Abstract
The vestibulo-ocular reflex (VOR) is the main retinal image stabilising mechanism during rapid head movement. When the VOR does not stabilise the world or target image on the retina, retinal image slip occurs generating an error signal that drives the VOR response to increase or decrease until image slip is minimised, i.e. VOR adaptation occurs. Visual target contrast affects the human smooth pursuit and optokinetic reflex responses. We sought to determine if contrast also affected VOR adaptation. We tested 12 normal subjects, each over 16 separate sessions. For sessions 1–14, the ambient light level (lx) during adaptation training was as follows: dark, 0.1, 0.2, 0.3, 0.5, 0.7, 1, 2, 8, 16, 32, 64, 128 and 255 lx (light level for a typical room). For sessions 15–16, the laser target power (related to brightness) was halved with ambient light at 0 and 0.1 lx. The adaptation training lasted 15 min and consisted of left/right active head impulses. The VOR gain was challenged to increment, starting at unity, by 0.1 every 90 s for rotations to the designated adapting side and fixed at unity towards the non-adapting side. We measured active and passive VOR gains before and after adaptation training. We found that for both the active and passive VOR, there was a significant increase in gain only towards the adapting side due to training at contrast level 1.5 k and above (2 lx and below). At contrast level 261 and below (16 lx and above), adaptation training resulted in no difference between adapting and non-adapting side gains. Our modelling suggests that a contrast threshold of ~ 1000, which is 60 times higher than that provided by typical room lighting, must be surpassed for robust active and passive VOR adaptation. Our findings suggest contrast is an important factor for adaptation, which has implication for rehabilitation programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vestibulo-ocular reflex (VOR) is the main retinal image stabilising mechanism during rapid head movement. During far viewing, the ideal VOR rotates the eyes in the opposite direction, but equal magnitude, of head velocity (gain = eye / head velocity). Classic studies in humans have shown that the VOR gain can change depending on viewing conditions, for example, when looking through magnifying lenses (Gauthier and Robinson 1975; Gonthor and Jones, 1976). The VOR gain changes (i.e. adapts) when visual-vestibular mismatch occurs. Under this circumstance, retinal image slip occurs, generating an error signal that drives the VOR gain to increase or decrease until image slip is minimised (Gauthier and Robinson 1975).
Studies examining the closely related smooth pursuit and optokinetic systems (e.g. Cohen et al. 1981; Büttner and Büttner-Ennever 2006), which like the VOR seek to stabilise images on the retina using an image slip error signal, have shown that visual contrast affects the perceived velocity of a stimulus and the visual following gain (= eye / target velocity). For example, in humans low contrast targets appear to move slower than high contrast ones (Thompson 1982 and Thompson 1983). The effect of contrast on smooth pursuit depends on the target speed. At low speeds, there is a linear increase in pursuit gain with increasing contrast (Spering et al. 2005). Whereas at high speeds (> 1°/s), there is a steep rise in gain as contrast rises two to three times above a threshold (Spering et al. 2005). Visual contrast also affects the slow-phase velocity of optokinetic nystagmus such that it increases with increasing contrast for a given speed (Sumnall et al. 2003). For both smooth pursuit and optokinetic nystagmus, contrast must pass a certain threshold for the detection of image motion. That threshold depends on the contrast sensitivity of the visual following system and changes depending on the target velocity and spatial frequency (Spoor et al. 2014; Leguire et al. 1991). As contrast increases, the internal estimate of stimulus speed reconstructed from prior retinal (movement of retinal image) and extra-retinal signals (efference copy and other proprioceptive cues) becomes more accurate (Waddington and Harris 2015).
The effects of visual target contrast on human VOR adaptation have not been studied. Presumably, there is a contrast threshold below which no adaptation occurs, which would be consistent with the findings in smooth pursuit and the optokinetic response. It is not clear, however, whether above this threshold the magnitude of VOR adaptation increases with increasing visual contrast during visual-vestibular mismatch training, or whether the magnitude of the increase stays constant, or a combination of both, i.e. VOR adaptation increases with contrast up until a saturation point where it becomes constant. We sought to determine the effects of contrast on human VOR adaptation across a broad range of contrast values.
