content was of 44.58 % at 240 °C. Nitrogen was found in very minor quantity. Sulfur was not detective because of its minor amount in lignocellulosic feedstock. The feedstock had 4.86 % of hydrogen, which started to increase when heated. Higher value of hydrogen of 5.54 % was observed at 210 °C. Loss of oxygen was seen when the feedstock was heated (Table 2).
Energy content and energy yield of hydrochar
The energy content of all, the HTC hydrochar samples is given in Table 3. Energy content is the amount of energy stored in a material. Increase in temperature has a direct effect on energy content of hydrochar. The energy content of dry feedstock is 16.42 MJ/kg. At 200 °C with reaction time of 30 min, the energy content value raised to 16.81 MJ/kg. The hydrochar energy content started to increase as temperature exceeded 200 °C. The highest energy content of 19.98 MJ/kg was observed at 240 °C, with an increase of 21 % compared to that of the feedstock. There was a slight decline in the energy content when heated beyond 240 °C. Several researchers have mentioned the increase in energy content with increase in temperature. Energy content showed 39 % increase by using loblolly pine [38], while 30–32 MJ/kg high energy content hydrochar was obtained by using microalgae in the HTC process [6].
It is also useful to calculate energy yield and energy densification. The energy densification can be defined as the ratio of energy content of hydrochar and feedstock. The energy densification of hydrochar samples is shown in Table 3, which explains that energy densification also increase with increase in temperature (Fig. 4). The minimum energy densification of 1.15 was at 200 °C, while the maximum of 1.36 was at 240 °C.
The energy yield of a hydrochar can be explained as the cross-product of yield of hydrochar and energy densification ratio. At 210 °C, the energy yield was 76 %, while the maximum hydrochar energy yield of 91.67 % was obtained at 240 °C. Because of the high values of energy content and energy densification values at 240 °C, maximum energy yield was also achieved at 240 °C.
Conclusion
The HTC carbonization of dry leaves solid hydrochar possesses relatively high the energy content compared to raw material. There was a significant increase in pore diameter of hydrochar from 8.26 to 21.79 nm, so it can be concluded that high-temperature hydrochar increase the pore diameter which can be more beneficial in holding moisture in soil and to increase water holding capacity as compared to raw feedstock. The energy content along with energy yield was also increased, despite the fact that the mass of yield was decreased. Highest energy content was obtained at 240 °C, which is about 21 % higher than that of the feedstock. Carbon content was increased from 41.35 to 44.58 %, while oxygen content was reduced. BET analysis also indicated an increase of surface area, and the maximum of 2.09 m2/g was obtained when heated at 250 °C. Therefore, it can be concluded that dry leaves can be used to obtain high-energy content hydrochar, which could be used for different energy purposes.
References
Ling PX, Zheng JS, Feng X, Run CS (2012) Hydrothermal carbonization of lignocellulosic biomass. Bioresour Technol 118:619–623
Lu X, Flora RVJ, Berge ND (2014) Influence of process water quality on hydrothermal carbonization of cellulose. Bioresour Technol 154:229–239
Dong R, Zhang Y, Chrisitianson LL, Funk TL, Wang X, Wang Z (2009) Product distribution and implication of hydrothermal conversion of swine manure at low temperatures. Trans ASABE 52(4):1239–1248
Peterson AA, Vogel F, Lachance RP, Froling M, Antal MJ, Tester JW (2008) Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies. Energy Environ Sci 1:32–65
Berge ND, Ro KS, Mao J, Flora JRV, Chappell MA, Bae S (2011) Hydrothermal carbonization of municipal waste streams. Environ Sci Technol 45(13):5696–5703
Heilmann SM, Davis HT, Jader LR, Lefebvre PA, Sadowsky MJ, Schendel FJ (2010) Hydrothermal carbonization of microalgae. Biomass Bio 34(6):875–882
Garcia AL, Torri C, Samori C, Vander SJ, Fabbri D, Kersten SRA (2012) Hydrothermal treatment (HTT) of microalgae: evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuel 26(1):642–657
Heilmann SM, Jader LR, Sadowsky MJ, Schendel FJ, Vonkeitz MG, Valentas KJ (2011) Hydrothermal carbonization of distiller’s grains. Biomass Bioenergy 35:2526–2533
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effect on soil biota—a review. Soil Biol Biochem 43:1812–1836
