Abstract
Here we introduce a series of behavioural tasks to assess inter-individual variability in behaviours exhibited by the cephalopod mollusc Octopus vulgaris. We propose that, by using octopus’ predatory behavioural response, it is possible to measure: (1) the ability to adapt to the captive condition (acclimatization), (2) the response towards novel stimuli (neophobia), (3) the capability of social learning, (4) the ability of solving problems (problem solving), and (5) the response to artificial stimuli (preferences, individual learning). To assure comparability and reproducibility of results, this battery of tests is here applied to a large sample of individuals in standardized experimental conditions. Such battery of tests serves as an in vivo screening that should be adopted not only to investigate cognitive abilities in specific behavioural domains, but also to monitor the welfare status of animals under captivity, thus to check sensory functions as well as motor abilities in other investigations within the fields of biology and neuroscience. Our aim was to provide a reliable tool to exploit this animal species for research in different fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behaviour is the final product of the nervous system. A good description of the behavioural repertoire of a species and of inter-individual differences in behaviour is required for the understanding of norms and syndromes and to define possible significant differences at the level of subpopulations within a given species (Sih et al. 2004a, b).
Differences in how individuals exhibit their behaviour appear related not only to ‘basic’ factors (i.e. age, sex, relative size) or to the environment in which single organisms live, but also to intrinsic ones; these are in agreement with the principle that natural selection may favour “the adoption of different strategies by different individuals” (Slater 1981, p. 35). “Massing animals to obtain a learning curve or a sequence diagram [as occurs in laboratory context, NoA] is only useful if there are insignificant differences among them: if not, the average animal which emerges may have a set of features that were not possessed by any single individual in the group. The possibility of animals possessing different strategies or personality profiles can only be explored when they are examined and compared as individuals” (Slater 1981, p. 46).
Common consensus has emerged over the last decades for the use of a standardized approach to characterize the behavioural phenotype of individuals within a given species when these are exposed to a diversified series of experiments. For example, the diffusion of transgenic and knockout mice requires discovering the biological function of a given gene. Under such circumstances, a standardized way to assess inter-individual variability in behaviour appears to help to characterize differences due to genetic manipulation. The key to success in these experiments is a robust, well-replicated or well-characterized phenotype (for review see for example: Crawley 1999, 2008). Biochemical, anatomical, physiological, pathological, and behavioural assays all contribute to the understanding of the consequences of a given mutation. Measures of the behavioural outcome are therefore essential for animals with, e.g. mutations in genes expressed in the brain. Mammals are not the sole cases; several invertebrate species and the so-called emerging model species are also benefiting of a standardized description of behavioural repertoire (e.g. Anagnostopoulos et al. 2001; Crawley and Paylor 1997; Hobert 2003; Skoulakis and Grammenoudi 2006; Swierczek et al. 2011).
There is a wealth of knowledge in behavioural neuroscience with respect to well-validated and carefully controlled methods for behavioural testing of sensory abilities, motor functions, learning and memory, traits relevant to anxiety, depression and schizophrenia, which have been tested in a number of vertebrate and invertebrate species. The Jackson Laboratory offers a searchable database of behavioural traits in mouse lines (JAX Mice Database and Mouse Phenome Database, www.jax.org).
In general, these behavioural assays are straightforward but require a high level of attention to detail. For example, behaviours are sensitive to a host of environmental factors, including handling, noise levels, and season. In addition, standardized and replicable behavioural testing across laboratories is also crucial (e.g. Crabbe et al. 1999; Gerlai 2019; von Kortzfleisch et al. 2019). In fact, when methods are appropriately conducted, the replicability of behavioural data is similar to the replicability of results obtained with other biological techniques (Wahlsten et al. 2003).
More in general, behavioural phenotyping is based on in vivo screening to monitor general health, sensory functions, and motor abilities and possibly specific behavioural domains to be tested. Therefore, groups of behavioural tasks are utilized for a reliable detection of differences in specific behavioural domains. Screening of general health and neurophysiological functions, such as reflexes and sensory abilities, motor functions, learning and memory, emotionality, nociception, psychiatric-like conditions and aggression, should be used to describe the behavioural profile of a given individual in detail, potentially at different stages of its development or captive condition in the laboratory (in the case of wild animals).
Only a handful of studies have been conducted to date testing individual differences in invertebrates (e.g. George and Brockmann 2019; Hedrick 2017; Liang et al. 2012),Footnote 1 and cephalopods in particular (Calvé 2005; Mather and Anderson 1993; Pronk et al. 2010; Sinn et al. 2008; Sinn and Moltschaniwskyj 2005; Sinn et al. 2001). For example, Octopus rubescens responses over a succession of tests (alerting, threat and feeding) were attributed to a shy-bold continuum in which three major behavioural components emerged (i.e. “activity”, “reactivity” and “avoidance”; Mather and Anderson 1993). Systematic shy-bold differences among individuals have also been found in the dumpling squid Euprymna tasmanica (Sinn and Moltschaniwskyj 2005) and in the cuttlefish Sepia officinalis (Calvé 2005). In O. bimaculoides, Sinn and colleagues (2001) showed that personality traits were possibly heritable in octopuses and that relatives behaved more similarly to each other than non-relative individuals. The above-mentioned works contributed to identify in individual cephalopods supposed “personalities” or temperaments that remain stable with time and do not seem to depend on their degree of habituation to the experimental setting (e.g. Borrelli 2007; Mather and Anderson 1993; Sinn et al. 2001).
The richness of the behavioural repertoire of octopuses and their allies (Barbato et al. 2007; Borrelli et al. 2006; Hanlon and Messenger 1996), and the tendency of different individuals to react to the same stimulus with different clear-cut responses seems promising to the aim of using a series of tests and appropriate experimental protocols to assess the general health and to better characterize the behavioural signature of a given individual. However, this would only be possible when standardized procedures are tested and made available to researchers.
Here, we analysed the behaviour of a large sample of Octopus vulgaris in a battery of eight consecutive experiments, spanning over 12 days. We exploited Octopus’ predatory behaviour to measure its: (1) ability to adapt to the captive condition (acclimatization), (2) response towards novel stimuli (neophobia), (3) capability of social learning, (4) ability to solve problems (problem solving), and (5) response to artificial stimuli (preferences, individual learning). Furthermore, for all tests, we considered the latency of attack as the main variable for scoring animals’ responses.
Our main goal was to standardize a series of tests in order to provide a rigorous protocol that would support scientists’ effort to reduce variation in the behavioural data that could obscure the effects of specific experimental treatments. This approach is novel for cephalopods, and to the best of our knowledge, and time is ripe to such systematization that will allow reproducibility and comparability of results across research groups and domains, thus guaranteeing a prolific exchange within the broad scientific community.
Materials and methods
Subjects
A total of 55 Octopus vulgaris (Mollusca, Cephalopoda) of both sexes (males = 22) were caught from different geographical sites of the Bay of Naples, Italy (time span: October 2002–October 2004). In order to standardize fishing procedures, we exclusively tested octopuses caught the same morning of the beginning of the experiments.
Experimental setting
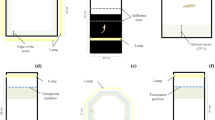
Following Fiorito and co-workers (1990), the experimental setting was designed in order to simulate natural conditions at 3–4 m depth (see Borrelli 2007 for details).
The tanks (60 × 100 × 50 cm) were located in a room whose access was allowed to experimenters only. They were made of dark grey PVC (colour components: Magenta = 10, Black = 50, Blue = 40) except for the front side consisting of a transparent glass panel (45 × 35 cm) to allow remote observation and video recording. A layer of sand, obtained directly from the coast off La Gaiola-Posillipo (Bay of Napoli, Tyrrhenian Sea), was adjusted on the bottom of each tank and a pair of bricks, set in a corner, served as the octopus’ den. All the tanks were firmly closed by a transparent cover (Plexiglas) with a hole to allow the inflow of seawater, which was filtered before inflow to avoid sediment accumulation.
At 1.40 m from the top of the tanks, three series of lamps (one series of Neodymlite tungsten ND60E27, two series of Neodymlite dichroic halogen MR16, Oy Airam AB, Finland) were positioned and programmed to switch on and off automatically according to the seasonal and daily rhythm at the latitude of the Bay of Naples.
