Abstract
Background
Peritoneal dialysis (PD) is essential for patients with end-stage renal disease. Peritoneal fibrosis (PF) is a complex inflammatory, fibrogenic process. No effective treatments are available to prevent these processes. Hepatocyte growth factor (HGF) possesses anti-inflammatory and anti-fibrotic properties. The aim of this study was to analyze whether HGF suppresses MGO-induced peritoneal inflammation and fibrosis in a mouse model.
Methods
PF was induced by intraperitoneal (IP) injections of MGO for 14 days. C57/BL/6 mice were divided into three groups: Sham group (only vehicle); Sham + MGO group (PF induced by MGO); and HGF + MGO group (PF mice treated with recombinant human-HGF). PF was assessed from tissue samples by Masson’s trichrome staining. Inflammation and fibrosis-associated factors were assessed by immunohistochemistry and quantitative real-time PCR.
Results
MGO-injected mice showed significant thickening of the submesothelial compact zone with PF. Treatment with HGF significantly reduced PM thickness and suppressed the expression of collagen I and III and α-SMA. Expression of profibrotic and proinflammatory cytokines (TGF-β, TNF-α, IL-1β) was reduced by HGF treatment. The number of macrophages, and M1 and M2 macrophage-related markers, such as CD86, CD206, and CD163, was reduced in HGF + MGO mice.
Conclusion
HGF attenuates MGO-induced PF in mice. Furthermore, HGF treatment reduces myofibroblast and macrophage infiltration, and attenuates the upregulated expression of proinflammatory and profibrotic genes in peritoneal tissues. HGF might be an effective approach to prevent the development of PF in patients undergoing PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritoneal dialysis (PD) is an essential treatment for patients with end-stage renal disease. According to the 2018 United States Renal Data System (USRDS) report, PD was needed for 2.7% renal replacements in Japan, and 7.0% in the United States. In Hong Kong, PD accounted for as much as 20–70% of modalities [1]. PD involves continuous dialysis, causes less burden on the cardiovascular system, and is better than other renal replacement therapy modalities for maintaining residual renal function. However, PD is known to cause functional and structural changes in the peritoneum [2,3,4]. PF is characterized by abdominal proliferation of α-smooth muscle actin (α-SMA) positive myofibroblasts, and significant accumulation of extracellular matrix (ECM) proteins [5]. Metalloproteinases (MMPs) are involved in ECM degradation, and play an important role in fibrosis, cancer growth, and invasion. MMP3 can decompose type III collagen and proteoglycan, and MMP9 can decompose α2 chain of type I collagen and acid insoluble type I and type III collagen [6]. Transforming growth factor-β (TGF-β) and MMPs are key molecules involved in fibrosis and remodeling [7,8,9]. PF has two cooperative processes caused by PD, fibrosis itself, and inflammation, the processes showing bidirectional mutual induction [10]. M1 macrophages and M2 macrophages are involved in peritoneal inflammation, with M1 macrophages playing an important pathogenic role in PF [11]. Marker expression of M1 macrophages includes CD86 and MHC-II, whereas that of M2 macrophages includes CD163 and CD206. F4/80 molecule is only expressed on the surface of tissue macrophages.
Methylglyoxal (MGO) is a major precursor of advanced glycation end products (AGEs) and cause of DNA, protein, and transcription modulators glycation. AGEs and these glycations upregulate the production of both inflammatory molecules and molecules that provoke tissue injury. Many factors have been reported to cause accumulation of MGO, including hyperglycemia, uremia, oxidative stress, aging, and inflammation [12]. Furthermore, peritoneal MGO injection has been established as a mouse model for PF [13]. The chlorhexidine model is a typical PH model, but compared to the chlorhexidine model, the MGO model has the same histological features as peritonitis patients, such as thickening of the medial subepithelial compact zone, filtration of inflammatory cells, and new blood vessels [14].
