Abstract
Electron microscopy revealed that glial cells in the posterior sub-esophageal mass of the brain in Sepia officinalis had a well-developed rough endoplasmic reticulum formed by long coverslips with rectilinear or curvilinear arrangements. The coverslips appeared dilated and have a large amount of adhered polysomes. Vesicular lamellae coexisted with the elongated lamellae of RER and dictyosomes of Golgi apparatus. Endocytosis was evidenced through the pale vesicles which were appeared next to the apical border of microvilli in some glial cells. Sub-cellular features of endocytosis, predominantly the fluid phase, were observed in the apical glial cell cytoplasm. Glial cells were related to phagocytosis of apoptotic neurons, endocytosis, pinocytosis and adsorption. These functions were proposed based on their ultrastructure characteristics and a significant number of vesicles with different shapes (oval to polygonal), sizes 0.052–0.67 µm and contents. Glycogen, MPS and lipid were detected in the glial cells. Alkaline phosphatase was not observed, while an activity of acid phosphatase was bound to lysosomes. ATPases were present in the glial cells along the lateral and basal plasma lemma as well as on the membranes of cell organelles. Unspecific esterase was clearly recognizable by electron microscopy. The monoamine and cytochrome oxidase activities were demonstrated, while the succinate dehydrogenase showed a weak enzyme activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glial cells are present in the CNS from the simplest invertebrates to humans (Lane 1981). The glial–neuron ratio increases with the size of the brain. The nematodes have a low percentage of glial cells, while in the fruit fly they represent app. 25%. In the brain of the mouse, this percentage increases to 65%, while that in the human brain and in the elephant the glia could represent more than 90%. Evolution indicates that the glia diversified and specialized for the neural functioning (Kettenmann and Ransom 1995; Imperadore et al. 2017). Ultrastructure studies of glial cells in cephalopods are described sporadically in the literature (Gray 1969; Reinecke 1976; Abbott et al. 1981; Bundgaard and Abbott 1981; Cardone and Roots 1990; Kettenmann and Ransom 1995; Imperadore et al. 2017). The fine structure of the brain components in cephalopods is not sufficiently covered (Gray 1970; Abbott et al. 1981). Electron microscopy revealed two types of glial cells in Octopus brain, the fibrous and protoplasmic glia; and also enigmatic dark cells (Gray 1969). The general morphology of the tick Boophilus microphis and cephalopod glial cells were described (Binnington and Lane 1980; Kettenmann and Ransom 2004). The physiology of neurotransmitters and brain anatomy of Octopus vulgaris were studied (Turchetti-Maia and Hochner 2019). The authors of this paper recommend two interesting publications which explain the functional organization of the brain parts of the cuttlefish Sepia officinalis (Boycott 1961; Bundgaard and Abbott 1981; Imperadore et al. 2017). The estrogen receptor of Octopus vulgaris is a constitutive transcriptional activator (Keay and Thornton 2006). The determination of the enzyme localization within the glial cells in cephalopods on ultrastructure level is entirely lacking in the literature. Ibrahim (2020), the first author of this study, investigated the fine structure of the central brain in the octopod Eledone cirrhosa (Lamarck, 1798) and think to cover the missing field of glial cells in the posterior sub-esophageal mass of the brain in Sepia officinalis. The immune cytochemical and biochemical evidence for the presence of serotonin containing neurons and nerve fibers in the Octopus arm was studied (Bellier et al. 2017). AMPA–kainate and NMDA-like glutamate receptors at the chromatophore neuromuscular junction of the squid were identified (Lima et al. 2003). The gyri of the Octopus vertical lobe have distinct neurochemical identities (Shigeno and Ragsdale 2015). Serotonin is a facilitating neuromodulator of synaptic transmission and reinforces long-term potentiation induction in the vertical lobe of Octopus vulgaris (Shomrat et al. 2010; Shomrat and Hochner 2015a). The available literature described the morphology of the brain in Octopus vulgaris and Loligo sp. (Young 1932, 1971a, 1976, 1977, 1979). Cephalopod nervous system shows a marked cephalization (Turchetti-Maia et al. 2017). The two pedal ganglia (ventral), the two pleurovisceral ganglia (lateral) and the two cerebral ganglia (dorsal) fuse to form the “brain.” It surrounds the esophagus and is comparable to the brain of the vertebrates; this represents an adaptive convergence. Protecting and wrapping cartilaginous ring-shaped case constitute the “skull” which is analogous to that of vertebrates (Graindorge 2008). Octo- and decapods have 8 and 10 brachial nerve cords, respectively, and these originate from the pedal ganglia (Shomrat et al. 2015b; Turchetti-Maia et al. 2017). At the base of each arm is a brachial ganglion, and all brachial nodes are joined by a brachial nerve ring. Brachial nerve cords are equivalent to the pedal cords of other molluscs (Budelmann and Young 1985). The fast movements are achieved thanks to the strong and synchronous contractions of the mantle muscles. These contractions are due to the impulses that arrive from a complex system of giant motor fibers (giant axons) (Shomrat et al. 2015b; Imperadore et al. 2017). The eyes of cephalopods are very developed (another adaptive convergence with the vertebrates), and from each one an optic nerve pinches off and terminates to each cerebral ganglion, there are some great ganglia (or lobes) that are many times bulkier than the cerebrospinal ganglia themselves (Shomrat et al. 2015b). In connection with the cerebral nodes, there is also a pair of upper mouth nodes, located above the buccal bulb. These in turn are connected with other lower mouth nodes located below it (Turchetti-Maia et al. 2017).
Our present work aims to investigate the fine structure of the glial cells in the posterior sub-esophageal mass of the brain in Sepia officinalis, to test which enzymes can be detected through cytochemical procedures and the fine structure localization can be demonstrated by electron microscopy. This study tests whether lipids and glycogen are present in the glial cells.
Materials and methods
Adults of Sepia officinalis were collected from the Mediterranean Sea of Alexandria, Egypt. n = 100/month during June–August 2018. Identification of the species was carried out according to Pyle and Döring (2017) and WoRMS (2017). Samples were transported in aquaria to the laboratory and dissected after carbon dioxide anesthesia. A tissue blister from the posterior sub-esophageal mass (Fig. 1) was isolated and fixed in phosphate-buffered glutaraldehyde (6%, pH 7.2) at 4 °C for 20–24 h or in Karnovsky fixative (Sigma-Aldrich®, St. Louis, MO, USA) (n = 10/month). This tissue blister was unified in all brains tested and marked in blue color in (Fig. 1). In sequence, the materials were post-fixed in OsO4 in the same buffer, followed by dehydration of the increasing series of acetone materials, inclusion in Araldite (Merck®, Darmstadt, Germany). Trimming of the blocks was done to obtain semithin (500 µm) and ultrafine (0.08 µm on average) sections. Sections were mounted in copper grids and contrasted with uranyl acetate and lead citrate (Pantin 1948). The ultrathin sections were analyzed and photographed under the electron microscope Philips CEM-100Ò Transmitter (Philips®, Germany).