Each tank was paired with the adjacent one by a transparent glass partition, allowing visual interaction during social learning phases. In all other cases, each animal was kept in isolation by an opaque panel slid between the two tanks to cover the glass partition.
A dark blue curtain (colour components: Magenta = 50, Black = 70, Blue = 100), dropping from the ceiling to the floor and running the entire length of the tanks, was positioned at a distance of 1.5 m from the frontal glass of each tank to hide both video equipment (video cameras, tripods, etc.) and the experimenter from the animals’ view; the curtain was cut by a series of slits through which only the lens of the video camera was pulled through allowing video recordings. On each tank, a second curtain dropped from the ceiling to the surface of the water at the level of the frontal glass. These curtains had a similar brightness to the tank walls and also helped in hiding the tester during the experiments.
Procedure
Each octopus was faced with a series of eight consecutive experiments (hereafter called battery) presented to all the animals in the same order (see Fig. 1) and lasting 12 days.
Animals were fed every other day. Under these conditions, octopuses show neither physiological nor motivational decline to attack live prey (e.g. Amodio et al. 2014; Borrelli 2007; Fiorito and Scotto 1992; Fiorito et al. 1990). In order to monitor for any potential difference in the overall food supply to each animal, we weighed each crab (Carcinus maenas, Crustacea, Decapoda) given to the octopuses on feeding days.
Arrival: day 1
Upon reception, each octopus was numbered, sexed, weighed (Borrelli 2007; Chapko et al. 1962) and housed in an experimental tank.
Acclimatization: days 2–6
Starting from the day following arrival and for five consecutive days, each animal was presented with a live crab attached to a cotton thread (tethered crab), a procedure commonly used by cephalopod experimenters to measure octopuses’ motivational levels and recovery in predatory performance (Amodio et al. 2014; Maldonado 1963, 1964). Once the prey landed on the bottom of the tank, it generally moved spontaneously; in case the crab stayed still (freezing behaviour) the thread was gently pulled to solicit the prey to move.
The crab was then promptly pulled out of the tank before the octopus could pounce on it, with the exception of days 3 and 5 in which the octopus was allowed to catch and feed on the prey (see Fig. 1).
A ceiling latency of 301 s was assigned to animals that failed to respond within a 5-min interval (trial duration), following which the crab was pulled out and the trial ended. On feeding days (i.e. days 3, 5), animals that did not attack the crab within the ceiling latency were not fed.
As a general criterion, the experiments following Acclimatization were carried out only on animals that succeeded in recovering their predatory behaviour: i.e. by attacking a crab readily by the end of the 5 days. The acclimatization was fixed to 5 days to include two feeding days, and because based on previous studies, this time was considered enough to allow the great majority of animals to be acquainted to the new condition. As an exclusion criterion, animals that did not attack a crab readily within Acclimatization were not tested further.
Neophobia 1st–day 6
Octopuses were tested for neophobia in two different circumstances: once before (Neophobia 1st) and the second time after the social learning experiment (Neophobia 2nd).
Following Greenberg (1983, 1984), the neophobia tests were designed to compare the time required by an octopus to attack a crab when presented alone or with a novel object.
A metallic cross (14 cm wide, 3 cm thick) was used as novel object during Neophobia 1st. The cross was attached to a cotton string and always presented in front of the animal (proximal position; the crab being distal). To test for potential fear towards novel objects, each octopus was presented with two blocks (morning block, afternoon block) of two trials each (Fig. 2).
The two-alternative morning/afternoon blocks to test neophobia in O. vulgaris. Each block consisted of two trials. During the first trial (crab) of each block, the animal was presented with the tethered crab as a measure of the octopus’ attack performance in “normal” conditions, and also to enhance the animal’s attention, and “prepare” it for the actual task. During the second trial of the block the octopus was presented either with the tethered crab again (CRA) or with the crab and a novel object, simultaneously (NOB). A coin flip, which was determined before the beginning of the morning block, assigned the type of presentation to each animal at the second trial (CRA or NOB)
Each trial lasted a maximum of 5 min (ceiling latency: 301 s). The time interval elapsed between the two trials of each block depended on the behaviour of the octopus. A timer was set for 2 min starting from when the crab was pulled out from the tank at the end of trial 1; if the animal was back home by the end of this 2-min interval, then the successive presentation (trial 2) was carried out immediately. If, on the other hand, the animal was not back in the den, an additional 2 min were added following which the second trial started anyway.
The two blocks were spaced apart by roughly 4 h.
Social interaction: day 7
The morning of the seventh day of the battery, the opaque partition separating the two adjacent tanks was removed to allow visual interaction between the demonstrator octopus (a trained O. vulgaris) and the experimental animal (hereafter called observer). Social interaction has been considered as a necessary phase to habituate the animals to each other and favour a steady-state equilibrium before the beginning of the social learning experiment (Fiorito and Scotto 1992).
Social learning (first session): day 8
Following the procedures of Fiorito and Scotto (1992), the social learning experiment (social 1) consisted of an observational phase (social) and a testing phase (in isolation). During the observational phase each observer (battery animal) watched a demonstrator (trained conspecific) solving the black-box problem; during the testing phase, the observer itself was presented with the black box to assess the solution of the problem following social demonstration (see below for details).
The “black-box problem” consisted of a cubic box (14.5 × 14.5 × 14.5 cm) made of black Plexiglas, with a drawer (5 × 14.5 cm) in one side. The drawer fitted perfectly inside the box so that when it was closed there was no visual cue (such as a knob or handle) to distinguish it from the rest of the box. A live, tethered crab was placed in the drawer as reward in both observational and testing phases. However, since the box was black, the prey was only detectable by tactile cues, i.e. only after pulling at the drawer’s surface, sliding it open and blindly exploring its contents. Moreover, in order to reach for the prey, octopuses were forced to pull and slide the drawer open by at least 5 cm since the internal compartment had a horizontal panel that blocked the entrance to the drawer by 2.5 cm.
Training of demonstrators
Demonstrator octopuses were trained in isolation for their capability to solve the black-box problem (see Supplementary Information for details).
Observational phase
During the observational phase each observer watched the demonstrator attack and solve the black-box problem, i.e. open the drawer and feed on the crab. Each observational trial lasted 10 min. The observational phase was repeated for two trials spaced apart by roughly 2.5 h (for exceptions, see Supplementary Information: Estimated visual field, centre visual field). Failures of the demonstrators during this phase caused the interruption of the battery for the corresponding experimental animals.
Each demonstrator was presented with the box in its own tank. In order to improve the observer’s perception of the object and of the specific cues necessary to solve the task, the object was placed in the centre of the observer’s visual field. Furthermore, it was slightly oblique in order to allow the animal to actually see the sliding movement and opening of the drawer.
Testing phase
The opaque panel was inserted, between the two tanks to isolate the observer from the demonstrator, 1 h following the last trial of the observational phase. The testing phase, in turn, started 1 h after the partition’s insertion.
The test consisted of a single presentation of the black box, placed at a distance of about 70 cm from the animal’s resting position in the den and with the drawer (rewarded) facing the octopus. The trial lasted 20 min. A ceiling latency of 1201 s was assigned to animals that failed to respond within the 20-min interval, following which the box was removed from the tank and the trial ended. If the octopus solved the problem it preyed on the crab; in case of failure, a crab was given to the animal anyway, 5 min from the removal of the black box.
Social learning (second session): day 9
The social learning experiment was repeated the following day (social 2) to test the effect of further social experience on the social learning capability of O. vulgaris. The experiment started early in the morning by removal of the opaque panel dividing the two adjacent tanks, in order to allow social interaction between demonstrators and observers.
The first observational trial was carried out at least 3 h from the beginning of the social interaction (but never after more than 4 h). In a similar way to the first session of the social learning experiment, observers were presented with two observational trials during which their demonstrators solved the black-box problem. Again, the object was presented, 2 h later, to the observers themselves for a 20-min trial.
Day 9 became a supplementary ‘feeding day’ if the experimental animal solved the problem it preyed on the crab; in case it failed, no further crab was given.
