Abstract
In this study, we present a systematic characterization of hair cell loss and regeneration in the chicken utricle in vivo. A single unilateral surgical delivery of streptomycin caused robust decline of hair cell numbers in striolar as well as extrastriolar regions, which in the striola was detected very early, 6 h post-insult. During the initial 12 h of damage response, we observed global repression of DNA replication, in contrast to the natural, mitotic hair cell production in undamaged control utricles. Regeneration of hair cells in striolar and extrastriolar regions occurred via high rates of asymmetric supporting cell divisions, accompanied by delayed replenishment by symmetric division. While asymmetric division of supporting cells is the main regenerative response to aminoglycoside damage, the detection of symmetric divisions supports the concept of direct transdifferentiation where supporting cells need to be replenished after their phenotypic conversion into new hair cells. Supporting cell divisions appear to be well coordinated because total supporting cell numbers throughout the regenerative process were invariant, despite the initial large-scale loss of hair cells. We conclude that a single ototoxic drug application provides an experimental framework to study the precise onset and timing of utricle hair cell regeneration in vivo. Our findings indicate that initial triggers and signaling events occur already within a few hours after aminoglycoside exposure. Direct transdifferentiation and asymmetric division of supporting cells to generate new hair cells subsequently happen largely in parallel and persist for several days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inner ear sensory hair cells are essential for hearing and balance. Hair cells are situated in pseudostratified sensory epithelia interspersed with supporting cells in a mosaic-like fashion. In mammals, lost cochlear hair cells are not replaced, which is the major reason why most forms of hearing loss are permanent (Brigande and Heller 2009). Mammalian vestibular hair cells regenerate at very low frequency through asymmetric division of resident somatic stem cells (Li et al. 2003; Warchol et al. 1993) as well as via direct transdifferentiation (non-mitotic phenotypic conversion) of supporting cells. However, these mechanisms are insufficient to restore the full complement of cells (e.g., Golub et al. 2012; Wang et al. 2015). On the other hand, the regenerative response in the avian inner ear is robust and sufficient to restore both structure and function to auditory and vestibular organs (Corwin and Cotanche 1988; Ryals and Rubel 1988). Moreover, avian vestibular sensory epithelia mitotically turnover lost hair cells, albeit at a low rate (Jorgensen and Mathiesen 1988; Roberson et al. 1992).
There is strong evidence for two distinct mechanisms by which new hair cells are produced in regenerating avian sensory epithelia, both of which appear to originate with the supporting cell population. In response to hair cell loss, a supporting cell may either directly convert into a new hair cell or it may divide, producing two daughter cells which may then differentiate asymmetrically (one into a new hair cell and the other into a supporting cell) or symmetrically (both into supporting cells). Avian hair cell turnover/replacement and regeneration were initially shown through direct evidence of asymmetric divisions of supporting cells observed in vivo (Corwin and Cotanche 1988; Jorgensen and Mathiesen 1988; Roberson et al. 1992; Rubel et al. 2013; Ryals and Rubel 1988; Stone et al. 1999; Stone and Rubel 2000; Stone et al. 2004; Weisleder and Rubel 1993) and in organ cultures (Oesterle et al. 1993). In addition to mitotic regeneration, direct transdifferentiation of supporting cells into hair cells is argued to be a significant regenerative mechanism in the chicken cochlea and utricle sensory epithelia (Adler et al. 1997; Adler and Raphael 1996; Cafaro et al. 2007; Roberson et al. 1996; Shang et al. 2010, reviewed in Stone and Cotanche 2007). Direct transdifferentiation entails a replenishment response for lost supporting cells, which has not yet been systematically investigated. It has been hypothesized that direct transdifferentiation is a rapid restorative response, whereas asymmetric division of supporting cells is a more sustained mechanism (Roberson et al. 2004). In the chicken cochlea, the two processes occur at different locations and possibly also differ in their onset and duration whereas both mechanisms have not been systematically assessed in such extent in balance organs (Cafaro et al. 2007).
The molecular mechanisms that trigger the onset of avian hair cell regeneration by either process are unknown. Despite identification of signaling pathways potentially involved in hair cell regeneration (Alvarado et al. 2011; Ku et al. 2014; Warchol et al. 2017), a thorough mechanistic insight of regeneration from the earliest events of initiation, realization, and ultimately termination is not known. Moreover, in vivo experiments to investigate regeneration require repeated systemic injections of ototoxic aminoglycoside to induce reliable hair cell loss throughout the utricle (e.g., Cafaro et al. 2007), which makes it difficult to define the precise dosage or onset of injury. This creates a challenge to discriminate differences in timing of direct transdifferentiation and asymmetric division of supporting cells. To address this challenge, we describe here a single surgical application of ototoxic aminoglycoside that leads to robust hair cell loss in the chicken utricle. Our results are in general agreement with previous studies as well as with in vitro observations (Cafaro et al. 2007; Oesterle et al. 1993; Stone and Rubel 1999; Stone et al. 2004), but they reveal additional insight into the timing of responses after a temporally defined onset of the ototoxic insult. We show that significant hair cell loss is already detectable 6 h after aminoglycoside application, that mitotic turnover of hair cells throughout the utricle is globally suppressed during the initial 12 h post-insult, and that direct transdifferentiation of supporting cells into new hair cells as well as mitotic hair cell regeneration via asymmetric divisions of supporting cells occurs simultaneously over the course of several days beginning around 12 h after streptomycin application. Our data further indicate that the pace of symmetric divisions of supporting cells is well coordinated with an unknown mechanism that keeps supporting cell numbers and density relatively constant during the duration of the regenerative response. We conclude that the establishment of a temporally defined onset of aminoglycoside ototoxicity in vivo provides a framework for the refined study of the two modes of hair cell regeneration in the chicken utricle.