We used the incremental VOR adaptation technique that relies on the subject performing active (self-generated) head impulses (Halmagyi and Curthoys, 1988) while visually tracking a laser target that moves in the opposite direction to the head at an incrementally increasing percentage of head speed (Schubert et al. 2008; Migliaccio and Schubert 2014 and 2013; Fadaee and Migliaccio 2016). With this technique, the visual-vestibular stimulus induces a retinal image error signal that gradually increases over the duration of the training, rather than starting and staying large throughout the training as has been the classic approach. Typically, we have set the gain demand needed to stabilise the retinal image of the target to gradually increase from 1.0 to 1.9 (Schubert et al. 2008; Migliaccio and Schubert 2014 and 2013; Fadaee and Migliaccio 2016). With this method, the VOR gain significantly increases after only 15 min of training. This technique allows us to explore the effect of contrast on human VOR gain increase adaptation in a way that was never practical using classic adaptation techniques, which required two or more hours of sometimes nauseating training (e.g. Gauthier and Robinson 1975; Gonshor and Jones, 1976). For each adaptation session, we set the target contrast by controlling either the ambient light level, target brightness or both during 16 separate sessions. The contrast levels ranged from 1.4 M (ambient light at 0 lx) to 16 (ambient light at 255 lx). In addition to examining the effect of contrast level on VOR adaptation, we sought to determine whether perception of contrast change affected adaptation.
Methods
Subjects
We tested 12 normal subjects across 16 separate sessions each (mean age 33 years, range 25–45 years). None of these subjects had any history or clinical signs of vestibular hypofunction. Participation in this study was voluntary and informed consent was obtained as approved by the University of New South Wales Human Ethics Committee.
Recording System
Head and eye rotations were measured using the EyeSeeCam system (Denmark), with the camera placed over the left eye (Bartl et al. 2009). The EyeSeeCam system consisted of a 220-Hz digital video camera, an infrared mirror to reflect the eye image to the camera and an inertial measurement unit to measure 3D (yaw, pitch and roll) angular head velocity. All components were rigidly mounted onto a lightweight swim goggle frame to minimise camera slippage relative to the head. In addition, we placed silicon putty (Surgipack, Australia) between the frame and face to further minimise slip and also add some comfort to the tight fit. The eye was illuminated via two on-board infrared LEDs. Horizontal and vertical eye positions were calibrated by having subjects fixate (goggle-mounted laser projected) visual targets at known angles with respect to the subject. The calibrated data were digitally filtered with a 50-tap zero-phase low pass FIR filter with a bandwidth of 50 Hz.
VOR Adaptation Training Protocol
We used the unilateral incremental VOR adaptation training protocol as has been previously described (Migliaccio and Schubert 2013 and 2014; Fadaee and Migliaccio, 2016). In brief, using the head impulse test (Halmagyi and Curthoys, 1988), we measured the active and passive VOR gain before and after active VOR adaptation training. Active head impulses are self-generated and predictable, whereas passive head impulses are externally applied and unpredictable to both timing and direction. Passive head impulses were delivered manually in the horizontal canal plane, i.e. leftward and rightward. Subjects were trained to perform active head impulses similar in profile to the passive head impulses (per Fig. 1 in Migliaccio and Schubert 2013). During VOR testing, a visual fixation target located straight-ahead and at eye level was provided. The fixation target was extinguished when the head rotated 0.6° away from its neutral (straight-ahead) position. Unilateral VOR adaptation training consisted of a series of active (self-generated) head impulses from a neutral starting position alternating leftward and rightward. The adapting side, leftward or rightward, was pseudo-randomised across subjects (e.g. leftwards for 6/12 subjects). For each active head impulse, subjects were instructed to maintain visual fixation of a laser target whose horizontal position was a function of horizontal head position, head impulse direction and adaptation gain (eye/head angular speed) demand. After head peak-velocity, other lower-latency vision stabilising systems, such as smooth pursuit, begin contributing to the compensatory eye movement. In order to drive only VOR adaptation, the laser target was extinguished once head peak-velocity was detected and reappeared only after the head returned to its neutral position. For rotations towards the non-adapting side the gain demand was fixed to unity (i.e. driving no adaptation), whereas for rotations towards the adapting side it increased from 1 (epoch 1) to 1.9 (epoch 10), i.e. an increment of 0.1 per 90 s epoch. The entire training period lasted 15 min.
