Abstract
Background
It is currently controversial whether neutrophil-to-lymphocyte ratio (NLR) has a prognostic role in patients with chronic kidney disease (CKD). We aimed to investigate whether NLR was an independent predictor of cardiovascular or all-cause mortality in CKD patients with or without hemodialysis by performing a meta-analysis.
Methods
Pubmed, Embase, and Cochrane Library databases are systematically searched for relevant literature that investigated NLR and subsequent cardiovascular or all-cause mortality risk in CKD with or without dialysis. Pooled hazard risk (HR) with 95% confidence interval (CI) was calculated for the high vs. low NLR category.
Results
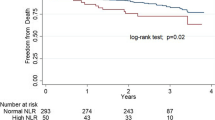
A total of thirteen studies enrolling 116,709 patients were identified and analyzed. In summary, high NLR was associated with an increased risk of all-cause mortality (HR 1.93, 95% CI 1.87–2.00; P < 0.00001) and cardiovascular mortality (HR 1.45, 95% CI 1.18–1.79, P < 0.001). Subgroup analysis indicated that high NLR are independently associated with all-cause mortality risk in dialysis patients (HR 1.94, 95% CI 1.87–2.01; P < 0.00001).
Conclusions
This meta-analysis indicates a high NLR is related to all-cause mortality and cardiovascular mortality in patients with chronic kidney disease. Dialysis patients with high NLR are candidates at high risk of mortality to allow for earlier interventions. Further large scale and more rigorously designed studies are warranted to confirm the prognostic value of NLR in the different stages of CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is an increasing health problem which affects 10.8–16% of the population in the world [1, 2]. CKD is a progressive disease without effective treatment, when it progresses to end-stage renal disease (ESRD), patients require renal replacement therapy including hemodialysis, peritoneal dialysis, and kidney transplant. People with CKD had increased risk of progressive renal failure, cardiovascular events, and mortality [3, 4], which is partly due to microinflammatory state afflicting patients with CKD and dialysis [5]. Studies have shown that patients with CKD tend to have high levels of inflammatory mediators, including high-sensitivity C-reactive protein, interleukin (IL)-6 and tumor necrosis factor-α [6]. These mediators stimulate mesangial, endothelial cell, and fibroblast, which produce large amounts of extracellular matrix, and then result in glomerular hypertension, tubulointerstitial fibrosis, and renal scaring [7, 8]. Inflammation is also a key factor in the initiation and progression of atherosclerosis and promoting cardiovascular mortality via leukocyte adhesion and infiltration of the vascular endothelium [9, 10].
Neutrophil-to-lymphocyte ratio (NLR) is a composite inflammatory marker calculated by dividing the neutrophil count by the lymphocyte count in peripheral blood. The neutrophil count represents innate immune, while the lymphocyte count indicates adaptive immune and nutrition [11,12,13]. The variation of NLR provides information on the change of immune system and the inflammation response. Previous literature revealed that NLR is a potential useful marker for determining inflammatory status and an elevated NLR plays an important role in predicting mortality for patients with tumors and cardiovascular diseases [14,15,16,17]. As for kidney disease, NLR has been developed as a complementary prognostic marker for assessing the cardiovascular risk in patients with stage 3–5 CKD. In addition, it is suggested that NLR are associated with the renal outcomes in CKD stage 1–4 [18]. Studies have shown that an increase in neutrophil count with a reduction in lymphocyte counts predicts adverse outcomes in hemodialysis patients [19] as well as peritoneal dialysis patients [20]. Therefore, early risk stratification for mortality is essential in those patients. However, the quality of these studies varies, some of these studies have small sample size, and the etiologies of CKD are also different. To the best of our knowledge, there exists no meta-analysis that addressed the relationship between NLR and mortality among patients with CKD with or without dialysis. As consistent and definitive data are not available, our study aims to use meta-analysis of the available evidence to investigate whether high NLR could predict cardiovascular or all-cause mortality in CKD patients.