Material and Methods
Experimental Model
Fertilized Rhode Island Red chicken eggs were purchased from AA Lab eggs Inc., Westminster, CA. For all experiments, successfully hatched chickens were moved within 12 h after hatching into a brooder box equipped with a heat lamp, food, and water and housed for seven additional days. Post-hatch chickens were euthanized by CO2 inhalation. Animal procedures were approved by the Stanford University Institutional Animal Care and Use Committee (IACUC).
Surgical Delivery of Streptomycin
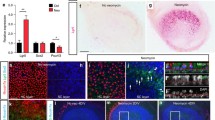
For unilateral surgical delivery of streptomycin into the vestibular promontory of post-hatch day chickens, we modified a previously reported approach (Dye et al. 1999; Zakir and Dickman 2006) (Fig. 1a). Anesthesia was conducted using a mouse nose cone and 1–3 % of isofluorane and an oxygen flow rate of 1 l/min. The level of anesthesia was monitored using pinch reflex, corneal reflex, and heart rate. Core temperature was maintained at 40 °C with a thermal pad. Surgical procedures were performed using aseptic technique with sterilized instruments, hardware, solutions, sponges, and drapes. After feather removal (Nair Hair Remover Cream), the skin was treated with diluted Betadine and alcohol (three rotations), and the animal was covered with Press’n Seal multipurpose sealing wrap. A small hole was made in the wrap to access the surgical area with care taken to ensure that no feathers occluded the opening. A small incision was made over the mastoid bone of the left ear (Fig. 1b). Microsurgery tools were used to create a small opening in the mastoid bone to expose the bony labyrinth with the lateral canal identified as landmark structure (Fig. 1c). Bony air cells were removed posterior to the round window and ventral of the lateral canal. An opening was made using a dental burr. The endosteum, a thin membrane that covers the inner surface of the bone and encloses perilymph, was kept intact as much as possible to minimize potential trauma caused by perilymph leakage (Fig. 1d). One to 2 μl of streptomycin (1–2 mg/μl in PBS) was slowly infused (Eppendorf, CellTram Vario microinjector) into the opening using a pulled glass capillary (25 μm in diameter) maneuvered into place with a micromanipulator (Eppendorf, Transferman NK2) (Fig. 1e). The opening was sealed with a muscle plug obtained from the exposed mastoid region and the removed mastoid bone was covered with the muscle tissue. The muscle layer was sutured with coated absorbable 4-0 Vicryl suture (Ethicon Inc.) and the skin was closed with 4-0 Ethilon suture (Ethicon, Inc.). After recovery, we ensured that the animals could stand and move freely. Unilateral vestibular hair cell ablation generally did not lead to inability to maintain upright posture after the surgery. At least three surgeries were conducted per time point.
In vivo single dose surgical ototoxic model for chickens. (a) Streptomycin was delivered unilaterally into the vestibular promontory of the chicken inner ear. Purple areas indicate the inner ear’s six main sensory epithelia. (b) The exposed mastoid bone of the left ear. (c) After opening the mastoid bone, the underlying tissues including the lateral canal were exposed. (d)A small opening was made to open the vestibule. The endosteum, a thin membrane that is attached to the inner surface of the vestibular bone, was kept intact to reduce potential trauma caused by leaking perilymph. (e) Streptomycin solution was slowly infused. (f) Quantification of supporting cells, hair cells, and EdU-labeled cells in whole utricles was performed on three selected extrastriolar (ES) and striolar (S) 10,000 μm2 regions along the anterior-posterior axis (red dashed squares indicate the typical positions of these sampling areas). Bp, basilar papilla. Sac, saccule. Utr, utricle. A, anterior. M, medial. P, posterior. c., canal. Amp, Ampullary cupulae.
Contralateral (uninjected) ears as well as PBS sham-injected ears were used as controls. In total, including pilot experiments, we performed 103 surgeries of which 75 (73 %) resulted in measurable signs of damage and regeneration. The utricles of 28 (27 %) animals were not affected by the surgical application of streptomycin and were considered as failed surgeries. These animals were not included in the study. We also assessed damage in the basilar papilla and found that this particular surgical application of streptomycin resulted in variable damage in this organ with 50 % of specimens showing hair cell loss and evidence of supporting cell divisions and hair cell regeneration. Thirty-two percent of basilar papillae had no lesion, whereas 18 % showed more severe damage. This suggests that the extent of basilar papilla damage is difficult to control using the described surgical approach.
EdU Injections
5-Ethynyl-2′-deoxyuridine (EdU, 100 mg/kg in PBS) was administered subcutaneously 0 h and 1, 2, 3, and 4 days post-streptomycin. Utricles were dissected 24 h after EdU administration. For the 6 h time point, utricles were dissected 6 h after EdU injection. For 10 day intervals, EdU was administered 12 h and 1, 2, and 3 days post-streptomycin and utricles were dissected after 10 days. EdU incorporation into DNA was detected using the Click-iT™ EdU Alexa Fluor 647 Imaging Kit (Thermo Fisher Scientific, C10340) in close alignment with a previously published protocol (Kaiser et al. 2009).