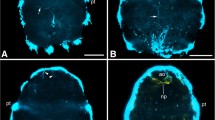
Computerized lateral view of the adult brain and associated structures in Sepia officinalis. The brain is pierced by the esophagus (es) and is divided into the sub-esophageal mass (SBM) and the supra-esophageal mass (SPM). The SBM is subdivided into the anterior (ASM), middle (MSM) and posterior (PSM) sub-esophageal masses. The optic lobe (OL) is situated on the lateral side of the optic tract region (OTR) in the SPM. bm, buccal mass; bN, brachial nerve; ibG, intrabrachial ganglion; ibL, inferior buccal lobe; pN, pallial nerve; sbL, superior buccal lobe; st, statocyst; vsN, visceral nerve
Cytochemical methods
Detection of carbohydrates
Samples (n = 10/month) for glycogen detection were subjected to histochemical reactions. Periodic acid Schiff (PAS) was used in order to detect neutral glycoconjugates and diastase + PAS, and to diagnose glycogen at pH 0.5 (Pearse 1968). This reaction oxidizes selectively glucose residues and produces aldehydes that react with Schiff’s reagent and produces a purple–magenta color. A suitable basic dye is often used as a contrast dye. PAS staining is mainly used to color structures containing a high proportion of carbohydrate macromolecules (glycogen, glycoprotein, proteoglycans) (Zakout et al. 2010). The PAS and the glycogen reactions were according to Khalid (2019). Acetylation/deacetylation was applied to check the Alcian blue color with subsequent PAS reaction. Acetylation (or ethanoylation) refers to the process of introducing an acetyl group (resulting in an acetoxy group) into a compound, that is, the substitution of an acetyl group for an active hydrogen atom. A reaction involving the replacement of the hydrogen atom of a hydroxyl group with an acetyl group (CH3CO) produces a specific ester, acetate. Acetic anhydride is generally used as an acetylating agent to react with free hydroxyl groups.
Detection of lipids (Pearse 1968)
Fragments of the posterior sub-esophageal mass were fixed in formalin calcium for 48 h n = 10/month. Samples were subjected to vacuum in a desiccator during the fixation process and dehydrated in an ethyl series. The histochemical samples were included in histological paraffin (Merck®, Darmstadt, Germany) (Hayashi 2011). The slides were assembled with glycerin gelatine. Using rotary microtome (820 Spencer, American Optical Corporation, Massachusetts, USA), 15-µm-thick frozen sections were obtained. Oil Red O, Sudan Black B and Nile Blue were applied according to Guigui and Beaudoin (2007). The control sections were extracted with pyridine before each reaction.
Enzyme cytochemical methods
Alkaline phosphatase
Alkaline phosphatase comprises a group of phosphohydrolase enzymes that have maximum activity at an alkaline pH close to 10. All ALP share a common protein structure, but differ in carbohydrate content. All isoforms of ALP are involved in the transport of metabolites across cell membranes. Alkaline phosphatase catalyzes the transfer of the phosphate group from the substrate p-nitrophenylphosphate (pNFF) to 2-amino-2-methyl-1-propanol (AMP), forming p-nitrophenol. The release rate of p-nitrophenol, which has a high absorbance at 405 nm, is proportional to the enzymatic activity of the alkaline phosphatase in the sample. Parts of the posterior sub-esophageal mass, n = 10/month, were rinsed with PBS pH 6.8 (79383, Sigma-Aldrich®, St. Louis, MO, USA). The sample was protected from light and stored at 2 to 8 °C. Samples were transferred to 2.5% glutaraldehyde buffered with 0.2 mol sodium phosphate and perfused with buffer pH 7.3. All samples were remained 6 h in fresh buffered glutaraldehyde and in 0.1 mol sodium cacodylate buffer pH 6.5 (Merk®, Germany) (Dhruv and Savio 2018). Incubation medium: 2.0 ml tris-maleate buffer 0.2 mol, pH 9.0 2.0 ml, Na-glycerophosphate solution 1.3 ml (G5422, Sigma-Aldrich®, St. Louis, MO, USA) and Pb (NO3)2 1% final pH 9.0. Control sections were treated with substrate-free medium. The incubation time was 5 min for the electron microscopic examination at 4 °C, The further treatment of glycerol gelatine sections before inclusion was carried out with treated ammonium sulfide (Pearse 1968). For electron microscopy, sections were inserted for 1 h in 1% OsO4 post-fixative and post-contrasted with 5% uranyl acetate.
Acid phosphatase
Acid phosphatase is the enzyme characteristic of lysosomes, and its presence allows to distinguish this organelle from other types of cytoplasmic vesicles. Samples (n = 10/month) for the detection of acid phosphatase were air-dried for at least 10 min and fixed in cold glutaraldehyde pH 7.2 (4 °C) for exactly 30 s. Samples were washed with double-distilled water under pressure allowing the excess water to drain. The incubation solution was prepared in situ as follows: 44 ml double-distilled water, 3 ml acetate buffer, 3 ml Naftol ASBI phosphate substrate (naphthol AsBi phosphoric acid solution, Sigma®, St Louis, USA) containing 12.5 mg/ml C18H15BrNO6P and 24 mg of the stable diazotized salt 2-methyl-4- (2-methylphenyl) -azobenzenedodiazosulfate (Fast Garnet GBC Sulfate Salt, Sigma Chemical Co, St Louis, USA). Dissolve by stirring for at least 5 min and filter the solution twice. After the incubation period, the slides were washed in running water. An effective way to wash is to deposit the slides at the ends of a Coplin glass, placing it under running water from a tap. In this way, the water jet is able to impact the bottom of the Coplin glass generating a multitude of air bubbles that drag up any unwanted deposit. After 10 min of washing, samples were passed through distilled water under pressure. The detection of acid phosphatase was carried out according to the method of (Jonek et al. 1977). Incubation medium: 0.12 g Pb (NO3)2, 100 ml 0.05 mol Na acetate buffer, pH 5.0 with 7.5% sucrose (0.22 mol), 10 ml of 3% Na-glycerophosphate solution with final pH 5.0. The control medium contained no glycerophosphate. The incubation period was 30 min at room temperature. Subsequently, 15-µm-thick frozen sections in Na acetate buffer (0.05 mol, pH 5.0) with 7.5% sucrose and 4% formalin in 1% acetic acid and finally rinsed in acetate buffer. Before inclusion in glycerol gelatine, sections were taken with treated ammonium sulfide solution (Pearse 1968). The ultrastructure cytochemistry test was detected on 50-μm-thick sections for 10-min incubation at room temperature. The flushing was carried out according to the previous scheme with replacement of 1% against a 2% acetic acid. For post-fixation, the sections were inserted for 2 h at 4 °C in 1% osmic acid. The post-contrasting took place partially with 5% uranyl acetate (Griffiths 1979).