Hepatocyte growth factor (HGF) is a mesenchymal cell-derived growth factor that affects various target cells [15,16,17]. It was originally recognized and cloned as a potent mitogen of primary cultured hepatocytes, and was purified as a hepatocyte mitogen from patients with fulminant hepatic failure. HGF has been reported to have an important role in liver, gastrointestinal tract, kidneys, lungs, and nervous system regeneration and repair [18]. It has been demonstrated that dialysate HGF concentration is significantly higher among patients with ultrafiltration failure [19]. Additionally, human peritoneal mesothelial cells constantly synthesize HGF, and treatment of human peritoneal mesothelial cells with HGF blocks glucose-induced mesenchymal epithelial transition (c-MET) [20]. HGF also has an anti-inflammatory and anti-fibrotic effect. While many studies have demonstrated the anti-fibrotic effect of HGF in various tissues [17, 19, 21,22,23], little research has been done on the anti-inflammatory and anti-fibrotic effects of HGF particularly in peritoneal tissue. To date, there is no effective treatment to prevent peritoneal inflammation and fibrosis.
In this study, we investigated the efficacy of HGF treatment in peritoneal tissue using an experimental mouse model of MGO-induced PF.
Materials and methods
Animal model of PF and administration of recombinant-HGF
PF animal model induced by MGO [14] was established in 7-week-old C57BL/6 J male mice weighing 17–20 g obtained from Charles River Japan (Kanagawa, Japan) to compare the degree of PF with or without HGF administration. All animal experimental procedures were performed in accordance with protocols and guidelines for animal experiments approved by the Institutional Animal Care and Use Committees of Kagoshima University (Permit Number: MD18007). Mice were maintained in a light- and temperature-controlled room (23 ± 1 °C) in the Laboratory Animal Center of Kagoshima University (Kagoshima, Japan). Mice were acclimatized to these conditions for 7 days prior to their use in experiments. Subsequently, the mice were divided into three groups (n = 5 per group): Sham, Sham + MGO, and HGF + MGO. Subcutaneous (SC) osmotic pumps (Alzet Model 1002, CA, USA) with a fluid capacity of 100 μL, delivery rate of 0.25 μl/h, were implanted on the backs of all the mice on day 0. The Sham group received saline (100 ml/kg) by IP injection for 14 days, and saline was delivered via osmotic pump. PF was induced in mice by MGO administration [12] [12]. The Sham + MGO group received IP injections of MGO (0.1 mmol/body, M0252, Sigma-Aldrich) for 14 days, and saline was delivered via osmotic pump. The HGF + MGO group received IP MGO injections (0.1 mmol/body, M0252, Sigma-Aldrich) for 14 days, and HGF (5 mg/ml, Eisai, Tokyo, Japan) was delivered via osmotic pump 14. Tissue and blood samples were collected from mice under a combination anesthesia of ketamine and medetomidine, intraperitoneally on day 14. Measurement of plasma HGF concentration was outsourced to BML (Kagoshima, Japan).
Histologic and immunohistochemical examination
To confirm that HGF release was functioning properly from SC pumps, proliferation cell nuclear antigen (PCNA) immunohistochemistry of liver tissue was performed. To analyze morphological peritoneal changes, 4-μm-thick peritoneal tissues collected from mouse abdomen were stained with Masson’s trichrome. Nine 200-μm fields were randomly selected at 200 × magnification. Thickness of the submesothelial zone was measured by image analysis software (Keyence, Osaka, Japan).
To examine collagen I, collagen III, and α-SMA peritoneal expression, immunohistochemical analysis was performed and the following primary antibodies were used: Rabbit polyclonal anti-type I Collagen antibody (Abcam, Cambridge, UK), Rabbit Polyclonal anti-type III Collagen antibody (Abcam, Cambridge, UK), Anti-α-SMA Antibody (Cell Signaling, Danbers, MA, USA), and Rat Monoclonal F4/80 Antibody (Bio-Rad, Hercules, CA, USA).
Nine 200-µm fields were randomly selected at 200 × magnification from the areas of peritoneum that stained positive for type I and type III antibodies. Samples were analyzed by an image analysis software (Keyence, Osaka, Japan), and a number of cells positive for α-SMA, CD68, and F4/80 in the submesothelial zone were counted.
