Abstract
Background
It remains unclear whether urinary albumin changes can predict subsequent kidney disease progression in people with diabetes.
Methods
This retrospective cohort study included 4570 Japanese adults with type 2 diabetes (T2D). The exposure was changes in urinary albumin-to-creatinine ratio (UACR) over 3 years, categorized into three categories: ≤ − 30%, minor change, or ≥ 30%. During the exposure period, eGFR decline was also examined and categorized into two categories: < 30% or ≥ 30% decline. The primary outcome was the composite of eGFR halving or initiation of kidney replacement therapy (KRT). The secondary outcome was the initiation of KRT.
Results
In the spline model, the hazard ratio for the primary outcome increased linearly on the log2 scale of UACR changes. When classified into six groups based on the categories of UACR changes and eGFR decline, people with a ≤ − 30% UACR change and < 30% eGFR decline had a 38% lower incidence of the outcome compared to those with a minor UACR change and < 30% eGFR decline. Meanwhile, the risk in those with a ≤ − 30% UACR change and ≥ 30% eGFR decline was 2.89 times. People with a ≥ 30% UACR change had the higher risk, regardless of whether a ≥ 30% eGFR decline occurred. Similar results were obtained in the secondary outcome.
Conclusions
UACR changes can be a useful surrogate for kidney disease progression in people with T2D. However, when setting a decrease in UACR as the surrogate, it may be necessary to simultaneously evaluate kidney function decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The establishment of a useful surrogate for end-stage kidney disease (ESKD) is an urgent requirement for people with diabetes owing to the long progression of diabetic nephropathy, which is a global public health concern [1,2,3,4]. Recently, a large cohort study of over 1.5 million individuals has shown that 30 and 40% eGFR declines were acceptable as a surrogate for ESKD [5,6,7]. Nevertheless, the incidence of these surrogates was low, especially in people without kidney insufficiency [5], indicating the necessity for clinical trials with a larger sample size, longer follow-up period, and higher costs.
A meta-analysis of 20 observational studies with 675,904 individuals has shown that changes in urinary albumin were associated with the subsequent progression to ESKD, regardless of whether diabetes exists [8]. This surrogate is more attractive in terms of its higher frequency of occurrence compared to the above eGFR-related surrogates [5, 8]. To our knowledge, only few studies have examined both urinary albumin changes and eGFR decline simultaneously as the surrogate in people with diabetes [9, 10]. In these studies, changes in urinary albumin unaccompanied by kidney function decline were not associated with the subsequent incidence of ESKD [9, 10]. If correct, the use of changes in urinary albumin as the surrogate should be reconsidered. Furthermore, in estimating changes in urinary albumin, there were a limited number of studies adjusting for imprecision in the determination of urinary albumin levels [8, 9], leading to regression dilution bias. In this study, we aimed to elucidate whether changes in urinary albumin can predict subsequent kidney disease progression in people with diabetes.
Materials and methods
Study design and ethical issues
We designed this single-center retrospective cohort study in adherence to the tenets of the Declaration of Helsinki. The local ethics committee approved the protocol, in which the need for informed consent was waived owing to the non-prospective interventional design (Approval No. 3932). Instead, the website of the institution offered participants an opportunity to opt out.
Participants and measurements
Initially, 7751 Japanese people aged ≥ 18 with type 2 diabetes (T2D) and no history of chronic kidney replacement therapy (KRT) before the index date were identified, who visited our outpatient clinic in Tokyo Women’s Medical University School of Medicine from August 1, 2003 to January 31, 2015. They all had records of body weight, blood pressure, hemoglobin A1c (HbA1c), serum creatinine, lipid parameters, urinary albumin, and urinary creatinine, all of which were measured on the same day. The earliest date of the collected data was set as the index date for each subject if multiple measurements were available during the above-mentioned period.