Adenosine triphosphatase (ATPase) (Pearse 1968)
Samples n = 10/month were fixed in 5% glutaraldehyde with 0.1 mol Na cacodylate buffer pH 7.2. Rinse with the same buffer containing 7% sucrose. The incubation time of light microscopic and for the electron microscopic sections was 15 min at room temperature. The Mg++, Mg++- Na+-K+- and Ca++- activatable ATPase was used with the developing medium, modified from (Martins and DeMeis 1985), diagnosed: 1: 0.83 mmol ATP; 10 mmol MgSO4; 80 mmol tris-maleate buffer pH 7.2; 2.4 mmol Pb (NO3)2; final pH 7.2. 2: medium (1) with the addition of 0.1 mol NaCl and 20 mmol KCl. 3: medium (1) in which MgSO4 was replaced by 5 mmol CaCl2. 4: medium (1) in which ATP is obtained by molar concentration of Na-glycerophosphate was exchanged. 5: medium (1) without MgSO4; this was an exclusion of the through spontaneous hydrolysis: medium (1) without ATP. Then the sections used for light microscopy were included in dis. H2O, treated with ammonium sulfide and covered in glycerol gelatine. For the electron microscopic examination, 50-μm-thick frozen sections after incubation in acetate–veronal buffer pH 7.4 with 7% rinsed sucrose and fixed with 1% OsO4 for 45 min. Partially the thin sections were examined in electron microscope before contrast in 5% aqueous uranyl acetate solution.
Unspecific esterase (Pearse 1968)
The samples (n = 10/month) were fixed in 5% glutaraldehyde and placed in 0.1 mol cacodylate buffer pH 7.2 with 7.5% sucrose. For the light microscopic examination, 15-μm-thick frozen sections were prepared (Mulyaningsih et al. 2017) and incubated room temperature in 49 ml 0.1 mol phosphate buffer pH 6.5, 1 ml of 1% solution of a-naphthyl acetate in 50% acetone, 50 mg real blue salt B, final pH 6.5. After the incubation, the sections were rinsed several times in tap water and covered in glycerin gelatine. Electron microscopic examination was the method of Crevier and Belanger, according to Mulyaningsih et al. (2017). Medium: 23 ml cacodylate buffer 0.05 mol pH 5.2, 0.5 ml MgCl2 1 mol, 1 ml thioacetic acid 0.24 mol, 0.2 ml Pb (NO3)2 0.1 mol, final pH 5.2. 7.5% sucrose was added to the medium. The control took place under the exchange of thioacetic acid with dis. H2O. The incubation time of the 50-μm-thick frozen sections was 60 min at 4 °C. After rinsing in distilled water and post-fixation in 1% osmium for one hour, the sections were embedded in Epon.
Monoamine oxidase (Pearse 1968)
Method according to Soep (1976): Native cryostat sections (20 μm) n = 10/month were incubated at 37 °C for 50 min in 25 mg tryptamine monohydrochloride, 4 mg sodium sulfate, 5 mg p-nitro blue tetrazolium chloride (Nitro-BT), 5 ml of 0.1 mol phosphate buffer pH 7.6, 15 ml and distilled water, The control medium does not contain tryptamine monohydrochloride. After rinsing in distilled water, sections were inserted in neutral 10% formalin and enclosed in glycerin gelatine.
Cytochrome oxidase
Method according to Jonek et al. (1977): Native cryostat sections n = 10/month were incubated for 45 min in 1 ml of ethanol 96%, 20 mg of a-naphthol acetate, 20 mg of 4-aminodiphenylamine, 29 ml of 0.2 mol Tris–HCl, buffer pH 7.4,70 ml and distilled water. The control sections were incubated in the same medium, but without 4-aminodiphenylamine. After sixty minutes of treatment with 10% cobalt nitrate in 10% formalin and two times in running water, the sections were covered in glycerol gelatine.
Succinate dehydrogenase
Method according to Kimura and Singer (1976): The incubation time of 20-μm-thick native cryostat sections, n = 10/month, was 20 min at 37 °C in 12.5 ml 0.2 mol phosphate buffer pH 7.6, 12.5 ml 0.2 mol succinic acid (disodium succinate), 12.5 ml distilled water, 12.5 ml saline solution: 34 ml distilled water, 1 ml 0.25 mol CaCl2, 1 ml of 0.05 mol of MgSO4, 10 ml 0.6 mol NaHCO3, 4 ml AlCl3. 50 mg p-nitro blue tetrazolium chloride (Nitro-BT). The control medium does not contain disodium succinate.
Results
Transmission electron microscopy of glial cells
The main cell types present in the posterior sub-esophageal mass in Sepia officinalis were glial cells. According to their morphology, glial cells were of several types. They were either longitudinally branched cells or endothelial-like or cuboidal. The cell body measured between 3 and 4 μm wide and 6 μm long. There was heterogeneity in the distribution of these three types inside the posterior sub-esophageal mass. The longitudinally branched cells were distributed centrally and have an elongated cell body with long processes parallel to neighboring axons. Their processes themselves had many ramifications. The endothelial-like glial cells were limited, while the cuboidal ones were the most numerous; both types were distributed everywhere. In their supra-nuclear cytoplasm, the rough endoplasmic reticulum (RER) was well developed and was formed by long coverslips with rectilinear or curvilinear arrangements. The coverslips appeared dilated at its extremities and sometimes in its middle parts, having a large amount of adhered ribosomes to its walls, and adjacent aggregates of free ribosomes forming polysomes (Fig. 2a). There were few coverslips of the RER, which represented dilated vesicles of this reticulum complex and have a smaller relative amount of adhered ribosomes. Vesicular lamellae coexisted with the elongated lamellae of RER itself and dictyosomes of Golgi apparatus. They were abundant and well developed in the supra-nuclear cytoplasmic mass. A large amount of vesicles with varied forms and contents surrounded the long lamellae of RER and Golgi sacs. Endomembrane-coated vesicles having clear or more electro-opaque contents and pale vesicles with smooth surface were observed (Fig. 2b). In some glial cells next to the apical border of microvilli, pale vesicles appeared in the apical membranes characterizing a process of endocytosis. Microtubules were evident, and junction complexes were formed between the glial cells and lateral plasma membranes adjacent at the apical level (Fig. 2c). Still in the supra-nuclear cytoplasm of several glial cells, multivesicular complexes encompassing dense bodies of different electrodes and fragment material were observed (Fig. 2d). Apparently, they are remnant of decomposed organelles (auto-phagocytosis). However, the possibility of occurrence of heterophagic digestion is present which is supported by the concomitant observation of homogeneous dense bodies, of different electrodes (Fig. 2e). In glial cell cytoplasm, at different levels, a large amount of lysosomes (Fig. 2f–h), pale vesicles, an aggregate of dense and coated vesicles were observed (Fig. 2f). Adjacent to lysosomes, elongated lamellae of the RER, polysomes, mitochondria, small and coated vesicles and pale multivesicular bodies were observed (Fig. 2g). Cisterns of the vesicular RER were located in the supra-nuclear cytoplasm of some glial cells, apparently circumscribing parts of the cytoplasmic content, having ribosomes attached to its outer walls (Fig. 2f). In the middle part of the posterior sub-esophageal mass, supra-nuclear cytoplasm of various glial cells was characterized by the occurrence of large cross-linking derived from the abundant RER and by appreciable amount of lysosomes and coated vesicles (Fig. 2g). The coverslips of RER appeared predominantly elongated, some of them dilated and with vesicular characteristics, showing dictyosomes of Golgi apparatus (Fig. 2h). Lysosomes were preceded by large amount of endosomes presented in the apical cytoplasm (Fig. 2e). Sub-cellular features of endocytosis, predominantly fluid phase, were observed in the apical glial cell cytoplasm, where apical areolas were observed, encompassing pale vesicles at the border of apical microvilli followed by the presence of pale vesicles, endosomes and multivesicular bodies being predominantly pale (Fig. 2i). Near the apical cytoplasmic border of some glial cells, sections were observed, with different structural parts of neurons in close contacts with the apical border of microvilli of glial cells, and/or with the plasma membrane of apical cytoplasm of glial cells (Fig. 2j, k). Such contact had lamellae RER concentricity within a predominance of circumferential association (Fig. 2n). RER lamellae, with circumferential arrangement, located at the basal level, delimited a space of the cytoplasmic content, in which mitochondria occurred, forming double-membrane vesicular tubes or two dense granules circumscribed by coverslips (Fig. 2n). The covering epithelium was formed predominantly by especial type of glial cells referred to as ependymal cells (Fig. 2m). The supra-nuclear cytoplasm in these cells presented large concentration of lysosomes, RER lamellar and many vesicles, predominantly small, but varied in shape 0.067 µm and content (Fig. 2m). The apical plasma membrane of these cells had internalized as small, clear coated vesicles, near the underlying cytoplasmic border of peripheral neurons. A few ependymal cells showed apical cytoplasmic extensions, projecting in the inside the brain between the apical edges of microvilli of peripheral glial cells, noting a large granule of lipid inclusion in the cytoplasmic content (Fig. 2n). In the infra-nuclear, cytoplasm, RER appeared circumferential, with their coverslips disposing so concentric and parallel to each other, delimiting a homogeneous and denser part of cytoplasmic content. Lamellae of RER itself appeared adjacent to the circumferential formation one of them contacted the nuclear envelope and the other involved a lysosome, with heterogeneous electro-density (Fig. 2o).