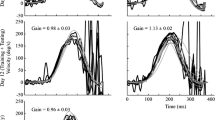
Post-adaptation active head impulses in a typical subject. The black traces denote the head velocity stimulus and the grey traces denote the inverted eye velocity response during active (self-generated) head impulses towards the adapting (left column) and non-adapting (right column) sides. Rows 1 to 4 show the VOR responses after adaptation training occurring with visual target contrast set at 1.4 M, 4.2 k, 2.2 k and 16, respectively. In parenthesis beside each contrast level is the ambient light level in lux and the target brightness as a percentage of maximum laser power. The post-training VOR gains towards the adapting side were significantly larger than the pre-training gain at all the contrast levels shown except level 16. In contrast, towards the non-adapting side, the pre- and post-training gains were the same regardless of contrast level. The asterisk denotes a significant increase (P < 0.05)
Data Collection
An improved digital version of the analogue portable laser target system described in Migliaccio and Schubert (2014) was used to control the laser target position directed onto a featureless matte-white projection screen (2.4 × 2.4 m) 1 m in front of the subject. The projection screen was positioned on a featureless matte-white wall so that no foveal or peripheral visual motion cues could be provided by the background. The laser diameter on the screen was 3.5 mm. The device consists of a head unit (strapped securely to the forehead) and a base (or control) unit. The head unit consists of a laser mounted in a fixed position relative to, and aimed at the centre of, an electrostatic MEMS micro mirror (Mirrorcle Technologies Inc., USA) and a 9D IMU (3D accelerometer, gyroscope and magnetometer; Pololu Corporation, Las Vegas). Information from the IMU is processed at 250 Hz using a gradient-descent fusion algorithm (Madgwick et al. 2011) to estimate instantaneous 3D head orientation and velocity. This algorithm is comparable to a Kalman filter and produces an orientation estimate with a typical latency of 6 ms during a head impulse with < 2 % root-mean-squared error. The head orientation is used to drive the mirror and hence laser target position with respect to the head. With head velocity > 50°/s and two sequentially decreasing head velocities, detection of head peak-velocity occurs with a delay of two samples, i.e. 8 ms. The base unit has a touch screen interface which allows the experimenter to set the training algorithm parameters, which can also be set via a Bluetooth or USB PC connection.
Unlike our previous studies where the adaptation training was performed under complete darkness, the light level and laser target intensity was varied for each subject session. The light source came from an incandescent lamp with diffuser and dimmer. We controlled the ambient light level to a precision of 2 decimal places with a lux meter (Iso-Tech, Taiwan) placed near the subject’s head facing the projection screen. Preliminary data from 3 subjects suggested that the effect of visual contrast on VOR adaptation training halved when the ambient light level was 0.5 lx compared to 0 lx. Based on these early results, we designed our experiment to have 7 out of 16 contrast levels (over separate sessions) with ambient light below or equal to 0.5 lx. Therefore, sessions 1 to 14 included an ambient light level set to 0 (dark; exact value 0.000865), 0.1, 0.2, 0.3, 0.5, 0.7, 1, 2, 8, 16, 32, 64, 128 and 255 lx (maximum room light), all with laser target power (related to target brightness) set to maximum (1 mW). For sessions 15 and 16, the background lighting was 0 or 0.1 lx, with laser target power set to 50 % of maximum (0.5 mW). The resulting 16 contrast levels were 1.4 M, 11.9 k, 8.6 k, 6.234 k, 4.2 k, 3 k, 2.3 k, 1.5 k, 495, 261, 139, 71, 46 and 16 for sessions 1–14 and 709.9 k and 6.231 k for sessions 15 and 16. Sessions were performed on separate days for each subject, sessions were not repeated, and the session presentation order was pseudo-randomised for each subject.
Perception of Change in Contrast
While the ambient light level was maintained at 0.1 lx, the target brightness was pseudo-randomly increased (50 to 100 %), decreased (100 to 50 %) or left unchanged (i.e. 3 conditions) at discrete transition times separated by 1 min. There were a total of 6 transitions, i.e. 2 per condition. Five seconds prior to each transition time subjects were instructed to close their eyes for 10 s. They were then instructed to open their eyes and look at the target for 45 s and then report whether target brightness had increased, had decreased or was unchanged.