Exclusion criteria at the index date were as follows: pregnancy (n = 46), malignant diseases (n = 134), history of unilateral nephrectomy (n = 4), biopsy-proven diagnosis of non-diabetic nephropathy (n = 2), and acute kidney injury or post-renal failure (n = 2). We also excluded 2993 participants who had no data of urinary albumin or serum creatinine over three years after the index date (n = 2582), experienced kidney outcome during the exposure period (n = 133), had no serum creatinine measurements in the follow-up period (n = 274), and had missing data on height or smoking status at the index date (n = 4). Eventually, 4570 participants were eligible for inclusion in this study (Supplementary Fig. 1). The characteristics at the index date of the 4570 eligible participants and 2993 participants with missing data are presented in Supplementary Table 1. In this study, a complete-case analysis was conducted.
Data obtained from blood and urine samples were measured using random and first morning samples, respectively. The following formula proposed by the Japanese Society for Nephrology was adopted to estimate GFR: eGFR (mL/min/1.73 m2) = 194 × age (years)−0.287 × serum creatinine level (mg/dL)−1.094 × (0.739 for women) [11].
Exposures and exposure period
Exposures were set as changes in urinary albumin-to-creatinine ratio (UACR) over three years from the index date, estimated using two UACR measurements. In determining the second UACR level for each participant, one year was allowed as a window, and the closest value to three years was selected. UACR changes over three years were treated as both continuous and categorical variables. In the former, UACR changes were expressed as fold changes which were calculated using the following equation: log2 (second UACR/first UACR). In the latter, those were categorized into three categories of the percentage changes as follows: ≤ − 30%, minor change (i.e., > − 30% to < 30%), or ≥ 30%. The above-mentioned treatment for UACR changes was done in previous studies [8,9,10, 12,13,14].
The eGFR decline over three years from the index date was also estimated using all eGFR values measured during the period in which the closest eGFR to three years within the same window for each participant was defined as the last eGFR. The eGFR decline was expressed as a percentage eGFR decline (%/3 years) using the following equation: 100 × 3 × [annual eGFR slope (mL/min/1.73m2/year)]/[eGFR (mL/min/1.73m2) at the index date]. The eGFR decline was treated as a continuous or categorical variable. In the latter, this was categorized into two categories as follows: < 30% decline (i.e., < 10% decline per year) or ≥ 30% decline (i.e., ≥ 10% decline per year), as done in previous studies [5, 9, 10, 14].
The exposure period for each participant was set as the duration from the index date, when UACR and eGFR were measured on the same day, to the date when the second UACR or last eGFR was measured, whichever occurred later (Supplementary Fig. 2).
Outcomes and follow-up period
The primary composite outcome was the first occurrence of a sustained decrease of at least 50% in eGFR from the index date for at least 90 days, or the initiation of chronic KRT. We also evaluated the time to the initiation of chronic KRT as a secondary outcome.
The follow-up period for each participant was set as the duration from the end of the exposure period to the date when the individual reached the outcome or the date when serum creatinine was last measured during the follow-up period without reaching the kidney outcome (Supplementary Fig. 2). The administrative censoring date was set to 31 January 2018.
Statistical analysis
All analyses were performed using the SAS software version 9.4 (SAS Institute, Cary, NC, USA). A two-tailed p value < 0.05 was considered statistically significant.
We used a linear mixed-effects model with a random intercept and slope to estimate the annual eGFR slope during the exposure period. The Cox proportional hazards model was used to estimate hazard ratios for each outcome, in which the following nine covariates were incorporated: sex, age, body mass index (BMI), systolic blood pressure, HbA1c, LDL cholesterol, UACR transformed into common logarithmic values and eGFR at the index date, and percentage decline in eGFR during the exposure period. In the analyses treating UACR changes as a continuous variable for each outcome, log2-transformed UACR change was modeled with the restricted cubic spline function, where four knots were placed at the 5th, 35th, 65th, and 95th percentile level. To mitigate the regression dilution bias, the hazard ratios at a − 30% and 30% UACR change were adjusted by dividing the log-transformed hazard ratio by an attenuation factor, referring to a previous study [8]. The attenuation factor was defined as the ratio of the variance of participants’ random effects to the total variance (i.e., sum of the variances of random effects and residual errors) estimated from the random intercept linear model fitted for all measurements of UACR during the exposure period [15]. The model adjusted for day at measurements as a spline function, while excluding it did not change the estimates. The variability of the estimates was evaluated by the parametric bootstrap samples, assuming a bivariate normal distribution for random effects and residual errors. In the analyses to compare the outcomes between six groups classified into three categories of UACR changes and two categories of eGFR decline, where the above eight variables (other than the percentage eGFR decline) were used as covariates, the interaction between the categories of UACR changes and eGFR decline with respect to each outcome was tested.