Neophobia 2nd–day 10
This experiment followed the same protocol previously described for Neophobia 1st, the only difference being in the novel object used (i.e. a metallic lid, 14 cm in diameter). Also in this case, the object was inserted proximal to the animal while the crab was distal.
Problem solving: day 11
To test problem solving, each octopus was presented, for one single trial (lasting 10 min), with a “multi-openable jar” consisting in a 3D box with the shape of a parallelepiped (20 × 15 × 15 cm) of clear, transparent Plexiglas with three different “plugs” (one for each side). The jar was placed as for other cases at roughly 70 cm from the octopus’ den with the free side of the object facing the animal (the other sides having the plugs). This was purposely done to avoid cueing the octopus towards one opening instead of another. A live, free-moving crab was placed in the jar as reward and was visible to the animal (transparent object), as opposed to the black box (opaque object) used for the social learning task.
Each plug protruded by 4 cm from the jar so that, even at a distance, the octopus could well distinguish the plugs from the rest of the jar. Of the three techniques, or operanda, to be opened the “pull” (positioned at the top of the jar) was to be simply unplugged, the “screw” (placed on one of the widest sides) was to be unscrewed and pulled away, while the “shutter” (on one of the shortest sides) was like a door in that it could be opened only by pulling on the right side (the one free of hinges). Each operandum was designed in order to require the same strength to be opened by the octopus. Animals could open one, two or all three plugs before being able to reach for the crab.
The multi-openable jar was similar, in principle, to that used in previous experiments (Fiorito et al. 1990, 1998b) but differed both for shape (a parallelepiped vs. a cylinder) and for number of possible openings (three vs. one solution). These characteristics made the jar more comparable to the objects used in problem-solving tests with other animals (e.g. in birds: Webster and Lefebvre 2001).
A ceiling latency of 601 s was assigned to animals that failed to respond within the 10-min interval, following which the jar was pulled out of the tank and the trial ended. For animals that solved the task (opened and removed prey) an additional 10 min were added starting from the seizure of the crab to analyse the potential exploration towards the jar following predation. In case of failure, a crab was given to the octopus anyway 5 min from the removal of the multi-openable jar.
Preferences and individual learning: day 12
Preferences
The procedure utilized for the preferences experiment derived from the protocol for simultaneous visual discrimination training in octopuses originally developed by Boycott and Young (1956). We applied this protocol but following the modifications of Fiorito and co-workers (Balzano 2003; Borrelli 2000; Coppola 1994; Fiorito and Scotto 1992; Maltese 1998).
Smooth plastic balls, white and red, were used as discriminanda. Since O. vulgaris is considered to be colour blind (Marshall and Messenger 1996; Messenger 1977), this should be considered as a brightness discrimination (Fiorito and Scotto 1992). Each ball (4 cm in diameter) was supplied with a pair of stainless steel probes protruding from its wall for 5 mm and was fixed at the extremity of a translucent nylon stick (80 cm in length). A cylinder handle, at the other end of the stick, allowed the experimenter to control positioning and movement of the stimuli by hand.
To test individual preferences, each octopus was simultaneously presented, for five consecutive trials, to the pair of stimuli. Both balls had a piece of fresh anchovy skewered to the pair of probes; therefore, the octopus received a reward for any choice made.
The tester introduced and removed the balls from the tank by hand. Stimuli were landed at approximately 35 cm from each other and at a distance of about 80 cm from the animal’s resting position in the den. They were inserted with the probes facing the frontal glass panel of the tank in order to hide the reward from the octopus. Once the stimuli landed on the bottom of the tank, they were kept still, in position, for approximately 10 s; in the absence of response by the octopus they were moved (one movement per second) first in place and then backwards and forwards with an up–down movement of the two objects. The vertical displacement of the balls was always within 2 cm from the bottom of the tank.
A trial started when the experimenter introduced the stimuli and ended when the animal pounced on one of the balls. Only one choice was possible in each trial; therefore, once the octopus attacked one of the two stimuli, the other one was quickly removed from the tank. A timer was set for 2 min starting from when the octopus pounced on one of the balls; if the animal was back home and willing to release the stimulus by the end of this interval, the trial was ended for the next one to be set up. If, on the other hand, the octopus was not back in its den and/or was still “possessive” towards the ball, an additional 2-min interval was added following which the trial was ended anyway. Trials were spaced by 1-min intervals. In case of no response (i.e. no attack) from the animal, a ceiling latency was fixed to 61 s.
Finally, consideration was given to the relative position (proximal, distal) of the two discriminanda in respect to the animal’s den that was used as reference point; the stimulus that resulted aligned with the den was considered proximal. The relative position of both stimuli was randomized for the first trial by a coin flip, after which (trials 2–5) the position of the two balls was alternated.
Individual learning
Each octopus was tested for its capability to learn individually by using a passive avoidance task. Octopuses learn to not respond to (i.e. not attack) a stimulus that is always associated with a negative reinforcement (electric shock), known as passive avoidance learning. The procedure utilized derives from that originally designed by Sanders and Barlow (1971) following the modifications of Fiorito and colleagues (De Simone 1996; Di Dato 2000; Zarrella et al. 2005, 2015).
The ball preferred by each animal during the previous preferences test was used, this time, as negative stimulus. According to this criterion, the task carried out as “Individual learning” test should be more appropriately considered as reversal learning: the octopus must learn not to attack the ball that, in its previous experience, had brought a reward. The individual learning experiment started at least an hour following the last trial of the preferences test.
A single session of trials (massed training) was carried out up to reaching criterion. The landing, positioning, and movement of the stimulus were accomplished as previously described. However, in this case, since a single ball was used, we preferred landing it at the centre of the tank with the electrodes, again, facing the frontal glass panel. A timer was set for 2 min as maximum duration of the trial (ceiling latency: 121 s), starting from when the ball landed on the bottom of the tank; 1 min was fixed as inter-trial interval.
At the beginning of the training session, an octopus readily attacked the ball; each attack (or contact) with the ball was punished with an electric shock (12 V AC of 2–3 s duration, about 300 mA) delivered through the electrodes of the ball by pressing a button fixed on top of the cylinder handle. The presentation of the negative stimulus continued up to when the animal did not touch the ball for six consecutive trials (criterion).
At the end of training, each octopus was tested for generalization (i.e. associative learning) and motivational effects potentially induced by the passive avoidance learning task. Therefore, 5 min after reaching criterion (i.e. following the last trial of training) the other ball was presented (the one not used for conditioning to avoid: unconditioned stimulus; 2-min trial duration). If the animal did not respond to the ball within the 2-min presentation, the ball was removed. Finally, each octopus was fed a crab 1 min afterwards (if no predatory response appeared after 5 min, the crab was removed).
Scoring performances and behaviours
All experiments were videotaped by remote-controlled colour 3-CCD video cameras (JVC, Model KY-F32, Japan) connected to video recorders (Panasonic, Model AGDV2700, Japan) through video timers (For-A, Model VTG-33, Japan). For each experiment, the video camera was hidden from the animal’s view and framed the frontal glass panel of the tank, which gave a complete view of the position and behaviour of the animal during the test. However, only during the social learning experiment the video camera framed a mirror, positioned on top of the tank, instead of the frontal glass panel. This allowed a bird’s eye view of the tank and of the animals, required to score each observational trial (see materials and methods), and to facilitate subsequent analysis of the performance of the octopuses (demonstrators and observers). Furthermore, a twin flat screen monitor was installed in the room to allow remote control and observation of the behaviour of the animals during the various phases of the battery.
For a detailed overview of the variables (quantitative and qualitative) measured through video recordings refer to Supplementary Information and Supplementary Table 1.
Statistical analysis
For all statistical analyses, we used SPSS (rel. 13.0, SPSS Inc-Chicago, 2004). All tests were two-tailed (unless otherwise stated) and the alpha was set at 0.05.
The data collected throughout the experiments was not normally distributed, as resulted by using a Shapiro–Wilk’s test.
Measures of central tendency for non-normally distributed data were given as the median (quartiles, ranges). Box and whisker plots were utilized, where boxes represented the interquartile range (25th–75th percentiles), bars within boxes the median values, whiskers the 10th and 90th percentiles, and stars the outliers.