Immunocytochemistry
For whole utricle labeling after EdU detection, the otolithic membranes of utricles were carefully removed using an eyelash or a gentle fluid stream generated with a 1 ml syringe and attached 30G 1/2 hypodermic needle. Utricles were fixed with 4 % paraformaldehyde (Electron Microscopy Science) in PBS overnight, rinsed three times with PBS for 15 min each, permeabilized for 30 min with Triton-X-100 (1 % in PBS), and blocked for 3–4 h with a PBS-based blocking solution containing 1 % BSA, 5 % normal donkey serum, and 0.1 % Triton-X-100. Utricles were then incubated at 4 °C overnight in blocking solution with primary antibodies (Goat anti-SOX2 (1:100), Santa Cruz Biotechnology, sc-17320; rabbit anti MYO7A (1:1000), Proteus Biosciences, 25-6790). Specimens were rinsed three times for 15 min with 0.2 % Triton-X-100 in PBS and incubated at room temperature for 1–2 h in blocking solution with secondary antibodies (Alexa Fluor 488 donkey anti-goat (1:250), A11055; Alexa Fluor 546 donkey anti-rabbit (1:250), A10040, Thermo Fisher Scientific). 4,6-Diamidino-2-phenylindole (DAPI, 1 μg/ml) was used to visualize nuclei. Finally, utricles were rinsed three times for 15 min with 0.2 % Triton-X-100 in PBS, and three times for 15 min with PBS. FluorSave™ Reagent (Calbiochem) was used to mount up to three utricles on a glass slide using a Secure-Seal™ Spacer (single well, 13 mm diameter, 0.12 mm deep, Invitrogen).
Microscopy, Image Analysis, and Cell Counts
For each time point, at least three independent surgical specimens were used. Per specimen, at least three 10,000 μm2 areas (100 × 100 μm squares) for the striola and equally three extrastriolar regions (Fig. 1f) were assessed with a Zeiss LSM 880 laser scanning confocal microscope with AiryScan and Zeiss acquisition and analysis software (Zen Black). A Plan-Apochromat objective (40×/1.3 NA oil) was used with 2.2 × zoom setting, and optical sections were collected every 0.40 μm to generate z-stacks. The stack data was loaded into Volocity 6.3 (PerkinElmer) for 3D reconstruction and cell counts. Hair cell and supporting cell layers were defined based on overall morphology, cell alignment, and the generally obvious layering of marker-positive cells. We used SOX2 labeling to identify type II hair cells (MYO7A+/SOX2+) and type I hair cells (MYO7A+/SOX2−). SOX2+/MYO7A−-labeled supporting cells were readily distinguishable. EdU was used as a marker for cells that underwent DNA replication. DAPI labeling was used to obtain total cell numbers. Cells with pyknotic nuclei were considered as dying cells and not included in the analysis. We defined cell pairs based on close proximity, which was unequivocal. An asymmetric pair was defined as one supporting cell and one hair cell. A symmetric pair consisted of two supporting cells or theoretically two hair cells. We did not observe a symmetric pair of two hair cells in the entire analysis.
Statistical Analysis
All data are presented as mean ± 95 % confidence interval (95 % CI). P values were computed with unpaired Student’s t tests and controlled for multiple testing using the false discovery rate approach (q values; Benjamini and Hochberg 1995). For 10 days interval EdU experiments, proliferation indices were calculated by dividing the number of EdU-positive cells by all cells multiplied by 500 per 10,000 μm2 area. For 24 h interval EdU experiments, the number of EdU-positive cells were divided by the number of SOX2-positive supporting cells multiplied by 500 per 10,000 μm2 area. Analyses and chart generation were performed with GraphPad Prism 7 (GraphPad Software, Inc. La Jolla, CA).
Results
Hair Cell Loss and Recovery After Single Surgical Application of Streptomycin
Seven-day-old chickens received a single dose of 1–2 mg streptomycin into the perilymphatic space superior to the roof of the left utricle (Fig. 1). Utricles were dissected at various time points over a 13 day period after surgery and numbers of hair cells were quantified in striolar and extrastriolar regions (Fig. 2a, b). Small but significant hair cell loss was detectable already 6 h post-surgery in the striola and was most extensive after 24 and 48 h. Striolar regions were more substantially and robustly affected than extrastriolar regions. We used confocal imaging to visualize the hair cell layer of affected striolar sensory epithelia and observed considerable loss of MYO7A-immunopositive cells and sparse distribution of the remaining hair cells (Fig. 2c). In extrastriolar regions, hair cell loss was more variable and less pronounced, but still significant (Tables 1 and 2).