Quantitative real-time PCR
The expression of TNF-α, IL-1β, TGF-β, MMP3, MMP9, CD86, CD163, and CD206 genes in peritoneal samples was analyzed using quantitative real-time polymerase chain reaction (qPCR). Total RNA was extracted from peritoneal tissues using TRIzol reagent (Ambion, Austin, TX, USA). RNA purity was confirmed by spectrophotometry, and A260/A280 ratios ranged from 1.9 to 2.1. After total RNA was reverse-transcribed to cDNA by Prime Script RT Reagent Kit (Takara, Otsu, Japan), qPCR was performed using the StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, CA, USA), and comparisons were obtained using the ΔΔCT method (Applied Biosystems, Foster City, CA, USA). Primers were designed based on published sequences (Supplementary Table 1), and β-actin was used as an internal control.
Western blot assay
Expression of c-MET and phospho-c-MET in peritoneal tissue was analyzed using total protein extracted from liver tissue. Protein concentration was measured using the DC Protein Assay (BIO-RAD, Hercules, CA, USA). Western blotting of the extracted proteins was performed by incubation with Anti-Rabbit primary antibodies of Anti-Met (SP260, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Anti-phospho-Met (Tyr1234/1235) (D26, Cell Signaling, Danbers, MA, USA), followed by incubation with Anti-Rabbit secondary antibody (Cell Signaling, Danbers, MA, USA). Internal reference and quantified bands were measured with Fusion Solo with Fusion Capt17 (Vilber-Lourmat, Marne-la-Vallée cedex, France).
Statistical analysis
Data were expressed as mean ± standard deviation (SD), unless specified otherwise. Statistical analyses were performed using SPSS software version 26 (SPSS Inc., Chicago, IL, USA). Kruskal–Wallis analysis of variance (ANOVA) was used to determine the statistical significance of the differences at p < 0.05.
Results
HGF suppresses body weight loss and thickening of MGO-induced PF
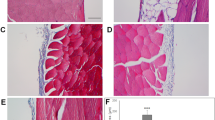
The Sham + MGO group exhibited a significantly lower body weight gain than the Sham and HGF + MGO groups (p < 0.05) (Fig. 1c). The peritoneal tissue of the Sham + MGO group showed significant thickening of the submesothelial compact zone compared with the Sham group (p < 0.05). Peritoneal tissue of the MGO + HGF group showed a significant decrease in thickening compared with the Sham + MGO group (p < 0.05) (Fig. 1). No mice died during these experiments in any of the groups.
Hepatocyte growth factor (HGF) suppresses thickening of peritoneal fibrosis in methylglyoxal (MGO)-injected mice. Representative photomicrographs of peritoneal tissues stained with Masson’s trichrome (200 ×) (a). Comparison of the average of nine randomly selected 200-μm fields of submesothelial zone at 200 × magnification. The peritoneal tissue of the Sham + MGO group showed significant thickening of the submesothelial compact zone compared with the Sham group. Peritoneal tissue of the HGF + MGO group showed significant decrease in thickening compared with the Sham + MGO group (*p < 0.05) (b)
HGF attenuates the expression of α-SMA, and type I and type III collagen
Accumulation of α-SMA expression cells was observed in the submesothelial compact zone in the Sham + MGO group. HGF administration significantly reduced the number of α-SMA positive cells in the HGF + MGO group compared to the Sham + MGO group (Fig. 2a).
Hepatocyte growth factor (HGF) inhibits α-smooth muscle actin (α-SMA) and reduces type I and III collagen expression in mice with peritoneal fibrosis. Immunohistochemical analysis of α-SMA (a), and type I and III collagen expression (b) in peritoneal fibrosis in mice. The number of α-SMA expressing cells and type I and III collagen pixels were significantly higher in Sham + MGO (methylglyoxal) group and lower in HGF + MGO group. Statistical analyses were performed using ANOVA (*p < 0.05)
A narrow range of positive areas of type I and type III collagen was observed in the Sham group. Both types of collagen were diffusely expressed at the submesothelial compact zone in the Sham + MGO group. Moreover, in the MGO + HGF group, positive areas of both collagen types were significantly reduced compared with the Sham + MGO group (p < 0.05) (Fig. 2b).