Within- and between-group comparisons were assessed with a Mann–Whitney U test and a Kruskal–Wallis test, respectively. Expected or unexpected trends were analysed by performing a Meddis test for trends or a Friedman ANOVA (Meddis 1984; Zar 1999). Whenever appropriate, when trends were significant Bonferroni corrected Wilcoxon matched-pairs signed-rank tests were used as post hoc procedure. Relationships between independent variables (e.g. latencies and types of attack) were assessed with Spearman correlations.
Octopuses’ performance (success/failure by sex, season, site of capture, etc.) or choice (e.g. crab vs. NOB, etc.) was analysed mainly with the G test (null hypothesis 50:50). Williams’ correction was used (unless otherwise stated).
Power analysis was conducted for all cases where marginally non-significant results could be explained by small sample size.
Results
Performance (quantitative analysis)
Acclimatization
Day 1 of Acclimatization (day 1 after arrival) was the most critical (median = 37.2 s, 22.2 and 125.3 s), with twelve animals (out of 55, 22%; six males and six females) that did not attack the crab and were scored 301 s (ceiling latency; see Supplementary Fig. 1a). By the end of acclimatization (day 5), however, all the animals attacked the crab within the ceiling latency (median = 5.9 s, 3.6 and 13.8 s; Fig. 3). This general and linear decline in latency to attack corresponded to a significant change over time (Meddis test for trends: Z = 18.83, N = 55, p < 0.001), showing that the octopuses resumed their “normal” predatory behaviour in captivity (see Supplementary Fig. 1b–c).
Box plots showing O. vulgaris latencies to attack (seconds) the crab during the 5 days of the Acclimatization. Boxes represent the interquartile range (25th–75th percentiles), bars within boxes the median values, whiskers the 10th and 90th percentiles, and stars the outliers. A general decline in latency to attack is observed over time (p < 0.001, after Meddis test for trends); see text for details
Neophobia
All the octopuses continued to promptly attack the prey, when presented alone (crab, CRA) or with the novel object (NOB), throughout the two neophobia experiments (refer to Supplementary Fig. 2), with the exception of one animal that did not attack the crab in the presence of the novel object during Neophobia 1st and was scored 301 s. As summarized in Fig. 4, the performance of O. vulgaris throughout the four trials of both morning (M_NOB group) and afternoon (A_NOB group) blocks remained stable, the main differences in performance explained by outliers, which responded with a high latency (67.5 and 130.4 s) and were responsible for the general trend as they showed very high latencies in each presentation (crab, CRA and NOB trials), reaching the peak in the NOB condition, where one of the two did not attack (Borrelli 2007).
Box plots showing the latencies to attack (seconds) the prey (crab, CRA) and/or novel object (NOB) by octopuses presented with the crab complex during morning (M_NOB group) or afternoon (A_NOB group) blocks of the Neophobia 1st (light bars) and Neophobia 2nd (dark bars) experiment. See text for details
Moreover, the repetition of crab presentations did not have any effect on the predatory performance of the animals throughout neophobia experiments (Wilcoxon matched-pairs signed-rank tests, crab morning vs. afternoon block: Neophobia 1st: Z = − 0.18, N = 55, p = 0.863; Neophobia 2nd: Z = − 0.98, N = 55, p = 0.332).
Considering no significant difference between the sequences (morning/afternoon) in which the crab complex had been presented to the octopuses, potential neophobia towards the novel object was tested by pooling the latencies of both blocks in: (1) those performed towards the tethered crab alone (CRA: Familiar), and (2) those performed towards the crab and the novel object (NOB: Novel). No significant difference emerged for Neophobia 1st (Wilcoxon signed-rank test: Novel vs. Familiar: Z = − 1.57, N = 55, p = 0.117), whereas an heterogeneity emerged in Neophobia 2nd (Novel vs. Familiar: Z = − 3.92, N = 55, p < 0.001). In the latter case, the latencies were relatively higher when the animals were faced with the novel object (median = 6.4 s, 4.7 and 10.4 s) than when they were presented with the crab alone (3.8 s, 3.1 and 5.0 s).
These results were further confirmed by distinguishing the presentations on the basis of the relative visibility of the object (Novel vs. Familiar: Neophobia 1st: “full”: T = 118.5, N = 25, p = 0.244; “partial”: T = 30.0, N = 12, p = 0.519; “poor”: T = 66.99, N = 18, p = 0.442. Neophobia 2nd: “full”: Z = − 3.06, N = 36, p = 0.002; “partial”: T = 3.0, N = 5, p = 0.313; “poor”: T = 16.29, N = 14, p = 0.020) and by measuring the choices made between crab and novel object when both were presented together (Neophobia 1st: 37% attacked the novel object, G1 = 3.64, p = 0.056, power = 98%; Neophobia 2nd: 9% attacked the novel object, G1 = 42.35, p < 0.001).
Social learning
During the social interaction (day 7), all the octopuses reached a steady-state equilibrium and were exposed, the following day, to the observational phase (results given in Supplementary Information: Social learning-Observational phase), followed by the testing phase (Supplementary Fig. 3).
Half of the octopuses (social 1: N = 27, 49%; social 2: N = 29, 53%) were successful in opening the drawer and preying on the crab after social demonstration by a conspecific octopus (demonstrator) on the first day of the experiment and when the test was repeated the following day (McNemar’s test: success vs. failure social 1, 2: p = 0.688). Among the unsuccessful animals, 40% did not even touch the box on social 1, remaining in the den for the whole trial duration, although only 31% did not touch or explore the problem box the following day (social 2). The results suggest that, in our experimental conditions, learning by observation was a difficult task for O. vulgaris and that the repetition of the demonstration had little effect on the animals’ social learning capabilities.
On social 1, 33 animals (out of 55, 60%) attacked the black box with a median latency of 135.2 s (24.6 and 372.5 s). Thus the box per se, which obscured the prey from sight, was not a sufficiently appealing visual stimulus to elicit an attack response in all the animals. Twenty-eight octopuses (51%) opened the drawer with a median latency of 255.2 s (79.0 and 465.6 s) and all, but one, also caught and ate the crab hidden inside taking a median of 260.2 s (94.3 and 500.1 s) to prey on the crab. We further tested whether the latency to attack the box and open the drawer was comparatively different between the octopuses that were successful and those that were unsuccessful in resolving the black-box problem. Not surprisingly, octopuses that failed in the task took on average a greater amount of time to touch the object (LA: 1201 s, 262.7–1201 s) and open the drawer (LOP: 1201 s, 376.7–1201 s) than those that succeeded (LA: 96.8 s, 23.3 and 240.8 s; LOP: 253 s, 74.6 and 482.7 s). The trend of the failures, in fact, was largely influenced by the proportion of animals that did not attack the box at all, let alone open the drawer.
When latencies were compared between days, the octopuses significantly improved their performance after a second exposure to the conspecific demonstrator. In fact, the animals scored a comparatively lower latency to attack (LA) and prey on the crab (LP) when the task was repeated the following day than when they were first exposed to the problem (Wilcoxon matched-pairs signed-rank test: social 1 vs. social 2: LA: Z = − 2.56, p = 0.010; LP: Z = − 2.28, p = 0.022). The latency to open the drawer, instead, was slightly above significance between days (LOP: Z = − 1.94, p = 0.052, Monte Carlo p = 0.056, 95% CIMC = 0.052–0.061). A more detailed analysis of the data revealed that on the first day octopuses spent more time to touch the object (LA: 501.1 s, 96.8 and 1201 s) than to find the solution to the problem (LOP: 1119.4 s, 253.0 and 1201 s; LP: 1201 s, 260.2 and 1201 s). On the second day instead, being more confident towards the box, they spent less time to touch the object (LA: 239.6 s, 10.9 and 1201 s) but took more time to open the drawer and prey on the crab (LOP: 873.3 s, 61.3 and 1201 s; LP: 988.7 s, 84.6 and 1201 s), which may explain the differences in significance of our results.