A semithin and TEM sections in the sub-esophageal mass of the brain in Sepia officinalis showing: a glial cells and nerve cell bodies. b RER with Golgi dictyosomes, free-floating ribosomes and various endocytosis vesicles. c Axons embraced by glial cells implicating massive arrays of microtubules. d Dense bodies of different electrodes. e Lysosomes and pale vesicles. f RER, polysomes, mitochondria, vesicles and pale multivesicular bodies. g Abundant RER, lysosomes and coated vesicles. h Coverslips of RER, dictyosomes of Golgi apparatus and endosomes. i fluid phase endocytosis. j, k Some neurons were in close contact with the apical border of microvilli of glial cells, j in circumferential association. k, l RER lamellae delimited a space in which mitochondria occurred. m Covering epithelium of the sub-esophageal mass. n Apical cytoplasmic extensions, projecting in the inside the brain between the apical edge of microvilli of peripheral glial cells. o Circumferential RER coverslips. Apical cytoplasm AC; apical microvilli AM; axons A; collagen fibrils CF; decomposed organelles DO; dense bodies of different electrodes DE; free-floating ribosome R; endocytosis vesicles EV; ependymal cells EC; lysosomes L; glial cells GC; granules G; golgi dictyosomes GD; lipid inclusion LI; microtubules MT; mitochondrion M; multivesicular body MB; nerve cell bodies NB; nuclear envelope NE; pale vesicles V; polysomes P; rough endoplasmic reticulum RER
Enzyme cytochemical test
Acid phosphatase (Fig. 3a, b)
Some of the investigated glial cells were examined individually through light microscopy. Black granules were observed in the supra-nuclear zone. More evenly dark-colored layer was seen in the apical cell districts. In both cases, these were the reaction products of acid phosphatase, which could be confirmed in control experiments. The electron microscopy revealed rounded, approximately 0.1–0.3 μm cell organelles with different electro-dense granules filled and surrounded by a membrane. It was after the definition of (Jonek et al. 1977) for lysosomes associated with lead phosphate precipitations of the cytochemical detection reaction for acid phosphatases.
Histological and TEM sections in the sub-esophageal mass of the brain in Sepia officinalis showing: a acid phosphatase granules. b Cell organelles with different electro-dense granules of acid phosphatase. c The ATPases were in the form of black vertical stripes indicated by arrows. d Electro-dense granules of the ATPases indicated by arrows. e Blue–black granules of monoamine oxidase over the whole cell membrane. f Blue–black products of cytochrome oxidase indicated by arrows. g Dark tinged form of esterases indicated by arrows. h Esterases were small granules within the cell organelles as lysosomes. i Blackish bluish dripping of lipids stained with Sudan Black B. j Red granular products of glycogen indicated by arrows (color figure online)
Alkaline phosphatase
In all investigated glial cells, we did not found a positive reaction by light and electron microscopy for alkaline phosphatase.
ATPases (Fig. 3c, d)
Results could be obtained by light and electron microscopy at an incubation time of 15 min at room temperature. By light microscopy, the reaction products of the ATPases can be described as lead sulfide precipitates in the form of black vertical stripes on the lateral cell boundaries. Controls (incubation without substrate) were invariably negative. Spontaneous hydrolysis of the substrate at 15-min incubation was not observed. In the electron microscopy, enzymatic activity occurred only at the lateral cell boundaries. The reaction products were in the form of electro-dense granules both lateral and basal plasma lemmas. It should be taken into account in these findings that part of the enzyme activity was due to non-specific phosphatases. The experiments in which ATP was replaced by Na-glycerophosphate were shown only a weak positive reaction.
Oxydoreductases
Detection of succinate dehydrogenase (Fig. 3e)
Weak and apically localized enzyme activities of succinate dehydrogenase were observed.
Detection of monoamine oxidase
In the glial cells, a high enzyme activity was observed in the form of blue–black granules over the whole cell membrane. In the apical zone, these occurred more frequently.
Detection of cytochrome oxidase (Fig. 3f)
The proof was positive, but here were blue–black reaction products less numerous in the glial cells than in the monoamine oxidase detection.
Unspecific esterases (Fig. 3g, h)
Light microscopy is non-specific in cytochemical detection. Esterases have only moderately strong activity in dark tinged form. Electron microscopy, on the other hand, shows as in the case of the proof that the acidic ones were Phosphatase and the reaction products were small granules within the cell organelles as lysosomes, which were surrounded by a membrane. Both light and electron microscopies confirm the enzyme detection as in the case of acid phosphatase and the ATPase.
Lipids and glycogen
Lipids (Fig. 3i)
Blackish bluish dripping inclusions could be observed in the supra-nuclear zone of glial cells stained with Sudan Black B. These districts stained in the apical cytoplasm and remained unstained in the control sections with Baker pyridine extraction. To distinguish between neutral and acidic lipids, Nile Blue stain was used. Red inclusions were not observed, so this reaction pointed to the occurrence of acidic lipids in the glial cells. The additional Oil Red coloring with negative reaction also suggested that glial cells lacked neutral fats.