HGF decreases TNF-α, IL-1β, TGF-β, MMP3, and MMP9 gene expressions
Expression of TNF-α and IL-1β genes were increased in the Sham + MGO group. Conversely, expression of both the genes was significantly decreased or tended to decrease in the HGF + MGO group, compared with the Sham + MGO group (p < 0.05) (Fig. 3).
Expression of TGF-β, MMP3, and MMP9 in HGF + MGO group was significantly reduced or showed a tendency to decrease, compared with the Sham + MGO group (p < 0.05) (Fig. 3).
The number of CD68- and F4/80-positive cells was increased in the Sham + MGO group, but was significantly reduced in the HGF + MGO group compared to the Sham + MGO group (p < 0.05) (Fig. 4a, b). HGF treatment significantly downregulated mRNA expression of the M1 markers (CD86) and M2 markers (CD206 and CD163) (Fig. 4c).
Discussion
In this study, HGF administration in mice suppressed the progression of MGO-induced PF. HGF treatment also reduced myofibroblast and macrophage infiltration, and attenuated the upregulated expression of proinflammatory and profibrotic genes in the peritoneal tissue. To our knowledge, this is the first study to demonstrate the anti-fibrotic effect of HGF on PF in vivo.
In PD, peritoneal tissues are constantly exposed to non-physiological fluids that cause various histological changes, such as shedding of peritoneal mesothelial cells, mesothelial layer thickening, fibrosis, infiltration of inflammatory cells, angiogenesis, and reduction of peritoneal function [2,3,4]. In addition, it has been reported that fibroblasts presenting in the lower layer of peritoneal mesothelial cells are mainly derived from fibroblasts observed during peritoneal injury, rather than transformation from mesothelial cells [24]. Hyperosmolarity from high glucose concentrations, GDPs, and dialysates containing AGEs reportedly cause peritoneal degradation. Conventional commercial dialysates, which contain GDPs and 2–33 μM MGO, have the potential to induce mesenchymal-like mesothelial cell formation [14]. GDPs have been used for developing many animal PF models. MGO is an extremely toxic GDP in PD fluid, and a strong promoter for AGE formation [12]. As mentioned above, AGEs induce inflammation and angiogenesis.
The MGO model was used in this study, and the expressions of proinflammatory and profibrotic markers, such as TNF-α, IL-1β, TGF-β, α-SMA, and collagen type I and III were found to be upregulated in the peritoneal tissue in this mouse model. Collagen is the main component of the ECM, with type I collagen being the most abundant of the collagen protein family in fibrosis [6]. Collagen fragments (polypeptides) enhance the production of IL-1β by human peripheral blood mononuclear cells [25]. Type I collagen stimulates mouse peritoneal macrophages to aggregate and produce proinflammatory molecules through reactive oxygen species upregulation.
TGF-β is known to induce differentiation of peritoneal mesothelial cells and fibroblasts into myofibroblasts with collagen-producing ability. It is believed that these actions result in the suppression of PF [26]. It had been reported that HGF has anti-fibrotic effects on various organs such as the liver, kidney, lung, and heart by inhibition of collagen I, collagen III, and TGF-β [16, 21, 27]. Thickening of the peritoneal mesothelial tissue was observed with MGO administration, and the accumulation of collagen I, collagen III, and TGF-β was observed through immunohistological analyses, suggesting that TGF-β-mediated fibrosis had progressed. Similarly, in the group that received MGO, the expression of F4/80 and CD68 increased in the peritoneal mesothelial tissue, suggesting an increase in inflammatory macrophages.
In peritoneal dialysis patients, it is considered that the peritoneal mesothelial cells are shed by contact with non-physiological dialysate, and it has been shown that the peritoneal mesothelial cells secrete HGF. Susan et al. reported that HGF dialysis effluent levels were higher in the low-glucose dialysate group and that there was no difference between the two groups after these patients switched to high-glucose-based dialysate. This result indicates that high glucose dialysate reduces HGF production in mesothelial cells [28]. In a rat model of chlorhexidine, Matsuoka et al. used rat peritoneal mesothelial cells transfected with full-length human-HGF cDNA into the expression vector to reverse the pathological changes in the peritoneum [29].