Problem solving
Forty-one animals (out of 55, 75%) passed the problem-solving task (opened the jar and preyed on the crab), while 14 octopuses (25%) failed in the problem-solving task of which six (11%) opened but did not prey on the crab and eight (14%) did not even open the medium (Supplementary Fig. 4). The mean latency to attack (Mann–Whitney test: U = 180, N1 = 41, N2 = 14, p = 0.038Footnote 2) and to open the jar (Z = − 0.01, N1 = 6, N2 = 41, p = 0.938) of successful and unsuccessful octopuses was not statistically significant, suggesting that success or failure in the task was probably not influenced by a difference in motivation to attack the jar or a difference in problem-solving abilities.
Preferences
All the octopuses attacked the artificial stimuli from the beginning and throughout the preferences experiment (Supplementary Fig. 5), the time of attack of the animals declining significantly over the five consecutive presentations of the balls (Fig. 5; Meddis test for trends: Z = 9.46, N = 55, p < 0.001).
Box plots showing the latencies to attack (seconds) the artificial balls during the five trials of the Preferences experiment. O. vulgaris associated the artificial stimuli with a rewarding experience as shown by a significant decline over time of the latency to attack the stimuli. See text for details
In addition, a series of Wilcoxon matched-pairs signed-rank tests, used as post hoc procedures, revealed significant differences between the first and the following trials (p < 0.0025, Bonferroni corrected significance level = 0.0025), but not for the other comparisons, suggesting that the animals had associated the artificial stimuli with a rewarding experience.
Individual learning
All the octopuses (N = 55) successfully reached criterion (i.e. learned to not respond to the ball for six consecutive trials) in a median number of 9.0 trials and with a range of 4–21 trials (Fig. 6, Supplementary Fig. 6).
A Meddis test for trends was carried out to compare the latencies to attack among trials. Since the fastest octopuses (N = 4) reached criterion at trial 4 (total trials = 9), the analysis was restricted to the first nine trials to include the whole data set. The results suggest that O. vulgaris rapidly learns to avoid a stimulus associated with a negative reinforcement (Z = 91.46, N = 55, p ≪ 0.001). A series of pairwise comparisons among the nine trials of the individual learning experiment revealed significant differences between each pair except for trial 1 versus trial 2, trial 3 versus trial 4, trial 5 versus trials 6 and 7 and, as expected, for each pair following trial 6 (Bonferroni corrected significance level = 0.00069). In addition, a Wilcoxon matched-pairs signed-rank test that compared the latency to attack the stimulus at the first trial and at the trial preceding criterion was consistent with previous results (Z = − 6.45, N = 55, p < 0.001).
Most of the octopuses (N = 44, 80%) touched the unconditioned stimulus, which was presented to all the animals following the training session, while the remaining 11 animals did not respond and were scored 121 s (ceiling latency). A further analysis conducted by excluding the 11 non-responders showed that, not surprisingly, the latencies to attack the ball at the test were significantly higher than the latencies to attack the ball at the first trial of the training session (Z = − 5.72, N = 44, p < 0.001) although they were significantly lower than the latencies at the trial preceding criterion (Z = − 2.10, p = 0.035). In fact, the results showed that the octopuses attacked the ball at the test (median = 49.9 s, 25.6 and 68.5 s) more rapidly than the ball at the trial preceding criterion (58.9 s, 43.1 and 70.0 s), although they remained slower at the test than at the first trial of the training session.
Discussion
The leit motif throughout the battery of experiments presented here was Octopus’ predatory behaviour. This was exploited both to study the recovery in predatory performance following capture (acclimatization) and to evaluate the possible interference of various stimuli (e.g. tethered crabs, boxes and jars, and plastic balls) on the octopuses’ attack response. The predatory performance of each animal was recorded throughout the eight experiments as a measure of the species inter-individual variability, in order to validate a battery of tests and to provide scientists of different research domains with a detailed protocol to work with the cephalopod mollusc Octopus vulgaris.
A consolidated practice of learning paradigms for octopuses and other cephalopods (mainly Sepia officinalis) is that an experimental protocol should start after a period of acclimatization for the animal in the captive situation. From the pioneering studies initiated at the end of the 1940 s (Boycott 1954) up to recent times, the acclimatization period is a variable length of time during which the animal is exposed to a novel environment (the tank and its surroundings) and presented (generally ad libitum or on a daily schedule) with a live prey (e.g. Amodio et al. 2014; Boycott and Young 1955; Messenger and Sanders 1972; Palmer et al. 2006). In our experiments, the acclimatization period was fixed to 5 days, a time which was sufficient for octopuses to adapt to captivity. A steady and progressive decline in latency to attack the tethered crab occurred over consecutive days and all the octopuses recovered their predatory performance by the end of the experiment. To the best of our knowledge, a fixed length of time for animals to acclimatize has never been applied to octopuses. Therefore, here we define the optimal temporal window to start the investigations after capture so as all future studies will benefit from an equal treatment of the animals augmenting the possibility to compare the effects of treatments and manipulations.
Exposure to novel stimuli may interfere with an animal’s decision-making processes (sensu Greenberg 1983; review in Greenberg and Mettke-Hofmann 2001; see also: Mettke-Hofmann et al. 2005, 2006). In common practice, neophobia is measured by comparing an animal’s response to food items presented near novel objects, as opposed to its response to food alone. In our experimental procedure, the food item (i.e. prey) was presented together with a metallic cross (Neophobia 1st) or a lid (Neophobia 2nd).
A detailed review of the literature carried out on several bird species allowed Greenberg and Mettke-Hofmann (2001) to assert that generalist species show less neophobia than more specialist ones. Despite the scarcity of field studies on octopuses’ behavioural habits, these animals are commonly considered generalist, opportunistic predators (review in Hanlon and Messenger 1996, 2018); in particular, O. vulgaris seems to show less feeding specialization and a higher versatility in foraging than other cephalopods (Mather 1984; see also: Nixon and Mangold 1998).Footnote 3 Thus, on the basis of these considerations, a low level of neophobia is expected to be observed in O. vulgaris. This hypothesis was confirmed in our conditions where all the animals continued to promptly attack the prey and with no significant change in predatory performance over successive presentations, both when the crab was presented alone and when it was presented in the presence of the novel object. However, contrary to expectations, fear towards novel stimuli slightly increased as the experiments proceeded so that the effect of the social “experience” (preceding Neophobia 2nd) is not to be underestimated.
Despite more than a century of research, imitative learning (or true vicarious learning) is still notoriously difficult to demonstrate. In the typical experiment, a naïve “observer” animal is exposed to a task being performed by a trained “demonstrator” animal, and subsequently tested in isolation to see if it has acquired the same behaviour. The critical point in experiments of this kind is the difficulty of excluding other possible explanations for a match between the behaviours of the demonstrator and the observer (for review see: Whiten and Ham 1992; Zentall 2001, 2006) that involve: (1) the demonstrator drawing the observer’s attention to a particular location or stimulus in the environment (i.e. local or stimulus enhancement); (2) the socially mediated acquisition of the association between a stimulus and a reinforcer (i.e. observational conditioning), or (3) the mere presence of the conspecific that may influence the observer’s behaviour (i.e. contagion or social facilitation).
The ability of octopuses to “copy” the behaviour of conspecifics has already been established (Fiorito and Scotto 1992) and was also replicated to test whether observational learning could somehow be related to the neural circuit known to modulate learning in the octopus (Fiorito and Chichery 1995).Footnote 4
Our results suggest that the observation of a trained demonstrator attacking the box and opening the drawer to catch a crab hidden inside facilitates the subsequent behavioural performance by naïve O. vulgaris. Therefore, we may suppose that at least social facilitation or stimulus enhancement (for review see: Whiten and Ham 1992; Zentall 2001, 2006) may be the factor that led half of our sample of octopuses to benefit from the vicarious experience.
The outcome of the social learning task in our battery, however, was a surprisingly low performance on average when compared to the accuracy reached by O. vulgaris after observational phases in other experimental contexts (Amodio and Fiorito 2013; see also Fiorito and Scotto 1992). An explanation can be found in the integration of visual and tactile information acquired vicariously that appears to be difficult in the octopus especially in the light of the design of O. vulgaris’ sensory-motor neural circuit. From the observers’ point of view, tactile cues were very difficult to deduce (and apply) simply by visual observation of the task. In contrast, previous experiments published on social learning (Fiorito 1993; Fiorito et al. 1998a; Fiorito and Chichery 1995; Fiorito and Scotto 1992) were essentially based only on a visual cue: observers, in isolation, chose the same ball they had seen their demonstrators choose during the observational phase.