Contrast Measurement
Weber contrast is a function of the target and background scene luminance (e.g. Whittle 1994; Kingdom 2011). Luminance can be measured with a digital camera. Digital cameras consist of a mosaic of photosensitive elements which produce a voltage linearly related to the number of incident photons during a timed exposure. We measured contrast using a Canon EOS 60D camera shooting RAW format images. The camera was equipped with an ND64 filter to prevent over-exposure. This filter was removed only to photograph the background luminance at 0 lx to reduce underexposure. RAW images were converted to tiff format maintaining exposure linearity, which we verified using a test image with 11 grey levels (adjusted R 2 = 0.9907). White balance, saturation level and darkness level were kept constant during conversion to ensure accurate comparison between images. For each scene, the laser spot luminance was photographed with the laser on and the background luminance was photographed with the laser off. It was necessary to vary exposure time depending on ambient light level to correctly expose photographs. For best results, laser spot photographs were exposed for 10 to 33 ms and background photographs were exposed from 0.33 to 30 s. The luminance component of each image (colour images comprise luminance and chrominance) was extracted and the median pixel values of the laser target and background regions were respectively used in the formula below:
The formula was derived from (Conrad 2003; Hiscocks 2014) with the assumption that the camera and filter parameters remained constant as represented in the equation above. We also ensured that the camera’s optical axis was perpendicular to the scene and aligned with the visual target to reduce vignetting. Weber contrast was calculated using the formula below:
Data Analysis
Horizontal angular eye position was differentiated and the onset of each head impulse was calculated by fitting horizontal angular head velocity magnitude to a polynomial curve versus time. The point where the magnitude of the fitted curve was greater than 2 % of the curve’s peak magnitude (typically this threshold was 4°/s) was defined as the impulse onset. Only head impulses with peak magnitude between 150 to 300°/s were included in the analysis. Traces with saccades occurring inside a window starting at 100 ms before impulse onset and ending at impulse peak magnitude (typically 100 ms after onset) were also removed. Eye traces containing blinks and other artefacts were removed, along with their corresponding head traces. The instantaneous VOR gain was calculated as the magnitude of eye velocity divided by head velocity. We defined the median of the instantaneous VOR gains calculated during the 30 ms period (at 220 Hz this corresponds to 6 to 7 instantaneous gain values) immediately prior to impulse peak magnitude as the VOR gain. The VOR gain change for each side (adapting or non-adapting) as a percentage was calculated as:
A positive result indicated an increase in VOR gain due to adaptation training.
Statistical Analysis
Statistical analysis was performed using SPSS version 23 (IBM, USA), SigmaPlot version 13 (Systat Software Inc., USA) and Excel 2013 (Microsoft, USA) software. We used a linear mixed model (LMM) with repeated measures to analyse the VOR gain data. Independent variables included the following: impulse type (‘active’, ‘passive’), time (‘pre-training’, ‘post-training’), head rotation side (‘adapting’, ‘non-adapting’), ambient light level (0, 0.1, 0.2, 0.3, 0.5, 0.7, 1, 2, 8, 16, 32, 64, 128 and 255), laser target brightness (50 %, 100 %) and contrast level (1.4 M, 709.9 k, 11.9 k, 8.6 k, 6.234 k, 6.231 k, 4.2 k, 3 k, 2.3 k, 1.5 k, 495, 261, 139, 71, 46, 16). The only dependent variable was gain. We also analysed the VOR gain percentage data with an LMM using the same independent factors as the LMM above but excluding the time variable. All variables were included in the LMM initially and those found insignificant were subsequently removed. Paired t tests were performed on the pre- and post-training VOR gains using least significant difference (LSD) to correct for multiple comparisons. Two-, three- and four-segment piecewise linear regression analysis was performed on the VOR gain percentage versus contrast data. Only the interaction effects found to be significant are included in the results. Pooled data are described as mean ± 1 SD, whereas pooled means are described as mean ± 1 SE.
Results
Pre-adaptation VOR
There were differences in the pre-adaptation VOR gains between subjects (LMM: F 11,108 = 7.7, P < 0.001). There were no differences between active and passive head impulses (LMM: F 1,137 = 0.02, P = 0.88). However, there was a significant difference of ~ 2 % in VOR gains between leftward (0.93 ± 0.07) and rightward (0.91 ± 0.06) head impulses (LMM: F 1,137 = 8.3, P < 0.005). Because all the gains were calculated from the left eye, this result confirms that the VOR gain is higher when the eye is adducting versus abducting as per Weber et al. (2008).