Dying and regenerating hair cells post-streptomycin. (a) Mean total hair cell numbers in the utricle striola of streptomycin-treated inner ears (orange) compared to untreated (black) and PBS-treated (blue) specimens. Counted were all hair cells labeled with antibodies to MYO7A. The fraction of SOX2-labeled type II (a’) and SOX2-negative type I hair cells (a”) of untreated, streptomycin-treated, and PBS-treated utricles is indicated for each time point. (b) Mean total hair cell numbers in extrastriolar regions. Error bars represent the 95 % confidence interval. *q ≤ 0.05, **q ≤ 0.01, ***q ≤ 0.001 (for additional details, see Tables 1 and 2). Representative confocal images of the hair cell layer of untreated and streptomycin-treated utricles 2 (c) and 13 days (d) after streptomycin application. Whole utricles and xy projections (10,000 μm2) of a representative striolar and an extrastriolar region are shown as indicated with squares marked S and ES, respectively. MYO7A (red) and SOX2 (green) immunolabeling was used to identify hair cells (types I and II), as well as supporting cells. DAPI (blue) was used to stain nuclei.
After the initial insult, we investigated recovery and regeneration of hair cells. In striolar regions, we found increasing numbers of hair cells 3 days post-surgery, followed by a steady increase over the course of additional 10 days. Thirteen days post-surgery, the striolar regions recovered to 77–78 % of the pre-insult as well as the control conditions. Extrastriolar regions, which were less prominently damaged, displayed a full complement of hair cells that was re-established between day 5 and day 10.5 post-surgery. At the latest time point analyzed, we found no obvious differences in extrastriolar hair cell density between controls and previously damaged utricles; in striolar regions, a noticeable difference in cell density between controls and surgery utricles remained (Fig. 2d).
The chicken utricle exclusively harbors type II hair cells in extrastriolar regions, while the striola consists of both type II and type I hair cells (Warchol and Speck 2007). Type II hair cells express SOX2 which is also found in all supporting cells, whereas type I hair cells lack SOX2 expression, which has been shown previously in mice (Hume et al. 2007). The undamaged striolar regions on average contained 30 % type I hair cells, ranging from 18 to 38 % (Tables 1 and 2). The number of type I hair cells was starkly reduced during the regenerative response period to the point that the fraction of type I hair cells was 0–7 % between 2 and 11 days after streptomycin application (Fig. 2a”, Tables 1 and 2). Twelve and 13 days after streptomycin application, we detected an increase of type I hair cells (MYO7A-positive and SOX2-negative) to a range between 9 and 18 %.
Supporting Cell Number Equilibrium After Ototoxic Insult and During Regeneration
The density of SOX2-labeled supporting cell nuclei was surprisingly invariant and did not significantly change immediately (6 h) and during the 13 days following the single streptomycin application (Fig. 3). Even in striolar regions where 24 h post-surgery, the hair cell density was reduced to 10 % of the control (Tables 1 and 2), we neither detected significant reduction of supporting cells nor qualitative changes in morphological organization (Figs. 3a and 4c).
Supporting cell numbers are not affected during regeneration. (a) Average numbers of SOX2-labeled supporting cells in the striola do not significantly change after streptomycin application when assessed at the time points indicated. (b) Likewise, no significant changes in supporting cell numbers were observed in extrastriolar regions. Orange indicates streptomycin-treated utricles, black represents untreated, and blue indicates PBS-treated utricles. Counted were SOX2-immunopositive cell nuclei in the supporting cell layer. Error bars represent the 95 % confidence interval of the mean. Additional details are shown in Tables 1 and 2.
Short interval EdU experiments reveal time course of cell cycle re-entry after damage. Quantification of EdU-labeled nuclei in the (a) striola and (b) extrastriolar regions post-streptomycin. To label DNA replication in cells, a single subcutaneous injection of EdU in PBS was administrated 24 h before analysis. For the 6 h and 1 day post-streptomycin time point, EdU was injected at time zero. When streptomycin-treated utricles (orange) were analyzed at 6 h and 1 day post-streptomycin, a significant decrease in the number of EdU-labeled nuclei was apparent in the striola as well as in extrastriolar regions compared with untreated (black) and PBS-treated (blue) utricles. Conversely, a significant increase of EdU-labeled cells in streptomycin-treated utricles in the striola as well as extrastriolar regions was observed between 2 and 5 days post-streptomycin when compared with untreated and PBS-treated utricles. Untreated and PBS-treated controls of 6 h and 3 days post-streptomycin showed no significant changes in the number of EdU-labeled cells. Cells labeled during this 24 h (6 h) EdU exposure were exclusively identified as SOX2-positive supporting cells. Error bars represent the 95 % confidence interval of the mean. **q ≤ 0.01, ***q ≤ 0.001 (for additional details, see Tables 1 and 2). Representative confocal images of the supporting cell layers of untreated and streptomycin-treated utricles 1 (c) and 3 days (d) post-streptomycin. Whole utricles and xy projections (10,000 μm2) of a representative striolar and an extrastriolar region are indicated with squares labeled S and ES, respectively. SOX2 (green) immunolabeling was used to identify supporting cells, DAPI (blue) stain was used to visualize cell nuclei, and EdU labeling (white) identified nuclei with genomic DNA that underwent replication. Notably, no EdU- and MYO7A-double-labeled hair cells were detected.