Neuroanatomical and experimental evidence show that visual and tactile inputs are classified, processed and stored separately over a series of intersecting neural matrices (Young 1991, 1995). Although some anatomical components are in common between the two systems (e.g. frontal and vertical lobes) there is no evidence of computational association between the two (Young 1991) as occurs in higher vertebrates, for example in mammals (e.g. Gottfried and Dolan 2004; Gottfried et al. 2004; Ohara et al. 2006; Suchan et al. 2006), but at the level of effectors, i.e. the peripheral nervous system (Allen et al. 1986; Bradley and Young 1975; review in Young 1995). Therefore, it is not surprising that the limit imposed by parallel neural processing corresponds to a limit in the integration of cues from different modalities and in the capability of recalling the required motor patterns necessary to solve the black box as derived from vicarious experience.
In any case, the black-box task requires less in terms of experimenter efforts and may easily be standardized and transferred in different experimental contexts.
Octopuses showed the capacity to solve problems, i.e. to apply known sensory modalities to a novel context, as has been shown in many birds and mammals (review in Reader and Laland 2003). In our experimental conditions, most of the animals (41 octopuses, the 74.5% of the sample) were successful in the task by opening one or more plugs (pull, screw, shutter) and by preying on the crab. The apparatus was constructed taking inspiration from the one designed by Webster and Lefebvre (2001) to test innovative problem-solving capabilities in columbiform–passeriform assemblages. The clear Plexiglas box of Webster and Lefebvre had three plugs that could be opened by pulling, pushing, or removing the lids. In analogy to what is required for birds, octopuses must integrate visual and mainly tactile-driven cues in order to manipulate the object properly. As mentioned above, in doing this Octopus uses species-specific motor patterns pertaining to the animals’ predatory behaviour, in analogy to what occurs in birds (Bouchard 2002; Webster and Lefebvre 2001). The combination of the pulling actions and the exploratory behaviour applied to the composed maze (sensu Fiorito et al. 1990) results in the novel aspect of the present work, alongside with the establishment of a protocol to be used in assessing personality.
Quite a number of studies have been focused on the visual discrimination capabilities of O. vulgaris (review in: Sanders 1975; Wells 1965, 1978). These works have shown that octopuses are capable of learning to discriminate between a large variety of artificial objects appearing in their context when the two discriminanda are either presented in succession (review in Sanders 1975) or simultaneously (review in Boal 1996). However, as far as we know, only a handful of papers have analysed the spontaneous preferences of octopuses towards one of two discriminanda presented simultaneously, in any detail (review in Boal 1996). In our experimental conditions, animals showed a marked preference for the red ball, a result in line with previous studies showing that O. vulgaris prefers black stimuli to white on light backgrounds (Bradley and Messenger 1977; Young 1968) and that red balls are preferred to white in a simultaneous discrimination task (Fiorito and Scotto 1992). Since O. vulgaris is considered to be colour blind (Marshall and Messenger 1996; Messenger 1977), the discrimination of objects with different shade can be considered a brightness discrimination. Further studies should be carried out in order to verify if light polarization may also guide octopuses in the discrimination of objects.
Notwithstanding, the marked preference towards the red ball resulting in our experiments was greater than in any other published work to date, which can be explained if both stimulus generalization and contextual learning occurred during the battery of experiments. In fact, they are not surprising if we consider that the octopus easily associates the ball with a reward (contextual learning) and that the dark ball resembles the crab more than the white one (stimulus generalization): a case of positive learning (sensu Maldonado 1963; but see Young 1956).
Octopuses were presented with the artificial ball they had preferred during preferences also to test their individual learning capabilities. In this case, as opposed to Preferences, any contact with the ball was negatively reinforced (mild electric shock). O. vulgaris successfully learned to not attack (i.e. avoid) an artificial stimulus associated with a negative reinforcement. Learning was measured by a linear and progressive increase in the latency to attack the ball; by the end of the experiment all the octopuses were not responding and had reached the ceiling latency.
A number of studies have been focused on avoidance either as a training protocol or as a defence mechanism in both invertebrates (e.g. Dalesman et al. 2006; Denti et al. 1988; Pritchatt 1968, 1970; Sinn and Moltschaniwskyj 2005) and vertebrates (e.g. Budaev and Zhuikov 1998; Cook et al. 1987; Dunlop et al. 2006; Frontali and Bignami 1973; Laska and Metzker 1998; Mineka 1979). Avoidance has not only been employed to test the behavioural responses, but also to investigate the biological machinery and neural correlates involved in the processing of such a simple, reliable training task (e.g. Asok et al. 2019; Fendt and Fanselow 1999; Knapska et al. 2006; Ohi 1975; Tinsley et al. 2004; Tovote et al. 2015). It has been also applied in octopus for similar aims (Zarrella et al. 2015).
This protocol also offers great advantages to explore inter-individual variability, and how and to what extent the shy-bold continuum may influence individual learning.
Our battery of experiments, thus, demonstrates that both positive and negative learning processes (Maldonado 1965) may occur in O. vulgaris. In fact, octopuses were mostly favoured to attack the stimuli (crabs, novel objects, opaque and transparent boxes) presented to them over following days but were also tested on their capacity to learn to not respond to a noxious stimulus (the last day of the experimental array). Both processes (positive and negative) are mediated by brain centres (e.g. Boycott and Young 1955; Maldonado 1965; Marini et al. 2017; Young 1961).
May we consider our battery of experiments as the ‘gold standard’ behavioural tasks that any cephalopod worker should use to phenotype an octopus? Standardized screens that rely on a small number of tests are useful but experimental design and choice of tests should always be related to the given research hypothesis to be tested. On the other hand, we are strongly convinced that the experiments presented here represent a valuable tool and offer standardized procedures for the estimation of animals’ performance and inter-individual variability. Standardization is highly required and desirable when behavioural studies should be combined with other approaches that may shed light on the biological machinery underlying behaviour and learning in these animals.
Notes
See also papers included in the Frontiers in Ecology and Evolution Research Topic ‘The Development of Animal Personality’, https://www.frontiersin.org/research-topics/2570/the-development-of-animal-personality.
Significance in latency to attack between successful and unsuccessful animals was influenced by an outlier (animal 03/39) that took 264.6 s to attack the jar and determined the whole trend of the octopuses that failed in Problem Solving.
See also for review, second part of Borrelli (2007).