Contrast Effect on a Typical Subject
Figure 1 displays the post-adaptation training VOR gains of one typical subject when the training occurred at different ambient light levels ranging from 0 lx (dark) to 255 lx (maximum room light). Both the vestibular stimulus (head velocity—black traces) and vestibular response (eye velocity—grey traces) are shown for rotations towards the adapting (left column) and non-adapting (right column) training sides. At 0 lx, the laser target contrast was at the maximum level of 1.4 M. At this level, VOR adaptation training resulted in a significant increase of 11 % on the adapting side (t test: P < 0.001), and a non-significant change on the non-adapting side (t test: P = 0.36). The VOR gain increase towards the adapting side remained significant at 1 lx (t test: 0.5 lx, P < 0.02; 1.0 lx, P < 0.05), whereas the non-adapting side VOR gain remained unchanged. At 255 lx, the laser target contrast was at the minimum level of 16. At this level, VOR adaptation towards both the adapting and non-adapting sides was no longer significant (t test: adapting side, P = 0.40; non-adapting side, P = 0.49).
Contrast Effect Across Subjects
Figure 2 shows the pre-adaptation (grey bars) and post-adaptation training (black bars) VOR gains towards the adapting side for both the active (top row) and passive (bottom row) VOR responses. For the active and passive VOR, the pre- and post-adaptation gains were significantly different (t test: P < 0.05 denoted by *) only for 8 lx and below. Analysis of all the group data showed that visual contrast level significantly affected the VOR gain (LMM: F 15,329 = 4.2, P < 0.001). Other significant factors were whether the gain was measured pre- or post-adaptation training (LMM: F 1,214 = 80.8, P < 0.001) and whether the head was rotated to the adapting or non-adapting side (LMM: F 1,214 = 80.8, P < 0.001). We replaced the contrast factor in the LMM with target brightness level and ambient light level to determine whether one or both of these factors affected the gain. Both laser target brightness level (LMM: F 1,325 = 5.3, P < 0.05) and ambient light level (LMM: F 13,327 = 3.6, P < 0.001) significantly affected the gain. There was a significant interaction between time and head rotation side (LMM: F 1,259 = 81.3, P < 0.001) indicating that the adaptation training only affected the adapting side.
Mean (± SD) pre-adaptation (grey bars) and post-adaptation (black bars) training active (top row) and passive (bottom row) VOR gains across all subjects (n = 12) for each contrast level. In parenthesis beside each contrast level is the ambient light level in lux and the target brightness as a percentage of maximum laser power. LMM analysis indicates that adaptation training significantly increases both the active and passive VOR gains towards the adapting side by similar amounts at all contrast levels above and equal to 1.5 k (left side of vertical dashed line). LMM analysis indicates that adaptation training does not significantly increase the VOR gain at all contrast levels below and equal to 495. The asterisk denotes a significant increase (t test between pre- and post-adaptation gains, P < 0.05)
Due to the variability of pre-adaptation gains across subjects, we normalised data for each subject/session by calculating the percentage VOR gain increase for each side (adapting, non-adapting). Figure 3 shows the active (top panel) and passive (bottom panel) VOR gain percentage increases across all 16 sessions for rotations towards the adapting (black bars) and non-adapting (grey bars) sides. For the active and passive VOR, the adapting and non-adapting side gain increases were significantly different (t test, P < 0.05 denoted by *) only for 8 lx and below. Using all the group normalised data, visual contrast remained highly significant (LMM: F 15,69 = 5.3, P < 0.001). There was also a significant interaction between head impulse side and contrast level (LMM: F 15,69 = 3.9, P < 0.001), suggesting contrast only affected the adapting side gain. After replacing the contrast factor in the LMM with target brightness level and ambient light factors, ambient light level became more significant (LMM: F 13,74 = 5.7, P < 0.001), whereas laser target brightness level was no longer significant (LMM: F 1,148 = 0.9, P = 0.35). When the six highest ambient light levels (8, 16, 32, 64, 128 and 255 lx) data were removed from the analysis, the only remaining significant factor was head impulse side (LMM: F 1,399 = 225.0, P < 0.001), suggesting that although VOR adaptation was significant, it did not change across ambient levels less than or equal to 2 lx (LMM: 0 to 2 lx, F 9, 79 = 1.2, P = 0.32), whereas at ambient levels more than or equal to 8 lx, VOR adaptation no longer occurred (LMM: 8 to 255 lx, F 5,73 = 1.3, P = 0.27). In summary, over the laser target contrast range 1.4 M to 1.5 k (i.e. 0 to 2 lx), VOR gain adaptation towards the adapting side was significant for both the active and passive VOR, and it was constant, averaging 8.5 ± 3.8 % for the active VOR and 8.0 ± 3.7 % for the passive VOR. (VOR gain adaptation towards the non-adapting side was 3.2 ± 3.1 and 3.4 ± 3.6 % for the active and passive VOR, respectively.) In contrast, over the contrast range 495 to 16 (i.e. 8 to 255 lx), VOR gain adaptation towards the adapting side was not significant for both the active and passive VOR, but was also constant, averaging 3.9 ± 2.9 % for the active VOR and 4.3 ± 3.4 % for the passive VOR, similar to the non-adapting side increases. (VOR gain adaptation towards the non-adapting side was 2.4 ± 3.1 and 2.9 ± 3.7 % for the active and passive VOR, respectively.)