We conducted a series of sensitivity analyses for the primary composite kidney outcome. First, the association of UACR changes with the outcome was examined in each group classified into the absence or presence of albuminuria or kidney insufficiency at the index date, in which albuminuria and kidney insufficiency were defined as UACR of ≥ 30 mg/g and eGFR of < 60 mL/min/1.73 m2 based on the Clinical Practice Guidelines proposed by Kidney Disease: Improving Global Outcomes (KDIGO), respectively [16, 17]. Second, in determining the cutoff value of percentage UACR changes during the exposure period, − 40–40% were used instead of – 30–30%. Third, eGFR slope during the exposure period was used instead of percentage eGFR decline, where it was categorized into two categories: < 5 or ≥ 5 (mL/min/1.73 m2/year), referring to previous studies [18, 19]. Fourth, in addition to the above variables, the following five variables were incorporated as covariates: smoking status (current/former vs never), use of ACE inhibitors or angiotensin receptor blockers, use of mineralocorticoid receptor antagonists and history of coronary artery disease or stroke at the index date, and number of creatinine measurements per year during the follow-up period. Finally, the analyses treating death as a competing risk were conducted using the Fine and Gray subdistribution hazards model.
Results
Participant characteristics
Participant characteristics are shown in Table 1 and Supplementary Tables 2 and 3. The distributions of percentage changes in UACR and eGFR during the median exposure period of 3.1 years (IQR: 3.0–3.3 years) are shown in Supplementary Fig. 3. The median annual eGFR slope (mL/min/1.73m2/year) during the exposure period was 1.2 (IQR: 0.2–2.4). The median number of creatinine measurements per person per year performed during the exposure period was 3.2 (IQR: 2.1–5.5). People with ≤ − 30% in UACR change had higher UACR at the index date, and steeper percentage decline in eGFR during the exposure period compared to the other two groups (Table 1). At the index date, 15 participants and nobody took glucagon-like peptide-1 receptor agonists and sodium–glucose cotransporter 2 inhibitors, respectively.
Summary of the follow-up period and outcomes
During the median follow-up period of 5.3 years (IQR: 2.5–8.9 years), 310 participants reached the primary outcome, including 296 with a halving of eGFR and 14 who initiated chronic KRT before experiencing a halving of eGFR. There were 95 participants with chronic KRT after experiencing an eGFR halving. Eventually, there were 109 cases of chronic KRT initiation (i.e., secondary outcome) during the median follow-up period of 5.6 years (IQR: 2.7–9.0 years). The median (IQR) numbers of creatinine measurements per person per year, performed during the follow-up period, were 4.3 (IQR: 2.7–6.5) and 4.4 (IQR: 2.7–6.7) in the analyses for the primary and secondary outcomes, respectively. There were 207 and 221 deaths before reaching the primary outcome and initiating chronic KRT, respectively.
Association of UACR changes during the exposure period with subsequent incidence of outcomes
In the spline models, the hazard ratios for both the primary and secondary outcomes increased approximately linearly on the log2 scale of changes in the UACR during the exposure periods (Fig. 1A, B). The hazard ratios for the primary outcome at -30% and 30% in UACR change during the exposure period in the spline curves were 0.72 and 1.33, respectively (Table 2). The adjustment by a median attenuation factor, which was 0.560 (parametric bootstrap 95% CI 0.547–0.572), strengthened the estimation of the hazard ratios at the two points (Table 2). Similar results were obtained in the analyses for the secondary outcome (Table 2).