Supporting Cell DNA Replication Is Initially Suppressed After Ototoxic Insult Followed by a Robust Proliferative Response
We used the thymidine analog EdU to quantify S-phase re-entry after streptomycin application. Our experiments confirmed an observation originally reported by Matsui and colleagues (Matsui et al. 2000) using cultured chicken utricles, which showed initial global reduction of EdU incorporation in the striolar as well as extrastriolar regions (Fig. 4a–c). Compared to untreated and PBS-treated controls, we observed a significant decrease of EdU-labeled supporting cells during the initial 6 h and during the first 24 h post-surgery. This reduction was in stark contrast to a considerable increase in EdU-labeled supporting cells after 24 h (Fig. 4d shows an example for S-phase re-entries between 2 and 3 days). Although the response appeared to be the highest in striolar regions between 1 and 2 days and between 2 and 3 days in extrastriolar regions, we found continuous high levels of DNA replication during a phase stretching from 24 h post-surgery until at least 5 days after the insult. This suggests that cell regeneration in the utricle in vivo is sustained for multiple days after hair cell loss. In these 24 h labeling experiments, we observed EdU incorporation only in SOX2-positive supporting cells but not in MYO7A-positive hair cells (27 streptomycin-treated utricles, 34 controls, and 7 PBS-treated controls), suggesting that MYO7A upregulation in new hair cells generated from SOX2-expressing supporting cells requires more than 24 h.
Fates of Newly Generated Cells
The 24 h labeling experiments indicated that supporting cells begin with DNA replication 1 day after streptomycin application, but this analysis was not designed to reveal the fates of newly generated cells. Upregulation of marker genes and cell differentiation happen within a week when assessed in cultured damaged utricles (Ku et al. 2014; Matsui et al. 2000), and we hypothesized that 10 days would be a reasonable time to reveal the fates of new mitotically generated cells. We first assessed cells generated due to natural turnover/production in utricles without ototoxic insult, analyzed 10 days after EdU injections, which happened from post-hatch days 7.5–10. Mitotically generated new cells were solely generated via asymmetric divisions of supporting cells in striolar as well as extrastriolar regions (Fig. 5a–c, untreated). Asymmetric fates were discernable by occurrence of EdU-positive cells organized as closely situated pairs of a single MYO7A-expressing cell with hair cell morphology in the hair cell layer and an accompanying SOX2-immunopositive supporting cell located in the supporting cell layer (Fig. 5c, xz projection). This observation confirms that new hair cells in the post-hatch chicken utricle are generated by asymmetric cell divisions (Roberson et al. 1992) and that this hair cell generation is not associated with specific regions of cell production, but rather evenly distributed throughout the damaged regions (Fig. 5c).
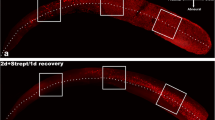
Long interval EdU experiments allow cell fate determination during regeneration. A single subcutaneous injection of EdU in PBS was administrated 10 days before each analysis. Asymmetric pairs of EdU-labeled nuclei were defined as pairs consisting of one supporting cell and one hair cell juxtaposed in the supporting cell and hair cell layers, respectively. Symmetric pairs of EdU-labeled nuclei were defined as closely positioned supporting cells. Quantification of asymmetric and symmetric pairs in the striola (a) and extrastriolar (b) regions post-streptomycin. Counts from streptomycin-treated utricles are represented as orange bars compared to untreated utricles shown as black bars. Shown are the average counts from at least three independent specimens; error bars represent the 95 % confidence interval of the mean. *q ≤ 0.05, **q ≤ 0.01, ***q ≤ 0.001 (for additional details, see Tables 1 and 2). Representative confocal images of supporting cell (SC) and hair cell (HC) layers of untreated (c) and streptomycin-treated (d) utricles 11 days after streptomycin application. Whole utricles, xy projections (10,000 μm2), and x-z projections of the striola and extrastriolar regions are shown. MYO7A (red) was used to identify hair cells, SOX2 (green) was used to identify supporting cells and to distinguish hair cell subtypes, DAPI (blue) stain visualized all nuclei, and EdU-labeled nuclei indicative of S-phase re-entry are shown in white. Generally, we observed mitotic pairs of EdU-labeled nuclei. Examples of asymmetric (arrow) and symmetric (arrowhead) pairs are indicated in the xz projections.
Streptomycin-induced hair cell loss elicited increased asymmetric as well as symmetric cell divisions. In striolar regions, we found that most new hair cells were generated from asymmetrically dividing supporting cells that were already marked with a single systemic EdU injection 12 h post-surgery (Fig. 5a; the 10.5 day time point represents the 12 h EdU injection; 73 % of new hair cells at this time point arose from asymmetric divisions, Tables 1 and 2). Symmetrically dividing supporting cells were also first labeled by the 12 h post-surgery EdU injection, but more robustly occurred 1, 2, and 3 days after streptomycin application (Fig. 5a, d). Based on the observation that symmetric supporting cell divisions mostly occurred after hair cell loss and that the density of SOX2-positive supporting cells did not differ from controls (Fig. 3), we conclude that supporting cells that directly transdifferentiated into new hair cells are replenished by symmetric divisions. Consequently, the number of symmetric EdU-positive pairs may serve as an indirect measure of the rate of direct transdifferentiation of supporting cells into new hair cells.
In extrastriolar regions, we detected a similar response and an apparent temporal decoupling of the onsets of cell divisions that result in asymmetric new hair cell regeneration versus symmetric supporting cell divisions. At 12 h post-surgery, the fate of newly generated EdU-labeled cells was almost entirely trackable to asymmetrically dividing supporting cells (Fig. 5b, 10.5 day time point; 97 % of new hair cells at this time point arose from asymmetric divisions, Tables 1 and 2); almost no symmetric pairs of supporting cells were detected at this early time point. This pronounced difference might be due to the milder damage of extrastriolar regions, or a true difference in the response pattern of the different utricle regions. At days 1, 2, and 3 post-surgery, we observed slightly lower and decreasing numbers of asymmetrically generated new hair cells, 85, 82, and 74 %, respectively—accompanied by increased numbers of supporting cells generated through symmetric divisions (Fig. 5b, d, Tables 1 and 2). We never observed unambiguous pairs of EdU-labeled and MYO7A-positive hair cells denoting absence of symmetric generation of two hair cells from a single cell division.