Methods
Search strategy
This meta-analysis was conducted in accord with the PRISMA guidelines [21]. An electronic search of the PubMed, Embase, and Cochrane Library databases was searched from their inception to May 1st, 2020 with no language restrictions. The following key words and/or medical subject heading terms searched were used: (neutrophil to lymphocyte ratio OR neutrophil/lymphocyte ratio OR neutrophil lymphocyte ratio) AND (chronic kidney disease OR renal insufficiency OR end-stage renal disease OR renal replacement therapy OR dialysis) AND (mortality OR death OR prognosis). A manual search of related articles was performed to expand the search range.
Study selection
Two independent investigators (GA and YW) performed the initial screening of titles and abstracts. Full-length articles of identified studies were retrieved. The inclusion criteria in our meta-analysis were as follows: (1) the prognostic significance of peripheral blood NLR for CKD with or without undergoing hemodialysis was assessed; (2) risk ratio (RR) or hazard ratio (HR) and their 95% confidence interval (CI) comparing high NLR to low NLR was available, or data regarding outcomes from which it could be evaluated; (3) outcome measures were cardiovascular or all-cause mortality. Studies were excluded if they were (1) case reports, conference abstracts, editorials, nonclinical studies, and reviews; and (2) duplicated publications. If the same patient population was provided in a few articles, we selected only the most comprehensive study.
Data extraction and quality assessment
The studies that fulfilled our inclusion criteria were independently extracted by two main investigators (GA and YW). Disagreements between the reviewers were resolved by discussion and consensus. The extracted data were as follows: name of the first author, the year of publication, study design, region, number of participants, age of patients, percentage of male participants, primary outcomes, hazard ratio, and 95% CI and follow-up duration. The primary endpoints were long-term all-cause mortality and cardiovascular mortality. Risk of bias/quality of studies was assessed using the Newcastle–Ottawa Scale (NOS) by two independent investigators (YW and QX) [22]. The studies are evaluated on three ways using the NOS, namely comparability, selection, and outcome confirmation. The maximum score is nine stars, and NOS scores greater than 6 is considered of high quality [22]. This study is registered with PROSPERO, number CRD 42020177106.
Statistical analysis
The collected data from the included studies were analyzed using the RevMan 5.3 (Cochrane Collaboration) and Stata 12.0 (StataCorp). Reported HRs and 95% CIs were extracted from included studies. Hazard ratios (HRs) with 95% CIs were used as the summary estimate for dichotomous outcomes. Heterogeneity among studies was evaluated using Cochran’s Q test and the I2 statistic, with an I2 less than 25%, 25% to 50%, and greater than 50% indicating low, moderate, and high heterogeneity, respectively. The fixed effect model (Mantel–Haenszel) was applied to calculate pooled estimates among studies, when low and moderate heterogeneity exists. When substantial heterogeneity existed, the random-effect model (DerSimonian and Laird) was preferred. Sensitivity analysis was conducted to investigate the stability of the outcome and was performed by sequentially excluding 1 study at a time. If substantial heterogeneity was presented in the meta-analysis, subgroup analyses and meta-regression were conducted to identify the potential sources of heterogeneity. Subgroup analyses were performed according to a priori groupings related to study design (retrospective compared with prospective studies), region (studies performed in the Asian countries compared with studies performed outside of Asian countries), number of participants (≤ 200 compared with > 200), cutoff value of NLR (≥ 3 compared with < 3), and patients type (dialysis compared with dialysis plus nondialysis CKD). To investigate whether publication bias affects the validity of the overall estimates, funnel plots were constructed and assessed by Begg’s and Egger’s tests. (P < 0.10 was considered indicative of statistically significant heterogeneity). P < 0.05 was considered statistically significant.