A. Multivariable-adjusted restricted cubic spline curves (95% CI) of the association between fold changes in UACR and the primary composite kidney outcome. B. Multivariable-adjusted restricted cubic spline curves (95% CI) of the association between fold changes in UACR and the secondary outcome. The two red dotted lines show a − 30% and 30% change in UACR during the exposure period, respectively. The primary composite kidney outcome was either a decrease in eGFR of ≥ 50% from the index date or the initiation of KRT, whichever came first. The secondary outcome was the initiation of chronic KRT. The 4 knots were placed at the 5th, 35th, 65th, and 95th percentile level of fold changes in UACR. The following variables were used as covariates: sex, age, BMI, systolic blood pressure, HbA1c, LDL cholesterol, UACR transformed into common logarithmic values and eGFR at the index date, and percentage decline in eGFR during the exposure period. UACR urinary albumin-to-creatinine ratio, KRT kidney replacement therapy
In the three groups classified into UACR changes during the exposure period, the risks for the primary outcome in people with ≤ − 30% and those with ≥ 30% in UACR change were 0.60 and 2.79 times, respectively, compared to those with a minor change in UACR, as was the case in the secondary outcome (Table 3).
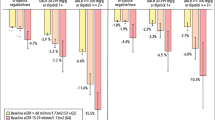
Next, the three groups were further classified into the six groups according to two categories of eGFR decline (< 30% or ≥ 30%) during the exposure period. There was a significant interaction between the categories of UACR changes and eGFR decline during the exposure period with respect to the subsequent primary outcome (p for interaction = 0.002). Compared to people with a stable change in UACR and eGFR (i.e., minor change in UACR and < 30% decline in eGFR), those with ≤ − 30% in UACR change and < 30% decline in eGFR had a 38% lower incidence of the subsequent primary outcome (Fig. 2A). Meanwhile, the risk in those with ≤ − 30% in UACR change and ≥ 30% decline in eGFR was 2.89 times (Fig. 2A). People with ≥ 30% in UACR change had the higher risk, regardless of whether a ≥ 30% eGFR decline occurred during the exposure period (Fig. 2A). Similar results were obtained in the analyses for the secondary outcome (Fig. 2B), although the interaction was not significant (p for interaction = 0.223).
A. Risk of the primary composite kidney outcome in the six groups classified based on categories of changes in UACR and decline in eGFR during the exposure period. B. Risk of the secondary outcome in the six groups classified based on categories of changes in UACR and decline in eGFR during the exposure period. C. Risk of the primary composite kidney outcome in the twelve groups classified based on categories of changes in UACR and decline in eGFR during the exposure period. “Minor” indicates a > − 30% to < 30% change in UACR during the exposure period. The primary composite kidney outcome was either a decrease in eGFR of ≥ 50% from the index date or the initiation of KRT, whichever came first. The secondary outcome was the initiation of chronic KRT. To estimate the hazard ratios, the following variables were used as covariates: sex, and age, BMI, systolic blood pressure, HbA1c, LDL cholesterol, UACR transformed into common logarithmic values and eGFR at the index date. UACR urinary albumin-to-creatinine ratio, KRT kidney replacement therapy, dec decline, HR hazard ratio
Finally, to examine effects of eGFR decline during the exposure period in people with ≤ − 30% in UACR change in more detail, the eGFR decline was further classified into four categories as follows: < 15% decline, ≥ 15 to < 22.5% decline, ≥ 22.5 to < 30% decline, and ≥ 30% decline. In this analysis of the twelve groups, even if a ≤ − 30% change in UACR occurred, people with a ≥ 15% decline in eGFR during the exposure period had a higher risk of the subsequent primary outcome compared to those with a stable change in UACR and eGFR (Fig. 2C).