Glycogen (Fig. 3j)
The PAS reaction after (Peter 1985) was clearly positive in the form of red granular products that were uniform in the cytoplasm of the glial cells. In the glial cells with long peripheral processes, PAS-positive substances could be found. But thereafter, the positive PAS reaction remained unchanged, and a positive failure was recorded, concluding that glycogen was not the proven polysaccharide. As the sections after prior acetylation and irreversible esterification colored amino alcohol groups, the PAS reaction was negative. At subsequent deacetylation, the hydroxyl groups by oxidation converted back into aldehydes and were in the form of a pale red coloration visible in the sections of glial cells of cuboidal or endothelial forms. It followed that in the case of the positive PAS reaction not glycogen, but un-substituted glycol groups play a role. A clear blue coloration of these glial cells with the Alcian blue and subsequent PAS staining indicates the presence of acidic MPS.
Discussion
Glial cells of the posterior sub-esophageal mass in Sepia officinalis were characterized by a developed RER with long lamellar, straight and curvilinear tanks. They had a concentric arrangement with characteristic vesicular cisterns with few attached ribosomes. Perhaps glia have a possible implication in the secretion of proteins (Bundgaard and Abbott 1981; Elisabet et al. 2013). The excess ribosomes and polysomes attached to the coverslips of RER present in the supra-nuclear cytoplasm would express a high protein synthesis capacity (Baskin 1971). RER in supra-nuclear cytoplasm showed long lamellae associated with dictyosomes of Golgi apparatus. Coated vesicles in nereid polychaetes presented in the cytoplasm adjacent to these organelles (Baskin 1971). The association between RER and Golgi sacs with adjacent dense vesicles may be related to the synthesis and secretion of glycoproteins in Sepia officinalis (Abbott et al. 1981). The secreted proteins pass directly through Golgi apparatus before being released into the tubular lumen (Bios-e-16 2013). However, studies of autoradiographic images, with marked isotopes, showed the silver grains initially on RER and successively on Golgi apparatus: apical cell surface and tubular lumen (Bentivoglio 1989). The exact transport mechanism of proteins, i.e., the nature of the carrier of secreted macromolecules from Golgi sacs to the cell surface is still not clearly defined. It was suggested to possible involvement of pale vesicles of smooth surface, adjacent to Golgi apparatus, in the transport of secreted proteins (Amgen 2019), or would be the small, dense coated vesicles, responsible for this process (Abbott et al. 1981). Our observations tend to agree with the first interpretation reserved for pale vesicles rather than participation of the endocytotic apparatus of cells. Lamellae of RER delimited a space of the cytoplasmic content containing mitochondria, polysomes and electro-dense granules, circumscribed by vesicles, also suggesting the synthesis of protein material. The presence of circumferential RER with electro-dense material had been characterized in cells of four sea hare species, namely Aplysia Juliana, A. parvula, D. dolabrifera and A. californica (Prince and Johnson 2015). Several functions were proposed to these glial cells in this study: phagocytosis of apoptotic neurons, fluid phase endocytosis activities (or pinocytosis) and adsorptive endocytosis (or phagocytosis). The action of glial cells was therefore ambivalent: stimulation of neurogenesis and induction of neuronal apoptosis. These glial cells were equipped with a large panel of receivers and can therefore take into account any modification of their environment. These findings were comparable to the glial-like cells in Octopus vulgaris (Young 1971b; Cardone and Roots 1990; Kettenmann and Ransom 2004). That same kind of activity was evidenced in supra-nuclear cytoplasm of all glial cells in invertebrates (Binnington and Lane 1980; Kettenmann and Ransom 1995; Kettenmann et al. 2010), insects (Moussa and Banhawy 1958; Richard et al. 1984), lobsters (Holtzman et al. 1970) and gastropods (Sun and Tsai 2011). The first step in endocytosis was characterized by the presence of an apex formed between the microvilli, which internalize as small vesicles in the apical cytoplasm and interrelated sub-cellular structures to the endocytosis (Lane and Swales 1976). Moreover, at the level of apical and supra-nuclear cytoplasm the presence of sub-cellular endocytotic processes was typically characterized following previously proposed models for this functional activity (Guerra 1992; Hochner and Shomrat 2012). Suggestions were assumed that the cytoplasmic processes of glial cells would be mere fixing artifacts (Clayton 1962; Lane and Treherne 1972; Reinecke 1976; Murphy and Pearce 1987; Nixon and Young 2003; Perry and Barron 2013) or inert structures and no striking functional meaning (Stephens and Young 1969). Regarding the presence of lipid inclusions in the supra-nuclear cytoplasm of some glial cells, they are non-nervous elements and might represent a functional reserve (Clayton 1962; Young 1979; Williamson and Chrachri 2004; Wentzell et al. 2009).
The cytochemical evidence of this study suggested that the cubic glial cells and their peripheral processes contained PAS-positive granules of glycogen nature in their cytoplasm. The negative PAS reaction after acetylation and the weak positive reaction after the acetylation indicated the presence of Glycol groups out. Blue coloration of the glial cells cytoplasm with the Alcian blue staining suggested the occurrence of acid MPS; for review, see Baumann and Pham-Dinh (2001) and Ceprian and Fulton (2019). The detection of lipids in our study can be assumed with the result of Hayashi (2011) and unlike to Zimmermann et al. (2012), who gave a strong positive response of alkaline phosphatase (GOMORI method) (Dhruv and Savio 2018). We found in electron microscopic study that lead precipitates were observed presumably due to unspecific reactions. A similar result (Zimmermann et al. 2012) indicated that the level of glycogen and alkaline phosphatase fluctuates in glial cells and was involved in glycogen assembly and degradation. In numerous studies, in small laboratory animals on CNS, it was found that a positive reaction of acid phosphatase (Packard and Albergoni 1970; Jonek et al. 1977; Griffiths 1979; Cardone and Roots 1990; Zimmermann et al. 2012). Uptake of glucose and its conversion to glycogen by neurons and glial cells in the leech central nervous system were studied (Wolfe and Nicholls 1976). The hydrolase acidic phosphatase was regarded as carrier and function with other lysosomal enzymes for intracellular digestion. Different results brought in previous studies on the ATPase activity in glial cells (Medzihradsky et