Mean (± SD) adapting (black bars) and non-adapting (grey bars) side pre- to post-adaptation training VOR gain increase as a percentage for the active (top row) and passive (bottom row) VOR across all subjects (n = 12) for each contrast level. In parenthesis beside each contrast level is the ambient light level in lux and the target brightness as a percentage of maximum laser power. LMM analysis indicates there is a significant difference in percentage gain increase between the adapting and non-adapting sides at all contrast levels above and equal to 1.5 k (left side of vertical dashed line). The asterisk denotes a significant increase (t test between adapting and non-adapting side percentage increase, P < 0.05)
Figure 4 shows a scatter plot of VOR gain percentage increase towards the adapting side (after pooling active and passive gains) versus the contrast level. In order to determine the contrast threshold where VOR adaptation was no longer significant, we performed piecewise linear regression on the data shown. Two-segment regression (R 2 = 0.26) determined a knot at 836, indicating that at this contrast level, there was a significant difference between the two lines respectively modelling the data below and above this contrast level. Three-segment modelling (R 2 = 0.27) was performed to determine if there was a transition period between two contrast levels with gradually increasing (i.e. sloped) VOR gain adaptation. This analysis revealed a lower knot at 854 and an upper knot at 1.3 M. Four-segment modelling (R 2 = 0.28) revealed three knots: 225 (lower), 1197 (middle) and 1.2 M (upper). Taken together, this data suggests there was a transition period starting at ~ 225 and ending at ~ 1000 (i.e. between 836 and 1197) where the VOR gain percentage increase gradually increased. Below 225, there was no-significant adaptation and above 1000 there was maximal adaptation.
Scatterplot of VOR gain percentage increase towards the adapting side (after pooling active and passive gains) versus the contrast level plot on a log10 scale. Two-segment piecewise regression (R 2 = 0.26) determined a knot at 836, indicating that at this contrast level there was a significant difference between the two lines respectively modelling the data below and above this contrast level. Three-segment analysis revealed a lower knot at 854 and an upper knot at 1.3 M. Four-segment modelling (R 2 = 0.28) revealed three knots: 225 (lower), 1197 (middle) and 1.2 M (upper). Overall, our analysis suggests there was a transition period starting at ~ 225 and ending at ~ 1000 (i.e. between 836 and 1197) where the VOR gain percentage increase gradually increased. Below 225, there was no-significant (but constant) adaptation and above 1000, there was maximal adaptation
Perception of Change in Contrast at High Contrast (Low Ambient Light) Levels
While the ambient light level was maintained at 0.1 lx, the target brightness was pseudo-randomly increased (50 to 100 %), decreased (100 to 50 %) or left unchanged. Our analysis shows that subjects could perceive the difference in laser brightness levels (Pearson chi-square test, P < 0.01, phi = 0.421 [large effect]). However, there was no difference between active and passive VOR gain increases towards the adapting side after training at 0.1 lx at either 50 or 100 % laser brightness (LMM: F 1,86 = 1.1, P = 0.30). The pooled (active and passive) VOR gain increase towards the adapting side at 0.1 lx was 7.5 ± 3.0 % at 50 % laser brightness and 9.0 ± 3.4 % at 100 % laser brightness, a difference that was not significant (t test: P = 0.27).
Discussion
Our findings suggest that in humans, robust unilateral VOR adaptation occurs when the visual target used during visual-vestibular mismatch training has contrast level 1.5 k and above. At contrast levels between 1.5 k to 1.4 M, the VOR gain increase due to adaptation training did not change, suggesting that at these levels the effect of contrast on VOR adaptation had saturated. When target contrast was reduced to 261, the active and passive VOR gain increase towards the adapting side was no longer significant and was similar to the non-adapting side, suggesting that 261 was below the threshold needed for adaptation to occur. Overall, our results suggest there is a contrast threshold for VOR adaptation to occur and that above that threshold there is a contrast level at which VOR adaptation saturates.