Discussion
Our goal was to quantitatively characterize the in vivo regenerative response of post-hatch chicken utricle sensory epithelia to a single application of aminoglycoside antibiotic. We aimed to reveal a time course of distinguishable damage and regeneration-related events such as direct transdifferentiation and asymmetric divisions of supporting cells that take place in the two major anatomical regions of the utricle sensory epithelium. Our results demonstrate that surgical application of a single aminoglycoside dose into the chicken vestibular promontory is a feasible approach to elicit hair cell loss and regeneration.
Global Suppression of Baseline DNA Replication During the First 12 h Post-Damage
Our EdU-labeling experiments revealed a baseline rate of asymmetric supporting cell divisions leading to new hair cell production in the undamaged post-hatch chicken utricle. In response to streptomycin application, we found a significant initial decrease of baseline DNA replication throughout the utricle (Fig. 6a). Cessation of cell cycle re-entry in response to aminoglycoside treatment has been previously reported in cultured chicken utricles (Matsui et al. 2000). We found that this suppression also happens in vivo where it occurred throughout the utricle sensory epithelium. This suggests a global signal that arises after aminoglycoside application and suppresses the baseline generation of new hair cells. Our 12-h-to-10.5-day EdU cell fate experiments suggest that cell cycle re-entry and increased EdU incorporation occur as early as 12 h post-surgery, which suggests a time window for global suppression within the first 12 h after streptomycin application. We can only speculate about the origin and nature of such a signal, which could simply be a direct effect of aminoglycoside on supporting cells or a paracrine acting signal released by damaged hair cells affecting the whole utricle sensory epithelium, among other possibilities. Certain potential hair cell-originating signaling molecule candidates have been identified in avian utricle culture after aminoglycoside treatment (Alvarado et al. 2011; Ku et al. 2014; Tsue et al. 1994), although a possible mechanistic link of any candidate to the initial suppression of S-phase re-entry remains to be established.
Orchestration of chicken hair cell regeneration. (a) Within a few hours after aminoglycoside-induced damage (red), mitotic turnover is globally suppressed. This suppressive period may serve as one of the earliest responses during the first steps of regeneration (blue). (b) Mitotic hair cell regeneration via asymmetric divisions of supporting cells and phenotypic conversion/transdifferentiation of supporting cells into new hair cells may occur largely in parallel. (c) We hypothesize that transdifferentiating supporting cells are being replenished by symmetric divisions of supporting cells. Illustrations were inspired by Burns and Corwin (2013) and Meyers et al. (2009)
Asymmetric Division of Supporting Cells Is the Main Regenerative Response to Aminoglycoside Damage
With the established damage paradigm of ≈ 90 % hair cell loss in striolar regions and up to 72 % loss of extrastriolar hair cells, we found that the predominant mode of hair cell regeneration in the chicken utricle in vivo is asymmetric division of supporting cells, which agrees with previous notions (Roberson et al. 1992; Stone et al. 1999; Stone et al. 2004). In striolar regions, we estimate that initially up to 73 % of hair cells are regenerated via asymmetric divisions whereas in extrastriolar regions, this ratio initially reaches 97 %. These high rates of asymmetric divisions gradually decline over the course of the first 3 days post-damage to 47 % in the striola and 74 % in extrastriolar regions. Our calculations are based on our EdU cell fate experiments and do not include cells that re-entered the cell cycle after 4 days post-damage, when symmetric supporting cell divisions might become more prevailing. Nevertheless, we conclude from our experiments that most utricle supporting cells that respond to aminoglycoside-induced hair cell loss are re-entering the cell cycle and asymmetrically generate a hair cell and a supporting cell. This response behavior is typical for a somatic stem cell and raises the question whether the majority of supporting cells in the post-hatch chicken utricle are facultative somatic stem cells (Grompe 2012). We should note that our analyses assume that the bioavailability of EdU after a single systemic injection is less than 24 h. Pharmacokinetic studies for EdU and BrdU in rodents estimate half-lives of these tracers of a few hours, based on serum levels (Cheraghali et al. 1994; Matiašová et al. 2014). In the newborn mouse utricle, the bioavailability of systemically injected EdU has been directly determined to be 1 h or less (Burns et al. 2012). The EdU-labeling results shown in our study suggest a bioavailability of EdU of less than 12 h, which is a conclusion based on the timing between suppression of cell cycle re-entry (Fig. 4a, b; 6 h and 1 day) and the increase of responding cells observed 12 h post-streptomycin (Fig. 5a, b; 10.5 days).