al. 1972; Martins and DeMeis 1985; Cardone and Roots 1990; Khalid 2019). Peroxidase uptake by glial cells in de-sheathed ganglia of the cockroach was proved (Lank and Trbherne 1969). Our study revealed only an ATPase activity by light microscopy at the lateral and basal cell boundaries, which could be confirmed by electron microscopy. Occasionally, reaction products in the apical zone of glial cells were likely non-specific. This was shown by the experiments in which ATP was replaced by glycerophosphate and also apical reactions were recognizable. In addition, the absence of ATPase activity on the cell membrane sections was involved in the formation of detention complexes. At the adhesion complexes of all glial epithelia, no ATPase activity could be detected. As enzymes of biological membranes, the ATPases were by Ca++ ions and MG ions (Medzihradsky et al. 1972; Martins and DeMeis 1985). For the active intercellular transport, a Mg++-dependent serves ATPase, which continues through Na+ and K+ activation and represents a Na–K transport system (Peter 1985). Such transport ATPases could also be detected in sense that calcium activates potassium channels and both participate in secretion (Petersen andMaruvama 1984). The esterases are usually lysosomal-bound enzymes. Naphthyl acetate as a substrate is representable as a weakly granular reaction. It could be detected by electron microscopy with thioacetic acid as substrate. Extralysosomal esterases such as (Livingston et al. 1969) in plant and animal tissues as probably ribosomal-bound allylases were able to detect in the cubic glial cells of invertebrates. In earlier studies on oxidoreductases in invertebrate brains, lower vertebrates and various mammals (Soep 1976; Kimura and Singer 1976; Emmanuel et al. 2019) were able to get a regular enzyme activity of monoamine oxidase, cytochrome oxidase and succinate dehydrogenase be detected. Statements about the strength of the enzyme reactions can be carried out without “special control steps” according to Emmanuel et al. (2019). The observation in the present study indicates that the enzyme activity was related to resorptive processes or active secretion of substances into the subnuclear zone. The positive result of monoamine oxidase may be related to an adrenergic transmitter mechanism when seen to the function of this enzyme in terms of its inactivation of contaminants such as noradrenaline and serotonin and other biogenic amines think (Shomrat et al. 2010; Shomrat et al. 2015a). The main functions of glial cells were manifold and have been assumed in this study as adsorptive endocytosis, fluid phase endocytosis, resorption of the luminal fluid, protein and glycoprotein secretions; and water, saline, ions and macromolecules exchanges with neurons. Glial cells are assumed for different enzyme cytochemical behavior in the brain as, for example, primarily enzymes of oxidative metabolism for mechanical tasks, enzymes for glycolytic metabolism as expression for synthesis and ATPase activity. The ability of glial cells to secrete proteins including glycoproteins was related to developed organellar system associated with synthesis and secretion such as the presence of a remarkable rough endoplasmic reticulum (RER) associated with the appliance of Golgi apparatus. Other ultrastructural components were related directly to the complex RER–Golgi apparatus, such as dark and coated vesicles, exocytotic vesicles and transcytosis which were similarly observed in four species of sea hares (Prince and Johnson 2015). The use of markers of macromolecules placed in the microenvironment glial cells was useful to demonstrate amino acid transport and incorporation through the free surface of the cells epithelial (Elisabet et al. 2013; Liscovitch-Brauer et al. 2017). In addition, one pathway intracytoplasmic secretory protein was demonstrated in glial cells of Octopus vulgaris (Packard and Albergoni 1970; Young 1971b) and cell-to-cell transfer of glial proteins to the squid giant axon was observed (Lasek et al. 1977). The glial cells of the cerebral ganglia of Helix pomatia functioned in uptake of ferritin and ‘H-glutamate (Shivers 1976). Trans-glial channel-facilitated translocation of tracer protein across ventral nerve root sheaths of crayfish (Shivers 1976). The presence of apical cytoplasmic processes with meaning of possible cell secretion was noted in the hepatopancreas of Astacus leptodactylus (Hirsch and Jacobs 1930), the gonads of Octopus (Wells and Wells 1959), neuroglia cells in the brain of invertebrates (Lane 1981), the glial cells in the glial blood–brain barrier in the cuttlefish, Sepia offirinalis (Bundgaard and Abbott 1981), and the endodermal region of the GI tract in the freshwater shrimp Neocaridina heteropoda (Sonakowska et al. 2015).
This study concluded that the glial cells in the posterior sub-esophageal mass in Sepia officinalis were either longitudinally branched or endothelial-like or cuboidal. There were dilated vesicles of RER and dictyosomes of Golgi apparatus with an amount of adhered ribosomes. In glial cell cytoplasm, at different levels, a large amount of lysosomes, polysomes, mitochondria, pale vesicles and an aggregate of coated vesicles were observed. Parts of neurons were observed in close contact with the apical border of microvilli, and/or with the plasma membrane of apical cytoplasm of glial cells. Though light and transmission electron microscopies, this study proof the presence of acid phosphatase, ATPases, succinate dehydrogenase, monoamine oxidase, cytochrome oxidase, lipids, glycogen and acidic MPS in the glial cells.
References
Abbott N, Bundgaard M, Cserr HF (1981) Fine-structural evidence for a glial blood-brain barrier to protein in the cuttlefish, Sepia officinalis. J Physiol 316:52–53. https://doi.org/10.1007/BF01224761
Amgen F (2019) The endomembrane system, Science Biology Structure of a cell tour of a eukaryotic cell. Khan Academy. https://www.khanacademy.org/science/biology/structure-of-a-cell/tour-of-organelles/a/the-endomembrane-system
Baskin DG (1971) The fine structure of neuroglia in the central nervous system of nereid polychaetes. Zeitschrift für Zellforschung und Mikroskopische Anatomie 119:295–308. https://doi.org/10.1007/BF00306928
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–910. https://doi.org/10.1152/physrev.2001.81.2.871
Bellier J, Xie Y, Farouk SM, Sakaue Y, Tooyama I, Kimura H (2017) Immunocytochemical and biochemical evidence for the presence of serotonincontaining neurons and nerve fibers in the octopus arm. Brain Struct Funct 222:3043–3061. https://doi.org/10.1007/s00429-017-1385-3
Bentivoglio M (1989) The Golgi apparatus emerges from nerve cells. Trends Neurosci 21:195–200
Binnington KC, Lane NJ (1980) Perineurial and glial cells in the tick Boophilus microphis (Acarina: Ixodidae): freeze-fracture and tracer studies. J Neurocytol 9:343–362. https://doi.org/10.1007/bf01181541