Ido et al. found that, in the liver tissue of the rat model, injection of HGF induced tyrosine phosphorylation of c-MET, a specific receptor for HGF [30]. HGF has an important role in the regeneration of organs, and has an anti-inflammatory and anti-fibrotic effect in various tissues [17, 19, 21,22,23]. Though it has been reported that HGF reduced the effects of TGF-β-induced α-SMA and collagen I of human peritoneal fibroblasts in vitro [31], it is unclear if HGF has similar anti-inflammatory and anti-fibrotic effects on peritoneal tissue in vivo. Fibrosis results from inflammatory reactions. Macrophage aggregation, which occurs secondary to inflammation, is widely involved in subsequent fibrotic processes [32]. Macrophages play important roles in innate cellular immunity and produce proinflammatory cytokines, such as TNF-α and IL-1β under inflammatory conditions [33]. Macrophages can be polarized to exhibit different phenotypes by cytokine production and, accordingly, two macrophage subsets (M1 and M2) have been identified. M1 macrophages generate proinflammatory cytokines and function mainly in inflammatory reactions and tissue destruction. In contrast, M2 macrophages produce anti-inflammatory and profibrotic cytokines such as TGF-β, and promote tissue remodeling and fibrosis [34]. M1 macrophages upregulate CD86 protein levels, and produce proinflammatory cytokines, such as IL-1β and TNF-α. CD86 and CD80 are markers for M1 macrophages. CD206 and CD163 are markers for M2 macrophages which are involved in the anti-inflammatory and fibrotic processes [35, 36]. CD206 is a receptor involved in endocytosis, and is the most commonly used marker of M2 macrophages.
By inhibiting angiogenesis and inflammation, macrophage-derived TGF-β is suppressed, and the TGF-β protein levels in PD patients with peritonitis are increased [37]. PF and monocytes/macrophages are closely related. CD86- and CD206-positive macrophages are both present in peritoneal macrophages, and CD68 is highly expressed in blood monocytes and tissue macrophages. F4/80 is also expressed at high levels on the surface of various macrophage of various tissues in mice [38]. Some previous studies showed that monocytes express c-MET, which is a specific HGF receptor, and HGF affects the differentiation and function of monocytes and macrophages [39,40,41,42]. However, the effect of HGF-MET signaling against monocytes/macrophages in peritoneal tissues remains unclear. In our study, we found that HGF administration inhibits infiltration of M1 and M2 macrophages in peritoneal tissue (Fig. 4). These results suggest that HGF affects macrophage infiltration and polarization, causing attenuation of proinflammatory and profibrotic factors.
The study limitation is that it does not assess all downstream factors of HGF. Activation of MET by HGF induces kinase catalytic activity of MET and initiates transphosphorylation of Tyr1234 and Tyr1235. These two tyrosine residues bind to various signaling factors and initiate various biological activities driven by MET. Evaluation and investigation of these downstream factors of HGF will be considered.
In summary, we demonstrated that HGF attenuates MGO-induced PF in mice, and HGF treatment reduced myofibroblast and macrophage infiltration. Furthermore, we found that HGF attenuated the upregulated expression of proinflammatory and profibrotic genes in peritoneal tissue. Our results are promising for the future of PF treatment, and may shed light on a possible new approach for preventing PF by treatment with HGF in patients undergoing PD. Further studies are needed before these experimental findings can be translated into clinical applications.
References
Robinson BM, et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet. 2016;388:294–306.
Morgan LW, et al. Glucose degradation products (GDP) retard remesothelialization independently of D-glucose concentration. Kidney Int. 2003;64:1854–66.
Williams JD, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–9.
Witowski J, et al. Prolonged exposure to glucose degradation products impairs viability and function of human peritoneal mesothelial cells. J Am Soc Nephrol. 2001;12:2434–41.