Hoekman SK, Broch A, Robbins C, Zielinska B, Felix L (2013) Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass Convers Biorefinery 3:113–126
Yan W, Acharjee TC, Coronella CJ, Vasquez VR (2009) Thermal pretreatment of lignocellulosic biomass. Environ Prog Sustain Energy 28(3):435–440
Oliveria L, Blohse D, Ramke HG (2013) Hydrothermal carbonization of agricultural residues. Bioresour Technol 142:133–146
Lehmann J, Downie A, Crosky A, Munroe P (2009) Biochar for environmental management science and technology. Earthscan, London, pp 13–32
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2007) Agronomic values of greenwaste biochar as a soil amendment. Aust J Soil Res 45(8):629–634
Lehmann J (2007) A handful of carbon. Nature 447:143–144
Zhao L, Cao X, Masek O, Zimmerman A (2009) Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J Hazard Mater 256–257:1–9
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44(4):1295–1301
Graber ER, Harel YM, Kolton M, Cytryn E, Silber A, David DR, Tsechansky L, Borenshtein M, Elad Y (2010) Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 337:481–496
Kloss S, Zehetner F, Dellantonio A, Hamid R, Ottner F, Liedtke V, Schwanninger M, Gerzabek MH, Soja G (2012) Characterization of slow pyrolysis biochars: effects of feedstocks and pyrolysis temperature on biochar properties. J Environ Qual 41(4):990–1000
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102(3):3488–3497
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impacts of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428
Singh R, Shukla A, Tiwari S, Srivastava M (2014) A review on delignification on lignocellulosic biomass for enhancement of ethanol production potential. Renew Sustain Energy Rev 32:713–728
Sanchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27(2):185–194
Nanda S, Mohammad J, Reddy SN, Kozinski JA, Dalai AK (2014) Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Conv Bioref 4:157–191
Mafakheri F, Nasiri F (2014) Modeling of biomass-to-energy supply operations: applications. Chall Res Dir Energy Policy 67:116–126
Lui Z, Balasubramanian R (2012) Hydrothermal carbonization of waste biomass for energy generation. Procedia Environ Sci 16:159–166
Parshetti GK, Hoekman SK, Balasubramanian R (2013) Chemical structural and combustion characteristics of carbonization of palm empty fruits bunches. Bioresour Technol 135:683–689
Guo YP, Rockstraw DA (2007) Activated carbons prepared from rice hull by one-step phosphoric activation. Microporous Mesoporous Matter 100:12–19
Gaskin JW, Stener C, Haris K, Das KC, Bibens B (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans Asabe 51(6):2061–2069
Lee Y, Park J, Ryu C, Gang KS, Yang W, Park YK, Jung J, Hyun S (2013) Comparison of Biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour Technol 148:196–201
Wang Y, Wang L, Fang GD, Herath HMSK, Cang L, Xie Z, Zhou D (2013) Enhanced PCBs sorption on biochars as affected by environmental factors: humic acid and metal cations. Environ Pollut 172:86–93
ISO 1928 Solid mineral fuels—determination of gross calorific value by the bomb calorimetric method, and calculation of net calorific value
Singh B, Singh BP, Cowie AL (2010) Characterisation and evaluation of biochars for their application as a soil amendment. Aust J Soil Res 48:516–525
Abdullah H, Wu H (2009) Biochar as a Fuel: 1. Properties and grindability of biochars produced from the pyrolysis of mallee wood under slow-heating conditions. Energy Fuel 23:4174–4181
Yan W, Jason T, Tapas C, Acharjee, Charles J, Coronella, Vaquez VR (2010) Mass and energy balance of wet torrefaction of lignocellulosic biomass. Energy Fuel 24:4738–4742
Rouquerol F, Rouquerol I, Sing K (1999) Adsorption by powders and porous solids. Academic Press, London, p 13
Theis JK, Rilling MC (2009) Characteristics of biochar, biological properties, biochar for environmental management sciences and technology. Earthscan, London, p 85
Hoekman SK, Broch A, Robbins C (2011) Hydrothermal carbonization (HTC) of Lignocellulosic biomass. Energy Fuel 25:1802–1810
Acknowledgments
This project was supported by Korea Ministry of Environment (MOE) as “The Eco-Innovation 21 project (2013000150004)”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saqib, N.U., Oh, M., Jo, W. et al. Conversion of dry leaves into hydrochar through hydrothermal carbonization (HTC). J Mater Cycles Waste Manag 19, 111–117 (2017). https://doi.org/10.1007/s10163-015-0371-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-015-0371-1