Bios-e-16 (2013) Cell biology 04: the ecretory pathway. Harvard Extension’s Cell Biology course. https://www.cureffi.org/2013/02/24/cell-biology-04-the-secretory-pathway/
Boycott BB (1961) The functional organization of the brain of the cuttlefish Sepia officinalis. Proc R Soc Lond B Biol Sci 153:503–534. https://doi.org/10.1098/rspb.1961.0015
Budelmann BU, Young JZ (1985) Central pathways of the nerves of the arms and mantle of Octopus. Philos. Trans R Soc Lond B Biol Sci 310:109–122. https://doi.org/10.1098/rstb.1985.0101
Bundgaard M, Abbott NJ (1981) Fine-structural evidence for a glial blood-brain barrier to protein in the cuttlefish, Sepia offirinalis. J Neurocytol 21:260–275. https://doi.org/10.1016/0006-8993(81)91083-0
Cardone B, Roots BI (1990) Comparative immunocytochemical study of glial filament proteins (glial fibrillary acidic protein and vimentin) in goldfish, octopus, and snail. Glia 3(180):180–192. https://doi.org/10.1002/glia.440030305
Ceprian M, Fulton D (2019) Glial cell AMPA receptors in nervous system health, injury and disease. Mol Sci 20:23–39. https://doi.org/10.3390/ijms20102450
Clayton DE (1962) A comparative study of the non-nervous elements in the nervous systems of invertebrates. J Ent Zool 234:3–22
Dhruv L, Savio J (2018) Alkaline Phosphatase. StatPearls., https://www.ncbi.nlm.nih.gov/books/NBK459201/
Emmanuel P, PaulV, Deepika RJ (2019) Emerging trends in the industrial production of chemical products by microorganisms. Developments in Applied Microbiology and Biochemistry, https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/oxidoreductases
Graindorge N (2008) Vertical lobe and formation of the menesic trace in the cuttlefish, Sepia officinalis (Doctoral dissertation). Université de Caen Normandie
Gray EG (1969) Electron microscopy of the glio-vascular organization of the brain of octopus. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.1969.0002
Gray EG (1970) The fine structure of the vertical lobe of octopus brain. Philos Trans R Soc Lond B. https://doi.org/10.1093/oxfordhb/9780190456757.013.29
Griffiths G (1979) Transport of glial cell acid phosphatase by endoplasmic reticulum into damaged axons. J Cell Sci 36:361–389
Guerra A (1992) Mollusca, cephalopoda. In: Fauna Iberica, Vol. 1. Ed. Ramos, M.A., Museo Nacional de Ciencias Naturales CSIC, Madrid, 1–327. http://hdl.handle.net/10261/50383
Guigui K, Beaudoin A (2007) The use of Oil Red O in sequence with other methods of fingerprint development. J For Identif 57:550–581
Hayashi H (2011) Lipid metabolism and glial lipoproteins in the central nervous system. Biol Pharm Bull 34:453–461. https://doi.org/10.1248/bpb.34.453
Hirsch GC, Jacobs W (1930) Der Arbeitsrhythmus der Mitteldarmdrüse von Astacus leptodactylus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 12:524–558. https://doi.org/10.1007/BF00337896
Hochner B, Shomrat T (2012) An embodied view of octopus neurobiology. Curr Biol 22:887–892. https://doi.org/10.1016/j.cub.2012.09.001
Holtzman E, Freeman AR, Kashner LA (1970) A cytochemical and electron microscope study of channels in the Schwann cells surrounding lobster giant axons. J Cell Biol 44:438–444. https://doi.org/10.1083/jcb.44.2.438
Ibrahim G (2020) Fine structure of the central brain in the octopod Eledone cirrhosa (Lamarck, 1798) (Mollusca–Octopoda). Invertebrate neuroscience, revised in 2019
Imperadore P, Shah SB, Helen P, Makarenkova A, Fiorito G (2017) Nerve degeneration and regeneration in the cephalopod mollusc Octopus vulgaris: the case of the pallial nerve. Sci Rep 7:46564. https://doi.org/10.1038/srep46564
Jonek J, Chociłowski W, Kamínski M, Konecki J (1977) Quantitative determination of acid phosphatase activity detected in tissue sections according to the BURSTONE method. Acta Histochem 59(2):285–289. https://doi.org/10.1016/S0065-1281(77)80051-2
Keay JBJ, Thornton JW (2006) The Octopus vulgaris estrogen receptor is a constitutive transcriptional activator: evolutionary and functional implications. Endocrinology 147:3861–3869. https://doi.org/10.1210/en.2006-036
Kettenmann H, Ransom BR (1995) Neuroglia. Oxford University Press, Oxford
Kettenmann H, Ransom BR (2004) Neuroglia. The concept of neuroglia: a historical perspective. Oxford University Press, New York. https://global.oup.com/academic/product/neuroglia-9780199794591?cc=us&lang=en&
Kettenmann HHU, Noda M, Verkhratsky A (2010) Physiology of microglia. Physiol Rev 91:461–553. https://doi.org/10.1152/physrev.00011.2010
Khalid M (2019) Laboratory diagnosis of the causative dermatophytes of Tinea capitis (pdf). World J Pharm Res 8:85–99. https://doi.org/10.20959/wjpr20196-14850
Kimura JHT, Singer TP (1976) Studies on succinate dehydrogenase. J Biol Chem 232:4987–4993
Lane NJ (1981) Invertebrate neuroglia-junctional structure and development. J Exp Biol 95:7–33
Lane NJ, Swales LS (1976) Interrelationships between Golgi, gerl and synaptic vesicles in the nerve cells of insect and gastropod ganglia. J Cell Set 22:435–453
Lane NJ, Treherne JE (1972) Studies on perineurial junctional complexes and the sites of uptake of microperoxidase and lanthanum in the cockroach central nervous system. Tissue Cell 4:427–436. https://doi.org/10.1016/S0040-8166(72)80019-3
Lank NJ, Trbherne JE (1969) Peroxidase uptake by glial cells in desheathed ganglia of the cockroach. Nat Lond 333:861–862
Lasek RJ, Gainer H, Barker JL (1977) Cell-to-cell transfer of glial proteins to the squid giant axon: the glia-neuron protein transfer hypothesis. J Cell Biol 74:501–523. https://doi.org/10.1083/jcb.74.2.501
Lima PA, Nardi G, Brown ER (2003) AMPA/kainate and NMDA-like glutamate receptors at the chromatophore neuromuscular junction of the squid: role in synaptic transmission and skin patterning. Eur J Neurosci 17:507–516. https://doi.org/10.1046/j.1460-9568.2003.02477.x
Liscovitch-Brauer N, Alon S, Porath HT, Elstein B, Unger R, Ziv T et al (2017) Trade-off between transcriptome plasticity and genome evolution in cephalopods. Cell 169:191–202. https://doi.org/10.1016/j.cell.2017.03.025
Livingston DC, CoombsL MM, Franks M, Maggi V, Gahan PB (1969) A lead phthalocyanin method for the demonstration of acid hydrolases in plant and animal tissues. Histochemie 18:48–60. https://doi.org/10.1007/BF00309901
Mandon EC, Trueman SF, Gilmore R (2013) Protein translocation across the rough endoplasmic reticulum. Cold Spring Harb Perspect Biol 5:a013342. https://doi.org/10.1101/cshperspect.a013342
Martins OB, DeMeis L (1985) Stability and partial reactions of soluble and membrane-bound sarcoplasmic reticulum ATPase. Biol Chem 260:6776–6781
Medzihradsky F, Sellinger OZ, Nandhasri P, Esantiago J (1972) ATPase activity in glial cells and in neuronal perikarya of rat cerebral cortex during early postnatal development. J Neurochem 19:543–545. https://doi.org/10.1111/j.1471-4159.1972.tb01365.x