Margetts PJ, et al. Transient overexpression of TGF-β1 induces epithelial mesenchymal transition in the rodent peritoneum. J Am Soc Nephrol. 2005;16:425–36.
Thomas AH, et al. Collagen fragments modulate innate immunity. Exp Biol Med (Maywood). 2007;232:406–11.
Epstein FH, et al. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–92.
Padwal M, et al. Matrix metalloproteinase 9 is associated with peritoneal membrane solute transport and induces angiogenesis through β-catenin signaling. Nephrol Dial Transplant. 2017;32:50–61.
Fielding CA, et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40:40–50.
Zhou Q, et al. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016;90:515–24.
Li Q, et al. A pathogenetic role for M1 macrophages in peritoneal dialysis-associated fibrosis. Mol Immunol. 2018;94:131–9.
Nagai T, et al. Linagliptin ameliorates methylglyoxal-induced peritoneal fibrosis in mice. PLoS ONE. 2016;11:e0160993.
Kitamura M, et al. Epigallocatechin gallate suppresses peritoneal fibrosis in mice. Chem Biol Interact. 2012;195:95–104.
Hirahara I, et al. Methylglyoxal induces peritoneal thicking by mesenchymal-like mesothelial cells in rats. Nephrol Dial Transplant. 2009;24:437–47.
Nita I, et al. Hepatocyte growth factor secreted by bone marrow stem cell reduce ER stress and improves repair in alveolar epithelial II cells. Sci Rep. 2017;7:41901.
Komaki Y, et al. Hepatocyte growth factor facilitates esophageal mucosal repair and inhibits the submucosal fibrosis in a rat model of esophageal ulcer. Digestion. 2019;99:227–38.
Wang Z, et al. Antifibrotic effects of hepatocyte growth factor on endothelial-to-mesenchymal transition via transforming growth factor-betal(TGF-β1)/smad and Akt/mTOR/P70S6K signaling pathways. Ann Transplant. 2018;23:1–10.
Rodgers JT, et al. HGFA is an injury-regulated systemic factor that induces the transition of stem cells into GAlert. Cell Rep. 2017;19:479–86.
Yu MA, et al. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrol. 2009;20:567–81.
Shinohara M, et al. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101:1142–7.
Mizuno S, et al. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005. https://doi.org/10.1096/fj.04-1535fje.
Mizuno S, et al. Hepatocyte growth factor suppresses interstitial fibrosis in a mouse model of obstructive nephropathy. Kidney Int. 2001;59:1304–14.
Schievenbusch S, et al. Profiling of anti-fibrotic signaling by hepatocyte growth factor in renal fibroblasts. Biochem Biophys Res Commun. 2009;385:55–61.
Chen YT, et al. Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury. J Am Soc Nephrol. 2014;25:2847–58.
Zhang X, et al. Type I collagen or gelatin stimulates mouse peritoneal macrophages to aggregate and produce pro-inflammatory molecules through upregulated ROS levels. Int Immunopharmacol. 2019;76:105845.
Yang AH, et al. Myofibroblastic conversion of mesothelial cells. Kidney Int. 2003;63:1530–9.
Azuma J, et al. Angiogenic and antifibrotic actions of hepatocyte growth factor improve cardiac dysfunction in porcine ischemic cardiomyopathy. Gene Ther. 2006;13:1206–13.
Yung S, et al. Impact of a low-glucose peritoneal dialysis regimen on fibrosis and inflammation biomarkers. Perit Dial Int. 2015;35:147–58.
Matsuoka T, et al. Hepatocyte growth factor prevents peritoneal fibrosis in an animal model of encapsulating peritoneal sclerosis. J Nephrol. 2008;21:64–73.
Ido A, et al. Pharmacokinetic study of recombinant human hepatocyte growth factor administered in a bolus intravenously or via portal vein. Hepatol Res. 2004;30:175–81.
Jiang D, et al. Protective action of hepatocyte growth factor on transforming growth factor β-1-induced α-smooth muscle actin and extracellular matrix in cultured human peritoneal fibroblasts. Case Reports Clin Pract Rev. 2010;16:250–4.