Symmetric Division Replenishes Supporting Cells That Directly Convert into Hair Cells
Of all cell divisions observed in striolar regions between 12 h and 3–4 days post-streptomycin, less than 40 % resulted in symmetric pairs of SOX2-expressing supporting cells. This proportion was lower in extrastriolar regions, where it accounted for only 16 % of all tracked divisions. We interpret the occurrence of symmetric divisions of supporting cells as a replenishment mechanism, which in turn is an indirect measure of the extent of direct transdifferentiation of supporting cells into new hair cells. We also observed that the proportion of symmetric divisions of supporting cells increased between the second and third day post-damage, which suggests that replenishment of supporting cells will potentially be more prevalent toward the termination of the regenerative response. This interpretation is based on the observation that symmetric divisions of supporting cells are strongly increased after streptomycin-induced hair cell loss and our finding that the density of supporting cells during the whole regenerative time period is not changing. We cannot directly measure the rate of direct transdifferentiation, and consequently, we cannot correlate this measure with symmetric supporting cell divisions. As a result, hair cell regeneration in the chicken utricle via direct transdifferentiation remains a concept that requires direct proof.
The different ratios of asymmetric versus symmetric divisions between the striolar and extrastriolar regions suggest that the rate of direct transdifferentiation in these distinct areas is either generally different or that the differences are linked to the higher hair cell loss in striolar regions. In the latter scenario, the rate of supporting-cell-to-hair-cell phenotypic conversion would be proportionally linked to the degree of damage, which could be tested by the establishment of a refined dose-response paradigm.
Our results reveal an interesting insight into the precise orchestration of hair cell regeneration in the utricle. It appears that phenotypic conversion of supporting cells into hair cells and asymmetric division of supporting cells into hair cells and supporting cells get initiated largely in parallel between 12 and 24 h after streptomycin-induced hair cell loss. Replenishment of supporting cells that converted into new hair cells commences with a delay of a few hours in striolar regions and at least for another 12 h in extrastriolar regions. Throughout these processes, the overall supporting cell number and density in all regions of the utricle remain constant. This suggests that the individual events comprising the regenerative response in the utricle are inter-coordinated with respect to onset, extent, and speed. We anticipate that this coordination requires global and local crosstalk. Our study contributes to setting a frame work for future in vivo investigations of the concerted actions that happen in the regenerating chicken utricle in response to aminoglycoside damage.
Change history
03 January 2018
This article was updated to correct a formatting error in Table 1.
07 May 2018
This article was updated to correct a formatting error in Table��1.
References
Adler HJ, Raphael Y (1996) New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett 205:17–20
Adler HJ, Komeda M, Raphael Y (1997) Further evidence for supporting cell conversion in the damaged avian basilar papilla. Int J Dev Neurosci 15:375–385
Alvarado DM et al (2011) An RNA interference-based screen of transcription factor genes identifies pathways necessary for sensory regeneration in the avian inner ear. J Neurosci 31:4535–4543. https://doi.org/10.1523/JNEUROSCI.5456-10.2011
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 57:289–300
Brigande JV, Heller S (2009) Quo vadis, hair cell regeneration? Nat Neurosci 12:679–685. https://doi.org/10.1038/nn.2311
Burns JC, Corwin JT (2013) A historical to present-day account of efforts to answer the question: “what puts the brakes on mammalian hair cell regeneration?”. Hear Res 297:52–67. https://doi.org/10.1016/j.heares.2013.01.005
Burns JC, On D, Baker W, Collado MS, Corwin JT (2012) Over half the hair cells in the mouse utricle first appear after birth, with significant numbers originating from early postnatal mitotic production in peripheral and striolar growth zones. J Assoc Res Otolaryngol 13:609–627. https://doi.org/10.1007/s10162-012-0337-0
Cafaro J, Lee GS, Stone JS (2007) Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn 236:156–170. https://doi.org/10.1002/dvdy.21023
Cheraghali AM, Knaus EE, Wiebe LI (1994) Bioavailability and pharmacokinetic parameters for 5-ethyl-2′-deoxyuridine. Antivir Res 25:259–267
Corwin JT, Cotanche DA (1988) Regeneration of sensory hair cells after acoustic trauma. Science 240:1772–1774
Dye BJ, Frank TC, Newlands SD, Dickman JD (1999) Distribution and time course of hair cell regeneration in the pigeon utricle. Hear Res 133:17–26
Golub JS, Tong L, Ngyuen TB, Hume CR, Palmiter RD, Rubel EW, Stone JS (2012) Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J Neurosci 32:15093–15105. https://doi.org/10.1523/JNEUROSCI.1709-12.2012
Grompe M (2012) Tissue stem cells: new tools and functional diversity. Cell Stem Cell 10:685–689. https://doi.org/10.1016/j.stem.2012.04.006
Hume CR, Bratt DL, Oesterle EC (2007) Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns 7:798–807. https://doi.org/10.1016/j.modgep.2007.05.002
Jorgensen JM, Mathiesen C (1988) The avian inner ear. Continuous production of hair cells in vestibular sensory organs, but not in the auditory papilla. Naturwissenschaften 75:319–320