Moussa T, Banhawy M (1958) Studies on the Nissl substance, neurofibrillae and intracellular trabeculae of insect neurones. J R Microsc Soc 78:114–119
Mulyaningsih B, Umniyati SR, Hadianto T (2017) Detection of nonspecific esterase activity in organophosphate resistant strain of Aedes albopictus skuse (Diptera: Culicidae) larvae in Yogyakarta, Indonesia. Southeast Asian. J Trop Med Public Health 48:552–560
Murphy S, Pearce B (1987) Functional receptors for neurotransmitters on astroglial cells. Neuroscience 22:381–394. https://doi.org/10.1016/0306-4522(87)90342-3
Nixon M, Young JZ (2003) The brains and lives of cephalopods. Oxford University Press, Oxford
Packard A, Albergoni V (1970) Relative growth, nucleic acid content and cell numbers of the brain in Octopus vulgaris (Lamarck). Exp Biol 52:539–552
Pantin GFA (1948) Notes on microscopical technique for zoologists. Cambridge University Press, Cambridge
Pearse AGE (1968) Histochemistry: theoritical and applied, vol 1, 3rd edn. Churchill, London
Perry CJ, Barron AB (2013) Neural mechanisms of reward in insects. Annu Rev Entomol 58:543–562. https://doi.org/10.1146/annurev-ento-120811-153631
Peter LJ (1985) Structure, function and regulation of Na, K-ATPase in the kidney. Kidney Int 29:10–20. https://doi.org/10.1038/ki.1986.3
Petersen OH, Maruvama Y (1984) Calcium activated potassium channels and their role in secretion. Nature 703:693–696. https://doi.org/10.1038/307693a0
Prince J, Johnson PM (2015) Ultrastructural comparison of Processing of protein and pigment in the ink gland of four species of sea hares. J Mar Biol 2015:13. https://doi.org/10.1155/2015/847961
Pyle R, Döring M (2017) ZooBank. International Commission on Zoological Nomenclature. Checklist dataset https://doi.org/10.15468/pfqjk1. Accessed via GBIF.org on 24 Jan 2020
Reinecke M (1976) The glial cells of the cerebral ganglia of Helix pomatia L. (Gastropoda, Pulmonata). II. Uptake of ferritin and ‘H-glutamate. Cell Tiss Res 169:361–382
Richard L, Saint M, Carlson SD, Che C (1984) The glial cells of insects. Insect Ultrastruct. https://doi.org/10.1007/978-1-4613-2715-8_12
Shigeno S, Ragsdale CW (2015) The gyri of the octopus vertical lobe have distinct neurochemical identities. J Comp Neurol 523:1297–12317. https://doi.org/10.1002/cne.23755
Shivers RR (1976) Trans-glial channel-facilitated translocation of tracer protein across ventral nerve root sheaths of crayfish. Brain Ret 108:47–58. https://doi.org/10.1016/0006-8993(76)90163-3
Shomrat T, Hochner B (2015) Serotonin may convey positive and octopamine negative reinforcement signals to the learning network of Octopus vulgaris. Program No. 629.06. 2015 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience. https://books.google.com.sa/books?id=O_iEDwAAQBAJ&pg=PT9
Shomrat T, Feinstein N, Klein M, Hochner B (2010) Serotonin is a facilitatory neuromodulator of synaptic transmission and “reinforces” long-term potentiation induction in the vertical lobe of Octopus vulgaris. Neuroscience 169:52–64. https://doi.org/10.1016/j.neuroscience.2010.04.050
Shomrat T, Turchetti-Maia A, Stern-Mentch N, Basil J, Hochner B (2015) The vertical lobe of cephalopods: an attractive brain structure for understanding the evolution of advanced learning and memory systems. J Comp Physiol A 201:947–956. https://doi.org/10.1007/s00359-015-1023-6
Soep H (1976) The determination of monoamine oxidase activity. Biochem Afdeling Res Lab Dr C. Janssen, Beerse, Belgie 3:481–489
Sonakowska LWA, Poprawa I, Binkowski M, Śróbka J, Kamińska K et al (2015) Structure and ultrastructure of the endodermal region of the alimentary tract in the freshwater shrimp Neocaridina heteropoda (Crustacea, Malacostraca). PLoS ONE 10:e0126900. https://doi.org/10.1371/journal.pone.0126900
Stephens PR, Young JZ (1969) The glio-vascular system of, cephalopods. Philos Trans R Soc Lond B. https://doi.org/10.1098/rstb.1969.0001
Sun B, Tsai S (2011) Agonadotropin-releasing hormone-like molecule modulates the activity of diverse central neurons in a gastropod mollusk. Aplysia Californica Front Endocrinol 2:1–8. https://doi.org/10.3389/fendo.2011.00036
Turchetti-Maia TSA, Hochner AB (2019) The vertical lobe of cephalopods: a brain structure ideal for exploring the mechanisms of complex forms of learning and memory. In: JH Byrne (ed) The Oxford handbook of invertebrate neurobiology. https://doi.org/10.1093/oxfordhb/9780190456757.013.29
Turchetti-Maia A, Shomrat T, Hochner B (2017) The vertical lobe of cephalopods: a brain structure ideal for exploring the mechanisms of complex forms of learning and memory. In: Byrne JJ (ed) The Oxford handbook of invertebrate neurobiology. Oxford University Press, Oxford, pp 1–27
Wells MJ, Wells J (1959) Hormonal control of sexual maturity in Octopus. Exp Biol 36:1–35
Wentzell MM, Martínez-Rubio C, Miller MW, Murphy AD (2009) Comparative neurobiology of feeding in the opisthobranch sea slug, Aplysia, and the pulmonate snail, Helisoma: evolutionary considerations. Brain Behav Evol 74:219–230. https://doi.org/10.1159/000258668
Williamson R, Chrachri A (2004) Cephalopod neural networks. Neurosignals 13:87–98. https://doi.org/10.1159/000076160
Wolfe DE, Nicholls JG (1976) Uptake of radioactive glucose and its conversion to glycogen by neurons and glial cells in the leech central nervous system. J Neuropkytiol 30:1593–1609. https://doi.org/10.1152/jn.1967.30.6.1593
WoRMS T (2017) World register of marine species. http://www.marinespecies.org/
Young JZM (1932) On the cytology of the neurons of cephalopods. J Cell Sci 2:1–47
Young J (1971a) The anatomy of the nervous system of Octopus vulgaris. Clarendon Press, Oxford
Young JZ (1971b) The anatomy of the nervous system of Octopus vulgaris. Oxford University Press, London
Young J (1976) The nervous system of Loligo. II. Suboesophageal centres. Philos Trans R Soc Lond B:101–167. https://doi.org/10.1098/rstb.1976.0041
Young J (1977) The nervous system of Loligo. III. Higher motor centres: the basal supraoesophageal lobes. Philos Trans R Soc Lond B:351–398
Young J (1979) The nervous system of Loligo. Y.The vertical lobe complex. Philos Trans R Soc Lond B:311–354. https://doi.org/10.1098/rstb.1979.0008
Zakout YM, Salih MM, Ahmedm HG (2010) The effect of fixatives and temperature on the quality of glycogen demonstration. Biotech Histochem 85:93–98. https://doi.org/10.3109/10520290903126883
Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502. https://doi.org/10.1007/s11302-012-9309-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim, G., Luisetto, M. & Latyshev, O. Glial cells in the posterior sub-esophageal mass of the brain in Sepia officinalis (Linnaeus, 1758) (decapodiformes–sepiida): ultrastructure and cytochemical studies. Invert Neurosci 20, 16 (2020). https://doi.org/10.1007/s10158-020-00249-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10158-020-00249-z