Sensitivity analyses
In people with non-albuminuria and those with non-kidney insufficiency at the index date, the analyses in the above-mentioned six groups were not performed because of the small numbers of subjects with a ≥ 30% decline in eGFR during the exposure period (n = 19 and 28, respectively). The results of sensitivity analyses were consistent with the above-mentioned findings (Supplementary Tables 4 and 5, and Supplementary Figs. 4A–F and 5A–F).
Discussion
This single-center, retrospective cohort study of Japanese adults with T2D showed that UACR changes during the three-year exposure period, treated as both continuous and categorical variables, were significantly associated with the subsequent kidney outcomes independent of eGFR decline during the exposure period. Furthermore, the hazard ratios were adjusted using an attenuation factor. The analyses combining each category of the UACR changes and eGFR decline showed that people with ≤ − 30% in UACR change and < 30% decline in eGFR had a lower risk for the incidence of the kidney outcome, compared to those with a stable change in UACR and eGFR; however, the risk in those with ≤ − 30% in UACR change and ≥ 30% decline in eGFR was rather high. An increase of ≥ 30% in UACR was associated with the higher risk, regardless of whether a ≥ 30% eGFR decline occurred during the exposure period.
To our knowledge, the existing studies demonstrating a significant association of UACR changes with subsequent kidney disease progression did not consider effects of eGFR changes during the exposure period [8, 20,21,22,23,24]. In addition, only few studies have adjusted for imprecision in the determination of UACR when estimating the UACR changes [8, 9]. We consider that in the present study, the hazard ratios of the kidney outcomes adjusted by the attenuation factor was close to the true effects of the UACR changes, since the estimated coefficient of variation for UACR was by no means small even in a 24-h specimen or first morning spot urine specimen [25].
A cohort study of 8,766 people with T2D in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation Observational (ADVANCE-ON) showed that neither ≤ − 40% nor ≥ 40% in UACR changes during the two-year exposure period was associated with the subsequent progression to ESKD when those did not experience a ≥ 40% decline in eGFR during the exposure period [9], as was the case in a cohort study from Japan [10]. The findings of these studies are inconsistent with those of the present findings. However, the number of people who reached the kidney outcome in the two above studies was only 108 and 129, respectively [9, 10]. The recent population-based cohort study of 91,319 individuals, where 2541 experienced kidney outcomes, showed that in those with a stable eGFR (i.e., > − 30% to < 30% in eGFR changes) during the three-year exposure period, both − 30% and 30% in UACR changes during the exposure period were significantly associated with the subsequent kidney outcomes [14]. Based on the above results and together with the present findings, we believe that UACR changes can be a useful surrogate for subsequent kidney disease progression, irrespective of the presence of diabetes.
As shown in Fig. 2C, even people with a ≤ − 30% UACR change and 15–22.5% decline in eGFR (i.e., 5 to 7.5% decline per year) had the higher risk for the subsequent incidence of the primary outcomes in the present study, suggesting that compared to UACR changes, eGFR declines were more strongly associated with a subsequent kidney function decline. In the Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints (ALTITUDE) and the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) study, dual blockade of renin–angiotensin–aldosterone system reduced urinary albumin but not risk for kidney failure [26, 27], which might be partly explained by the present findings. We, therefore, consider that when setting an UACR decrease as a kidney outcome, kidney function decline needs to be evaluated simultaneously.
Consistent with previous studies [9, 10, 14], the present study showed that a combination of UACR increase and eGFR decline more strongly predicted subsequent incidence of kidney outcomes than each one, and the effects were synergistic with a significant interaction. However, the number of people who experienced both a ≥ 30% UACR change and ≥ 30% eGFR decline during the approximate three-year exposure period was only 1% in the present study.
The present study has several limitations. First, our cohort comprised an ethnically homogeneous population from a single Japanese university hospital. Second, we did not have information regarding histological examinations of people with albuminuria or kidney insufficiency. Finally, we could not evaluate time-dependent changes in laboratory data, blood pressure, BMI, and medications during the follow-up period.
Conclusions
The present study of people with T2D has provided evidence that UACR changes can be a useful surrogate for kidney disease progression. However, when setting an UACR decrease as a surrogate, it may be necessary to simultaneously consider kidney function decline.