Results
Identification of relevant studies
Through the literature search, a total of 124 potentially eligible studies were identified based on the predefined selection criteria, with 2 studies identified through manual searching of reference lists from these articles. After removal of duplicates, a review of the titles and abstracts of 78 articles was performed and then 54 studies were further excluded after screening the titles and abstracts. A total of 24 articles were obtained and read in full. Of these, a further 11 studies were excluded including 5 with insufficient data, three that were reviews or letters, three that did not provide survival analysis. Ultimately, 13 studies [23,24,25,26,27,28,29,30,31,32,33,34,35], comprising 116,709 patients with chronic kidney disease, were included in this meta-analysis. The process of study retrieval is summarized in Fig. 1.
Study characteristics and quality assessment
The demographic data of the patients in the included trials is present in Table 1. Among the 13 included studies, there were 3 studies from USA, 4 from China, 3 from Turkey, 1 from Japan, 1 from Australia, and 1 from Poland. Six studies [23,24,25, 30, 33, 34] had prospective designs. The cutoff values ranged from 1.75 to 3.9 in the included trials, with an average value of 3.13. Only one study divided the patients into two groups with respect to increased NLR and stable NLR without a cutoff value, and their basal NLR was 3.15 ± 1.76 [29]. One study enrolled patients with peritoneal dialysis [23]. Six studies [23, 26, 30,31,32,33] included patients undergoing hemodialysis, and the other studies included dialysis plus nondialysis CKD population. Twelve trials provided NLR data for predicting CKD from multivariate logistic regression analysis. One study by Catabay et al. enrolled more than 10,0000 hemodialysis patients, which is the largest number of patients among the included studies [31]. Twelve studies [23, 24, 26,27,28,29,30,31,32,33,34,35] used long-term all-cause mortality as the primary outcome; six studies [23, 25, 26, 32, 34, 35] used cardiovascular mortality as primary outcome. Included studies included confounding factors used in multivariate analyses, such as age, gender, systolic blood pressure, medical history of cardiovascular disease, and inflammatory markers. Five studies included C-reactive protein (CRP) as confounding factors in their multivariate analyses [24,25,26, 28, 32]. The quality of the included studies was high, with scores ranging from 8 to 9. The average number of NOS scores was 8.692.
Association of high NLR and all-cause mortality
A total of twelve included studies (116,623 patients) assessed the association of high NLR and all-cause mortality. As shown in Fig. 2, patients with high NLR was associated with increased risk of all-cause mortality (HR 1.93; 95% CI 1.87–2.00; P < 0.00001) compared with those with low NLR in a fixed effects model with low heterogeneity across studies (I2 = 6%; P = 0.39). The subgroup analysis according to the cutoff value of NLR revealed that the subgroup analysis revealed that a high NLR is associated with long-term all-cause mortality in patients with CKD in trials with cutoff value of NLR ≥ 3 (HR 2.5, 95% CI 1.67–3.37) and in those in trials with cutoff values of NLR < 3 (HR 1.93, 95% CI 1.86–2.00). The shape of the funnel plots showed some asymmetry (Fig. 3), but both of the Begg’s test (P = 0.451) and the Egger’s test (P = 0.995) did not show evidences of significant publication bias.
Association of high NLR and cardiovascular mortality
Six studies [13,14,15, 17] assessed the association of high NLR and cardiovascular mortality. A total of 1,684 patients were included. As shown in Fig. 4, patients with high NLR was associated with increased risk of cardiovascular mortality (HR 1.45, 95% CI 1.18–1.79, P < 0.001) compared with those with low NLR in a random-effect model with high heterogeneity across studies (I2 = 63%; P = 0.02). Sensitivity analyses by omitting each study at a time did not significantly change the direction of the overall effect size. Evidences of publication bias were not found according to the Begg’s test (P = 0.15) and the Egger’s test (P = 0.12).