Kaiser CL, Kamien AJ, Shah PA, Chapman BJ, Cotanche DA (2009) 5-Ethynyl-2′-deoxyuridine labeling detects proliferating cells in the regenerating avian cochlea. Laryngoscope 119:1770–1775. https://doi.org/10.1002/lary.20557
Ku YC, Renaud NA, Veile RA, Helms C, Voelker CC, Warchol ME, Lovett M (2014) The transcriptome of utricle hair cell regeneration in the avian inner ear. J Neurosci 34:3523–3535. https://doi.org/10.1523/JNEUROSCI.2606-13.2014
Li H, Liu H, Heller S (2003) Pluripotent stem cells from the adult mouse inner ear. Nat Med 9:1293–1299
Matiašová A, Sevc J, Mikeš J, Jendželovský R, Daxnerová Z, Fedoročko P (2014) Flow cytometric determination of 5-bromo-2′-deoxyuridine pharmacokinetics in blood serum after intraperitoneal administration to rats and mice. Histochem Cell Biol 142:703–712. https://doi.org/10.1007/s00418-014-1253-7
Matsui JI, Oesterle EC, Stone JS, Rubel EW (2000) Characterization of damage and regeneration in cultured avian utricles. J Assoc Res Otolaryngol 1:46–63
Meyers JR, Hu Z, Lu Z, Corwin JT (2009) Hair cell regeneration. In: Squire LR (ed) Encyclopedia of neuroscience, Academic Press, Oxford, pp 1005–1013
Oesterle EC, Tsue TT, Reh TA, Rubel EW (1993) Hair-cell regeneration in organ cultures of the postnatal chicken inner ear. Hear Res 70:85–108
Roberson DF, Weisleder P, Bohrer PS, Rubel EW (1992) Ongoing production of sensory cells in the vestibular epithelium of the chick. Hear Res 57:166–174
Roberson DW, Kreig CS, Rubel EW (1996) Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Audit Neurosci 2:195–205
Roberson DW, Alosi JA, Cotanche DA (2004) Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res 78:461–471. https://doi.org/10.1002/jnr.20271
Rubel EW, Furrer SA, Stone JS (2013) A brief history of hair cell regeneration research and speculations on the future. Hear Res 297:42–51. https://doi.org/10.1016/j.heares.2012.12.014
Ryals BM, Rubel EW (1988) Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240:1774–1776
Shang J, Cafaro J, Nehmer R, Stone J (2010) Supporting cell division is not required for regeneration of auditory hair cells after ototoxic injury in vitro. J Assoc Res Otolaryngol 11:203–222. https://doi.org/10.1007/s10162-009-0206-7
Stone JS, Cotanche DA (2007) Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol 51:633–647. https://doi.org/10.1387/ijdb.072408js
Stone JS, Rubel EW (1999) Delta1 expression during avian hair cell regeneration. Development 126:961–973
Stone JS, Rubel EW (2000) Temporal, spatial, and morphologic features of hair cell regeneration in the avian basilar papilla. J Comp Neurol 417:1–16
Stone JS, Choi YS, Woolley SM, Yamashita H, Rubel EW (1999) Progenitor cell cycling during hair cell regeneration in the vestibular and auditory epithelia of the chick. J Neurocytol 28:863–876
Stone JS, Shang JL, Tomarev S (2004) cProx1 immunoreactivity distinguishes progenitor cells and predicts hair cell fate during avian hair cell regeneration. Dev Dyn 230:597–614. https://doi.org/10.1002/dvdy.20087
Tsue TT, Oesterle EC, Rubel EW (1994) Diffusible factors regulate hair cell regeneration in the avian inner ear. Proc Natl Acad Sci U S A 91:1584–1588
Wang T et al (2015) Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat Commun 6:6613. https://doi.org/10.1038/ncomms7613
Warchol ME, Speck JD (2007) Expression of GATA3 and tenascin in the avian vestibular maculae: normative patterns and changes during sensory regeneration. J Comp Neurol 500:646–657. https://doi.org/10.1002/cne.21153
Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT (1993) Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 259:1619–1622
Warchol ME, Stone J, Barton M, Ku J, Veile R, Daudet N, Lovett M (2017) ADAM10 and γ-secretase regulate sensory regeneration in the avian vestibular organs. Dev Biol 428:39–51. https://doi.org/10.1016/j.ydbio.2017.05.014
Weisleder P, Rubel EW (1993) Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol 331:97–110. https://doi.org/10.1002/cne.903310106
Zakir M, Dickman JD (2006) Regeneration of vestibular otolith afferents after ototoxic damage. J Neurosci 26:2881–2893. https://doi.org/10.1523/JNEUROSCI.3903-05.2006
Acknowledgments
A special thank you to Stephanie Jensen (Stanford Veterinary Service Center) for her exceptional help and support in establishing the surgery protocol; to Jialin Shang (University of Washington) for assistance with tissue preparation; to J. David Dickman (Baylor) and Mark Warchol (Washington University) for consulting and enthusing discussions; and to Amanda Janesick, Giovanni H Diaz, and Byron H. Hartman for equally enthusing discussions and comments on the manuscript.
Funding
This work was supported by the Hearing Health Foundation’s Hearing Restoration Project and by the Stanford Initiative to Cure Hearing Loss. M.S. was supported in part by a fellowship (SCHM 2804/1-1) from the Deutsche Forschungsgemeinschaft; D.C.E. was supported in part by a Stanford Dean’s fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal procedures were approved by the Stanford University Institutional Animal Care and Use Committee (IACUC).
Additional information
This article was updated to correct a formatting error in Table 1.
Rights and permissions
About this article
Cite this article
Scheibinger, M., Ellwanger, D.C., Corrales, C.E. et al. Aminoglycoside Damage and Hair Cell Regeneration in the Chicken Utricle. JARO 19, 17–29 (2018). https://doi.org/10.1007/s10162-017-0646-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-017-0646-4