Lee HA, et al. Ethyl acetate extract from Asparagus cochinchinensis exerts anti-inflammatory effects in LPS-stimulated RAW264.7 macrophage cells by regulating COX-2/iNOS, inflammatory cytokine expression, MAP kinase pathways, the cell cycle and anti-oxidant activity. Mol Med Rep. 2017;15:1613–23.
Kigerl KA, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–44.
Wijesundera KK, et al. M1- and M2-macrophage polarization in rat liver cirrhosis induced by thioacetamide (TAA), focusing on Iba1 and galectin-3. Exp Mol Pathol. 2014;96:382–92.
Chen S, Lu Z, et al. Cathelicidin-WA polarizes E. coli K88-induced M1 macrophage to M2-like macrophage in RAW264.7 cells. Int Immunopharmacol. 2018;54:52–9.
Zhao Y, et al. Comparison of the characteristics of macrophages derived from murine spleen, peritoneal cavity, and bone marrow. J Zhejiang Univ Sci B. 2017;18:1055–63.
Mlambo NC, et al. Increased levels of transforming growth factor beta 1 and basic fibroblast growth factor in patients on CAPD: a study during non-infected steady state and peritonitis. Inflammation. 1999;23:131–9.
Bellón T, et al. Alternative activation of macrophages in human peritoneum: implications for peritoneal fibrosis. Nephrol Dial Transplant. 2011;26:2995–3005.
Beilmann M, Vande Woude GF, Dienes HP, Schirmacher P. Hepatocyte growth factor-stimulated invasiveness of monocytes. Blood. 2000;95:3964–9.
Jiang Q, et al. Differential responsiveness of cord and adult blood monocytes to hepatocyte growth factor. Clin Exp Immunol. 2001;125:222–8.
Chen Q, et al. Induction of met proto-oncogene (hepatocyte growth factor receptor) expression during human monocyte-macrophage differentiation. Cell Growth Differ. 1996;7:821–32.
Galimi F, et al. Hepatocyte growth factor is a regulator of monocyte- macrophage function. J Immunol. 2001;166:1241–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflicts of interest exist.
Human and animal rights
All procedures performed in studies involving animals were in accordance with the Kagoshima University Animal Experiment Rules. This article does not contain any studies with human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10157_2021_2067_MOESM1_ESM.pptx
Experimental protocol of the animal model of peritoneal fibrosis and the administration of recombinant-hepatocyte growth factor (HGF). The mice were divided into three groups (n = 5 per group). Peritoneal fibrosis was induced by intraperitoneal injection of methylglyoxal (MGO) (40 mM) for 14 consecutive days (a). All mice were implanted with an osmotic pump subcutaneously on the back. The contents of the pump were saline or HGF (b). Body weight loss was attenuated by HGF (*p< 0.05 vs. Sham+MGO) (c) (PPTX 2828 KB)
10157_2021_2067_MOESM2_ESM.pptx
Serum hepatocyte growth factor (HGF) concentration and proliferation cell nuclear antigen (PCNA) expression in liver tissue of mice with peritoneal fibrosis. Comparison of concentrations of serum HGF released from intraperitoneal osmotic pump versus subcutaneous osmotic pump on day 7 (n = 3) (a). PCNA immunohistochemical analysis of liver tissue was performed to confirm HGF release from the osmotic pump (n = 5) (b) (*p < 0.01, **p < 0.05) (PPTX 436 KB)
10157_2021_2067_MOESM3_ESM.pptx
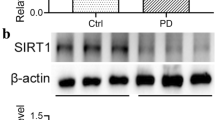
Expression of c-MET and phospho-c-MET in the peritoneal membrane of mice.c-MET and phospho-c-MET in the peritoneal membrane were compared by western blotting. β-actin was used as a loading control for western blot (n = 3) (PPTX 107 KB)
About this article
Cite this article
Yoshimine, H., Tanoue, S., Ibi, Y. et al. Hepatocyte growth factor ameliorates methylglyoxal-induced peritoneal inflammation and fibrosis in mouse model. Clin Exp Nephrol 25, 935–943 (2021). https://doi.org/10.1007/s10157-021-02067-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-021-02067-y