Our data suggest that the contrast threshold is between 261 and 495; however, our model suggests it is below this range at 225. The saturation threshold is between 495 and 1.5 k with our model suggesting it is about 1000, i.e. between 836 and 1197. The calculation of these ranges was limited because unlike contrast sensitivity studies in smooth pursuit and optokinetic nystagmus where these threshold levels are determined by briefly presenting each subject with consecutively varying in contrast stimuli and examining the ocular responses, our study required a separate experimental session on separate days for each contrast level. Also, the large variability in VOR gain increases made it difficult to determine these measures with more precision.
Contrast Perception
At contrast levels 11.9 k and above (ambient light levels 0.1 lx and below), several subjects reported difficulty tracking the laser target because they perceived the target to be too bright. We sought to determine whether other physiological factors, for example, pupil constriction due to target brightness, was reducing changes in contrast perception and limiting VOR adaptation, especially at the high contrast levels. Our data suggests that subjects could perceive changes in contrast when maintaining the ambient lighting fixed at 0.1 lx while varying the laser brightness 50 %. Thus, VOR gain adaptation does not seem to saturate due to an absent perception of change in contrast.
Our data suggest that once the internal estimate of target velocity with respect to the head approaches a certain level of accuracy VOR adaptation becomes closer to saturating. Above this transition level, better internal accuracy does not result in a closer to ideal VOR response. For example, the maximum VOR gain demand of 1.9, elicited during the last 90 s of the training (i.e. epoch 10), was much larger than the typical VOR gain in subjects after training of ~ 1.1. This may be in part not only due to the short duration of the training but also physiological limitations of VOR adaptation. For example, when the flocculus is lesioned, a site crucial for VOR gain modification, the VOR gain saturates at ~ 1.6 and vestibular plasticity is abolished (primates: Zee et al. 1981; cats: Luebke and Robinson 1994), suggesting that for our experiment a VOR gain increase of ~ 60 % would be the upper limit for VOR gain adaptation in normal subjects.
Implications for VOR Rehabilitation
Our findings show that tracking a laser dot with the size and brightness of a typical laser pointer in normal room lighting was not sufficient to drive significant VOR adaptation after 15 min of visual-vestibular mismatch training. Typical viewing under normal lighting is unlikely to have contrast levels as high as our stimulus. Our modelling suggests a contrast level of ~ 1000 is the minimum required for robust VOR adaptation, compare this to the maximum contrast level we obtained from a typical LCD monitor (24″ 1920 × 1080 resolution VS243 Asus, Taiwan) of 37.7 (3.5 mm diameter white dot on black background using the same contrast measurement technique described in methods) in an otherwise dark room. However, typical scenes of view do provide more small details and sharp edges, i.e. spatial frequency, than a single dot on a featureless background. Low image contrast may explain why classic human studies using normal lighting and magnifying or minifying lenses with full fields of view to drive VOR adaptation required long periods of training to significantly affect the VOR gain. Increasing contrast, in our case to 1.5 k, was sufficient for robust and rapid VOR adaptation to occur. However, it is unlikely that the increase in contrast was the only factor here. A prior study under similar lighting conditions to our maximum contrast level found that incremental adaptation training, rather than ‘all at once’ training (as occurs during classic lens training), resulted in significantly larger gain increases (Schubert et al. 2008), suggesting that visual contrast is but one of several factors affecting VOR adaptation. Our findings might also explain why many patients with incomplete vestibular hypofunction often have slow and incomplete recovery of VOR gains despite long exposure to the normal visual environment that typically drives the VOR gain to unity. We suggest that visual target contrast should be an important consideration during VOR adaptation/rehabilitation training.
In summary, we illustrate that VOR adaptation remains significant and constant across visual contrast levels above and equal to 1.5 k. Our data establish that VOR gain training using a bright target (as emitted by a typical laser pointer) can occur in moderate ambient lighting, i.e. 0.5–1 lx is the typical lighting of a room with curtains closed and lights turned off. These data provide critical ramifications for the development of rehabilitation tools that seek to improve the magnitude of a lesioned VOR.