References
Allen A, Michels J, Young JZ (1986) Possible interactions between visual and tactile memories in Octopus. Mar Behav Physiol 12:81–97
Amodio P, Fiorito G (2013) Observational and other types of learning in octopus. In: Menzel R, Benjamin PR (eds) Invertebrate learning and memory, Handbook of behavioral neuroscience, vol 22, pp 293–302. https://doi.org/10.1016/b978-0-12-415823-8.00023-x
Amodio P, Andrews P, Salemme M, Ponte G, Fiorito G (2014) The use of artificial crabs for testing predatory behavior and health in the octopus. ALTEX-Altern Anim Exp 31:494–499
Anagnostopoulos AV, Mobraaten LE, Sharp JJ, Davisson MT (2001) Transgenic and knockout databases: behavioral profiles of mouse mutants. Physiol Behav 73:675–689
Asok A, Kandel ER, Rayman JB (2019) The neurobiology of fear generalization. Front Behav Neurosci 12:329. https://doi.org/10.3389/fnbeh.2018.00329
Balzano O (2003) Contributo alla conoscenza della inibizione della sintesi proteica nei processi di memorizzazione a lungo termine in Octopus vulgaris (Mollusca, Cephalopoda). Università degli Studi di Napoli “Federico II”. Facoltà di Scienze Matematiche, Fisiche e Naturali
Barbato M, Bernard M, Borrelli L, Fiorito G (2007) Body patterns in cephalopods. “Polyphenism” as a way of information exchange. Pattern Recognit Lett 28:1854–1864
Boal JG (1996) A review of simultaneous visual discrimination as a method of training octopuses. Biol Rev 71:157–190
Borrelli L (2000) Effetti della punizione sull’apprendimento e la ritenzione di compiti di discriminazione visiva simultanea nel polpo Octopus vulgaris (Mollusca, Cephalopoda). Università degli Studi di Napoli “Federico II”. Facoltà di Scienze Matematiche, Fisiche e Naturali
Borrelli L (2007) Testing the contribution of relative brain size and learning capabilities on the evolution of Octopus vulgaris and other cephalopods. PhD Thesis, Stazione Zoologica Anton Dohrn, Italy and Open University, UK
Borrelli L, Gherardi F, Fiorito G (2006) A catalogue of body patterning in Cephalopoda. Stazione zoologica A. Dohrn. Firenze University Press, Napoli
Bouchard J (2002) Is social learning correlated with innovation in birds? An inter-and an intraspecific test. McGill University, Montreal
Boycott BB (1954) Learning in Octopus vulgaris and other cephalopods. Pubbl Staz Zool Napoli 25:67–93
Boycott BB, Young JZ (1955) Memories controlling attacks on food objects by Octopus vulgaris Lamarck. Pubbl Staz Zool Napoli 27:232–249
Boycott BB, Young JZ (1956) Reactions to shape in Octopus vulgaris Lamarck. In: Proceedings of Zoological Society of London, vol 126, pp 491–547
Bradley EA, Young JZ (1975) Are there circadian rhythms in learning by Octopus? Behav Biol 13:527–531
Bradley EA, Messenger JB (1977) Brightness preference in Octopus as a function of the background brightness. Mar Behav Physiol 4:243–251
Budaev SV, Zhuikov AY (1998) Avoidance learning and “personality” in the guppy (Poecilia reticulata). J Comp Psychol 112:92–94
Calvé MR (2005) Individual differences in the common cuttlefish, Sepia officinalis. Department of Biology Dalhousie University, Halifax
Chapko MK, Grossbeck ML, Hansen RL, Maher TD, Middleton RS, Simpson RW (1962) Devilfish. A practical guide to the dissection of octopus. Wayne Senior High School, New York
Cook M, Mineka S, Trumble D (1987) The role of response-produced and exteroceptive feedback in the attenuation of fear over the course of avoidance learning. J Exp Psychol Anim Behav Process 13:239–249
Coppola M (1994) Studio delle variazioni comportamentali del polpo comune Octopus vulgaris (Mollusca, Cephalopoda) durante l’apprendimento di compiti discriminativi. Università degli Studi di Napoli “Federico II”. Facoltà di Scienze Matematiche, Fisiche e Naturali
Crabbe JC, Wahlsten D, Dudek BC (1999) Genetics of mouse behavior: interactions with laboratory environment. Science 284:1670–1672
Crawley JN (1999) Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res 835:18–26. https://doi.org/10.1016/S0006-8993(98)01258-X
Crawley JN (2008) Behavioral phenotyping strategies for mutant mice. Neuron 57:809–818
Crawley JN, Paylor R (1997) A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav 31:197–211
Dalesman S, Rundle SD, Coleman RA, Cotton PA (2006) Cue association and antipredator behaviour in a pulmonate snail, Lymnaea stagnalis. Anim Behav 71:789–797
De Simone ML (1996) NO ed apprendimento per evitamento passivo in Octopus vulgaris (Mollusca, Cephalopoda). Università degli Studi di Napoli “Federico II”. Facoltà di Scienze Matematiche, Fisiche e Naturali
Denti A, Dimant B, Maldonado H (1988) Passive avoidance learning in the crab Chasmagnathus granulatus. Physiol Behav 43:317–320
Di Dato V (2000) Effetti dell’inibizione della sintesi proteica sull’apprendimento e memorizzazione di compiti di evitamento passivo in Octopus vulgaris (Mollusca Cephalopoda). Università degli Studi di Napoli “Federico II”. Facoltà di Scienze Matematiche, Fisiche e Naturali
Dunlop R, Millsopp S, Laming P (2006) Avoidance learning in goldfish (Carassius auratus) and trout (Oncorhynchus mykiss) and implications for pain perception. Appl Anim Behav Sci 97:255–271
Fendt M, Fanselow MS (1999) The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev 23:743–760
Fiorito G (1993) Social learning in invertebrates. Response. Science 259:1629
Fiorito G, Chichery R (1995) Lesions of the vertical lobe impair visual discrimination learning by observation in Octopus vulgaris. Neurosci Lett 192:117–120
Fiorito G, Scotto P (1992) Observational learning in Octopus vulgaris. Science 256:545–547
Fiorito G, von Planta C, Scotto P (1990) Problem solving ability of Octopus vulgaris lamarck (Mollusca, Cephalopoda). Behav Neural Biol 53:217–230
Fiorito G, Agnisola C, d’Addio M, Valanzano A, Calamandrei G (1998a) Scopolamine impairs memory recall in Octopus vulgaris. Neurosci Lett 253:87–90
Fiorito G, Biederman GB, Davey VA, Gherardi F (1998b) The role of stimulus preexposure in problem solving by Octopus vulgaris. Anim Cogn 1:107–112
Frontali M, Bignami G (1973) Go-no go avoidance discriminations in rats with simple “go” and compound “no go” signals: stimulus modality and stimulus intensity. Anim Learn Behav 1:21–24
George EA, Brockmann A (2019) Social modulation of individual differences in dance communication in honey bees. Behav Ecol Sociobiol 73:41. https://doi.org/10.1007/s00265-019-2649-0
Gerlai R (2019) Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacol Biochem Behav 178:30–38. https://doi.org/10.1016/j.pbb.2018.02.005
Gottfried JA, Dolan RJ (2004) Response to small: crossmodal integration–insights from the chemical senses. Trends Neurosci 27:123–124
Gottfried JA, Smith AP, Rugg MD, Dolan RJ (2004) Remembrance of odors past: human olfactory cortex in cross-modal recognition memory. Neuron 42:526–527
Greenberg R (1983) The role of neophobia in determining the degree of foraging specialization in some migrant warblers. Am Nat 122:444–453
Greenberg R (1984) The winter exploitation systems of Bay-breasted and Chestnut-sided warblers in Panama, vol 116. University of California publications in zoology, University of California Press, Berkeley
Greenberg R, Mettke-Hofmann C (2001) Ecological aspects of neophobia and neophilia in birds. In: Nolan VJ, Thompson CH (eds) Current ornithology, vol 16. Kluwer Academic/Plenum Publishers, New York, pp 119–178
Hanlon RT, Messenger JB (1996) Cephalopod behaviour. Cambridge University Press, Cambridge
Hanlon RT, Messenger JB (2018) Cephalopod behaviour, 2nd edn. Cambridge University Press, Cambridge. https://doi.org/10.1017/9780511843600
Hedrick AV (2017) Editorial: the development of animal personality. Front Ecol Evol 5:14. https://doi.org/10.3389/fevo.2017.00014
Hobert O (2003) Behavioral plasticity in C. elegans: paradigms, circuits, genes. J Neurobiol 54:203–223
Knapska E, Walasek G, Nikolaev E, Neuhausser-Wespy F, Lipp HP, Kaczmarek L, Werka T (2006) Differential involvement of the central amygdala in appetitive versus aversive learning. Learn Mem (Cold Spring Harbor) 13:192–200
Laska M, Metzker K (1998) Food avoidance learning in squirrel monkeys and common marmosets. Learn Mem (Cold Spring Harbor) 5:193–203