Data Availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Fox CS, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–73.
Afkarian M, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316:602–10.
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–33.
American Diabetes Association Professional Practice Committee. 11 Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S175–84.
Coresh J, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–31.
Levey AS, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–35.
Levey AS, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the national kidney foundation in collaboration With the US food and drug administration and european medicines agency. Am J Kidney Dis. 2020;75:84–104.
Coresh J, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7:115–27.
Ohkuma T, et al. Combination of changes in estimated GFR and albuminuria and the risk of major clinical outcomes. Clin J Am Soc Nephrol. 2019;14:862–72.
Oshima M, et al. Combined changes in albuminuria and kidney function and subsequent risk for kidney failure in type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9: e002311.
Matsuo S, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Waijer SW, et al. Short-term changes in albuminuria and risk of cardiovascular and renal outcomes in type 2 diabetes mellitus: a post hoc analysis of the EMPA-REG OUTCOME Trial. J Am Heart Assoc. 2020;9: e016976.
Oshima M, et al. Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: a post hoc analysis from the CREDENCE Trial. J Am Soc Nephrol. 2020;31:2925–36.
Neuen BL, et al. Changes in GFR and albuminuria in routine clinical practice and the risk of kidney disease progression. Am J Kidney Dis. 2021;78:350–60.
Keogh RH, et al. STRATOS guidance document on measurement error and misclassification of variables in observational epidemiology: part 1-Basic theory and simple methods of adjustment. Stat Med. 2020;39:2197–231.
Inker LA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–35.
Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO. Clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;2020(98):S1–115.
Levin A, et al. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2013;85:49–61.
Greene T, et al. Performance of GFR slope as a surrogate end point for kidney disease progression in clinical trials: a statistical simulation. J Am Soc Nephrol. 2019;30:1756–69.
Sumida K, et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol. 2017;12:1941–9.
Carrero JJ, et al. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int. 2017;91:244–51.
Jun M, et al. Changes in albuminuria and the risk of major clinical outcomes in diabetes: results from ADVANCE-ON. Diabetes Care. 2018;41:163–70.
Viazzi F, et al. Changes in albuminuria and renal outcome in patients with type 2 diabetes and hypertension: a real-life observational study. J Hypertens. 2018;36:1719–28.
Heerspink HJL, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7:128–39.
Witte EC, et al. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20:436–43.
Parving HH, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–13.
Fried LF, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–903.
Acknowledgements
This study was supported in part by Daiichi Sankyo Co., Ltd. We are grateful to E. Tauchi, S. Yamashita and I. Nyumura (Division of Diabetology and Metabolism, Department of Internal Medicine, Tokyo Women’s Medical University School of Medicine) for their assistance in data collection.
Funding
We have not received any funding for the present study.
Author information
Authors and Affiliations
Contributions
YY and KH contributed to the conception and design of the study. YY, KH, TM, YY, NY and HM contributed to the data collection and preparation. KH and TS contributed to the analysis and interpretation of data. YY and KH were responsible for drafting the manuscript. KH, TM, Y. Yamamoto, NY, HM, TS and TB revised the manuscript. All authors have approved the final version of the manuscript. TB is the guarantor of this work, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have nothing to disclose.
Ethical approval
All procedures performed in the present study were in accordance with the ethical standards of the institutional research committee of Tokyo Women’s Medical University School of Medicine (Approval No. 3932) and with the tenets of the Declaration of Helsinki of 1964 and its later versions.
Informed consent
As the present study was an observational and historical but not a prospective interventional study, the ethics committee waived the need for direct informed consent. Instead, we offered participants the opportunity to opt out to participate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yokoyama, Y., Hanai, K., Mori, T. et al. Changes in urinary albumin as a surrogate for kidney disease progression in people with type 2 diabetes. Clin Exp Nephrol 27, 465–472 (2023). https://doi.org/10.1007/s10157-023-02328-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-023-02328-y