References
Bartl K, Lehnen N, Kohlbecher S, Schneider E (2009) Head impulse testing using video-oculography. Ann N Y Acad Sci 1164:331–333
Büttner U, Büttner-Ennever JA (2006) Present concepts of oculomotor organization. Prog Brain Res 151:1–42
Cohen B, Henn V, Raphan T, Dennett D (1981) Velocity storage, nystagmus, and visual-vestibular interactions in humans. Ann N Y Acad Sci 374:421–433
Conrad, J. (2003) Exposure metering: relating subject lighting to film exposure. Available online:http://www.largeformatphotography.info/articles/conrad-meter-cal.pdf (accessed on 18 January 2017)
Fadaee SB, Migliaccio AA (2016) The effect of retinal image error update rate on human vestibule-ocular reflex gain adaptation. Exp Brain Res 234:1085–1094
Gauthier GM, Robinson DA (1975) Adaptation of the human vestibuloocular reflex to magnifying lenses. Brain Res 92:331–335
Gonshor A, Jones GMJ (1976) Short-term adaptive changes in the human vestibulo-ocular reflex arc. Physiol 256:361–379
Halmagyi GM, Curthoys IS (1988) A clinical sign of canal paresis. Arch Neurol 45:737–9
Hiscocks, P.D. (2014) Measuring luminance with a digital camera. Available online: http://www.ee.ryerson.ca/~phiscock/astronomy/light-pollution/luminance-notes-2.pdf (accessed on 18 January 2017)
Ito M, Shiida T, Yagi N, Yamamoto M (1974) Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res 65:170–174
Kingdom FA (2011) Lightness, brightness and transparency: a quarter century of new ideas, captivating demonstrations and unrelenting controversy. Vis Res 51:652–673
Leguire LE, Zaff BS, Freeman S, Rogers GL, Bremer DL (1991) Contrast sensitivity of optokinetic nystagmus. Vis Res 31:89–97
Luebke AE, Robinson DA (1994) Gain changes of the cat’s vestibulo-ocular reflex after flocculus deactivation. Exp Brain Res 98:379–390
Madgwick SO, Harrison AJ, Vaidyanathan A. (2011) Estimation of IMU and MARG orientation using a gradient descent algorithm. IEEE International Conference on Rehabilitation Robotics, pp. 1–7
Migliaccio AA, Schubert MC (2013) Unilateral adaptation of the human angular vestibulo-ocular reflex. J Assoc Res Otolaryngol 14:29–36
Migliaccio AA, Schubert MC (2014) Pilot study of a new rehabilitation tool: improved unilateral short-term adaptation of the human angular vestibulo-ocular reflex. Otol Neurotol 35:310–316
Schubert MC, Della Santina CC, Shelhamer M (2008) Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res 191:435–446
Spering M, Kerzel D, Braun DI, Hawken MJ, Gegenfurtner KR (2005) Effects of contrast on smooth pursuit eye movements. J Vis 5:455–465. https://doi.org/10.1167/5.5.6
Spoor M, Hosseini B, van Alphen B, Frens MA, van der Geest JN (2014) Human gaze following response is affected by visual acuity. J Ophthalmol 2014:543478. https://doi.org/10.1155/2014/543478
Sumnall JH, Freeman TC, Snowden RJ (2003) Optokinetic potential and the perception of head-centred speed. Vis Res 43:1709–1718
Thompson P (1982) Perceived rate of movement depends on contrast. Vis Res 22:377–380
Thompson P (1983) Discrimination of moving gratings at and above detection threshold. Vis Res 23:1533–1538
Waddington J, Harris CM (2015) Human optokinetic nystagmus and spatial frequency. J Vis 15:7. https://doi.org/10.1167/15.13.7
Weber KP, Aw ST, Todd MJ, McGarvie LA, Pratap S, Curthoys IS, Halmagyi GM (2008) Inter-ocular differences of the horizontal vestibulo-ocular reflex during impulsive testing. Prog Brain Res 171:195–198
Whittle P (1994) The psychophysics of contrast brightness. In: Gilchrist AL (ed) Lightness, brightness, and transparency. Lawrence Erlbaum Associates, Hillsdale, NJ, pp 35–110
Zee DS, Yamazaki A, Butler PH, Gucer G (1981) Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol 46:878–899
Acknowledgements
A.A. Migliaccio was supported by The Garnett Passe and Rodney Williams Memorial Foundation Senior/Principal Research Fellowship in Otorhinolaryngology and Project Grant (2013-15), and NHMRC Development Grant APP105550.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muntaseer Mahfuz, M., Schubert, M.C., Todd, C.J. et al. The Effect of Visual Contrast on Human Vestibulo-Ocular Reflex Adaptation. JARO 19, 113–122 (2018). https://doi.org/10.1007/s10162-017-0644-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-017-0644-6