Liang ZS, Nguyen T, Mattila HR, Rodriguez-Zas SL, Seeley TD, Robinson GE (2012) Molecular determinants of scouting behavior in honey bees. Science 335:1225–1228. https://doi.org/10.1126/science.1213962
Maldonado H (1963) The positive learning process in Octopus vulgaris. Zeitschrift für vergleichende Physiologie 47:191–214
Maldonado H (1964) The control of attack by Octopus. Zeitschrift für vergleichende Physiologie 47:656–674
Maldonado H (1965) The positive and negative learning process in Octopus vulgaris Lamarck. Influence of the vertical and median superior frontal lobes. Zeitschrift für vergleichende Physiologie 51:185–203
Maltese A (1998) Aspetti comparativi nell’apprendimento di un compito di discriminazione visiva simultanea: polpi (Octopus vulgaris; Mollusca, Cephalopoda) e delfini (Tursiops truncatus; Mammalia, Cetacea). Università degli Studi di Napoli “Federico II”. Facoltà di Scienze Matematiche, Fisiche e Naturali
Marini G, De Sio F, Ponte G, Fiorito G (2017) Behavioral analysis of learning and memory in cephalopods. In: Byrne JH (ed) Learning and memory: a comprehensive reference, volume 1—learning theory and behavior (Menzel, Randolf-volume Editor), 2nd edn. Academic Press, Elsevier, Amsterdam, pp 441–462
Marshall N, Messenger J (1996) Colour-blind camouflage. Nature 382:408
Mather JA (1984) Development of behaviour in Octopus joubini Robson, 1929. Vie et Milieu 34:17–20
Mather JA, Anderson RC (1993) Personalities of octopuses (Octopus rubescens). J Comp Psychol 107:336–340
Meddis R (1984) Statistics using ranks: a unified approach. Blackwell Publishing, USA
Messenger JB (1977) Evidence that Octopus is colour blind. J Exp Biol 70:49–55
Messenger JB, Sanders GD (1972) Visual preference and two-cue discrimination learning in Octopus. Anim Behav 20:580–585
Mettke-Hofmann C, Ebert C, Schmidt T, Steiger S, Stieb S (2005) Personality traits in resident and migratory warbler species. Behaviour 142:1357–1375
Mettke-Hofmann C, Rowe KC, Hayden TJ, Canoine V (2006) Effects of experience and object complexity on exploration in garden warblers (Sylvia borin). J Zool 268:405–413
Mineka S (1979) The role of fear in theories of avoidance learning, flooding, and extinction. Psychol Bull 86:985–1010
Nixon M, Mangold K (1998) The early life of Sepia officinalis, and the contrast with that of Octopus vulgaris (Cephalopoda). J Zool 245:407–421
Ohara S, Lenz FA, Zhou YD (2006) Modulation of somatosensory event-related potential components in a tactile-visual cross-modal task. Neuroscience 138:1387–1395
Ohi S (1975) The effects of actinomycin D on the brain RNA synthesis and on the passive avoidance latency in the goldfish. Jpn J Psychol 46:191–198
Palmer ME, Calvé MR, Adamo SA (2006) Response of female cuttlefish Sepia officinalis (Cephalopoda) to mirrors and conspecifics: evidence for signaling in female cuttlefish. Anim Cogn 9:151–155
Pritchatt D (1968) Avoidance of electric shock by the cockroach Periplaneta americana. Anim Behav 16:178–185
Pritchatt D (1970) Further studies on the avoidance behavior of Periplaneta americana to electric shock. Anim Behav 18:485–492
Pronk R, Wilson D, Harcourt R (2010) Video playback demonstrates episodic personality in the gloomy octopus. J Exp Biol 213:1035–1041
Reader SM, Laland KN (2003) Animal innovation: an introduction. In: Reader SM, Laland KN (eds) Animal innovation. Oxford University Press Inc., New York, pp 3–35
Sanders GD (1975) The cephalopods. In: Corning WC, Dyal JA, Willows AOD (eds) Invertebrate learning. Cephalopods and echinoderms, vol 3. Plenum Press, New York, pp 1–101
Sanders GD, Barlow JJ (1971) Variations in retention performance during long term memory formation. Nature 232:203–204
Sih A, Bell A, Johnson JC (2004a) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sih A, Bell AM, Johnson JC, Ziemba RE (2004b) Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277
Sinn DL, Moltschaniwskyj NA (2005) Personality traits in dumpling squid (Euprymna tasmanica): context-specific traits and their correlation with biological characteristics. J Comp Psychol 119:99–110
Sinn DL, Perrin NA, Mather JA, Anderson RC (2001) Early temperamental traits in an octopus (Octopus bimaculoides). J Comp Psychol 115:351–364
Sinn DL, Gosling SD, Moltschaniwskyj NA (2008) Development of shy/bold behaviour in squid: context-specific phenotypes associated with developmental plasticity. Anim Behav 75:433–442
Skoulakis EMC, Grammenoudi S (2006) Dunces and da Vincis: the genetics of learning and memory in Drosophila. Cell Mol Life Sci 63:975–988
Slater PJB (1981) Individual differences in animal behavior. In: Bateson PPG, Klopfer PH (eds) Perspectives in ethology. Springer, Boston, pp 35–49
Suchan B, Linnewerth B, Koster O, Daum I, Schmid G (2006) Cross-modal processing in auditory and visual working memory. NeuroImage 29:853–858
Swierczek NA, Giles AC, Rankin CH, Kerr RA (2011) High-throughput behavioral analysis in C. elegans. Nat Methods 8:592–598
Tinsley MR, Quinn JJ, Fanselow MS (2004) The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: comparing pavlovian fear conditioning and inhibitory avoidance. Learn Mem (Cold Spring Harbor) 11:35–42
Tovote P, Fadok JP, Luthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16:317–331. https://doi.org/10.1038/nrn3945
von Kortzfleisch VT, Kästner N, Prange L, Kaiser S, Sachser N, Richter SH (2019) Have I been here before? Complex interactions of age and test experience modulate the results of behavioural tests. Behav Brain Res 367:143–148. https://doi.org/10.1016/j.bbr.2019.03.042
Wahlsten D et al (2003) Different data from different labs: lessons from studies of gene–environment interaction. J Neurobiol 54:283–311
Webster SJ, Lefebvre L (2001) Problem solving and neophobia in a columbiform–passeriform assemblage in Barbados. Anim Behav 62:23–32
Wells MJ (1965) Learning in the octopus. Symp Soc Exp Biol 20:477–507
Wells MJ (1978) Octopus: physiology and behaviour of an advanced invertebrate. Springer, Berlin
Whiten A, Ham R (1992) On the nature and evolution of imitation in the animal kingdom: reappraisal of a century of research. Adv Stud Behav 21:239–283
Young JZ (1956) Visual responses by Octopus to crabs and other figures before and after training. J Exp Biol 33:709–729
Young JZ (1961) Learning and discrimination in the octopus. Biol Rev 36:32–96
Young JZ (1968) Reversal of a visual preference in Octopus after removal of the vertical lobe. J Exp Biol 49:413–419
Young JZ (1991) Computation in the learning system of cephalopods. Biol Bull 180:200–208
Young JZ (1995) Multiple matrices in the memory system of octopus. In: Abbott JN, Williamson R, Maddock L (eds) Cephalopod neurobiology. Oxford University Press, Oxford, pp 431–443
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Zarrella I, Borrelli L, Fiorito G (2005) Passive avoidance in Octopus vulgaris (Mollusca, Cephalopoda). National Congress of the Italian Society for Neuroscience and Joint Italian-Swedish Neuroscience Meeting Abstracts:592
Zarrella I, Ponte G, Baldascino E, Fiorito G (2015) Learning and memory in Octopus vulgaris: a case of biological plasticity. Curr Opin Neurobiol 35:74–79
Zentall TR (2001) Imitation in animals: evidence, function, and mechanisms. Cybern Syst 32:53–96
Zentall TR (2006) Imitation: definitions, evidence, and mechanisms. Anim Cogn 9:335–353
Acknowledgements
The authors are grateful to Prof. L. Lefebvre (McGill University, Montreal, Canada) for his support and advice in the construction of the experimental battery for Octopus, following his pioneering studies on birds. This work is part of the PhD project of Dr L. Borrelli (Borrelli, L., 2007. Testing the contribution of relative brain size and learning capabilities on the evolution of Octopus vulgaris and other cephalopods. PhD Thesis, Stazione Zoologica Anton Dohrn, Italy and Open University, UK). The study has been supported by the Stazione Zoologica Anton Dohrn (Napoli, Italy) and Fondazione Banco di Napoli (Italy) to GF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borrelli, L., Chiandetti, C. & Fiorito, G. A standardized battery of tests to measure Octopus vulgaris’ behavioural performance. Invert Neurosci 20, 4 (2020). https://doi.org/10.1007/s10158-020-0237-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10158-020-0237-7