Subgroup and sensitivity analysis on all-cause mortality
As shown in Table 2, a subgroup analysis was conducted on the basis of the latent confounding factors, such as patient type, study design, cutoff value of NLR, sample size, and region. The association between high NLR and increased risk of all-cause mortality was significant not only in dialysis patients (HR 1.94; 95% CI 1.87–2.01), but also in the dialysis plus nondialysis CKD population (HR 1.88, 95% CI 1.47–2.41). Stratification by study design revealed that high NLR predicted increased all-cause mortality in prospective studies (HR 2.66, 95% CI 1.82–3.89) and in retrospective ones (HR 1.93, 95% CI 1.86–2.20). Results of the subgroup analysis on the basis of sample size suggested that high NLR was connected with increased risk of all-cause mortality when the sample size was ≥ 400 (HR 1.93, 95% CI 1.87–2.21) and sample size < 400 (HR 1.94, 95% CI 1.52–2.49). In addition, the association between NLR and all-cause mortality risk was consistently found in the region subgroups. We noticed that the study by Catabay et al. [31] enrolled the largest number of patients among the included studies. To appraise the impact of each study on the overall outcome (HR) of all-cause mortality, sensitivity analyses showed that the removal of the study by Catabay did not change the direction of the overall estimates (HR 1.83, 95% CI 1.56–2.15, I2 = 11%; P < 0.00001). Meta-regression was performed to examine the following potential confounders: age, gender, study design, and region (studies performed in the Asia compared with studies performed outside of Asia). No significant impact of the potential confounders on the results of meta-analysis was detected (all P > 0.05). Similarly, sensitivity analyses by excluding other individual study at a turn did not significantly alter the direction of the overall effect size or the degree of between-study heterogeneity.
Discussion
To investigate the prognostic value of NLR in patients with CKD, we performed this meta-analysis by combining the current literature. In the present study, we incorporated 13 studies of CKD patients with or without dialysis. The result of this study suggested that elevated NLR was a significant predictor of cardiovascular mortality and all-cause mortality in CKD patients. Sensitivity analysis and subgroup analysis in our study did not significantly alter the overall results. To our best knowledge, this is the first meta-analysis to evaluate the prognostic value of NLR in CKD.
The potential mechanism which explains the prognostic value of NLR in CKD is mainly due to systemic inflammatory response. A high NLR indicates relative increased neutrophils and decreased lymphocytes. Neutrophils are proinflammatory cells, which could release inflammatory cytokines and proteolytic enzymes, activate macrophages, and promote foam cell formation, these inflammatory events facilitated the destruction of cardiomyocytes and caused ischemic changes in vessels [36,37,38,39,40]. Lymphocytes played an important role in the regulation of immune system. Studies have demonstrated that inflammation could increase the apoptosis of lymphocytes [41], which resulted in a high risk of infection [42, 43] and adverse cardiovascular outcomes [44]. Therefore, NLR may reflect the balance between inflammatory response and immune function. Meanwhile, it is an easily available and cost-effective index for clinical practice, which makes it an attractive prognostic index for patient with CKD.
The role of NLR has been studied in various conditions, including type 2 diabetes, cancer, pulmonary arterial hypertension, obstructive sleep apnea syndrome, chronic obstructive pulmonary disease, spontaneous intracerebral hemorrhage, and peripheral arterial occlusive disease [45,46,47,48,49,50]. These studies indicated that NLR has a prognostic value in a broad range across different diseases. Although the exact mechanisms behind the relationship between an elevated NLR and poor prognosis have not been illuminated clearly, it could be associated with both increased neutrophil-dependent inflammatory response and reduced lymphocyte-mediated immunoreaction [51]. Patients with end-stage renal disease (ESRD) have elevated serum levels of inflammatory mediators, including CRP, tumor necrosis factor-α (TNF-α), and interleukin (IL)-6 [52, 53]. When compared, prognostic power for cardiovascular disease event among NLR, inflammatory markers, such as CRP and IL-6, NLR was the most superior marker for cardiovascular disease [25]. In addition, Chen et al. found that NLR, but not CRP or platelet–lymphocyte ratio, is an important prognostic predictor of all major clinical outcomes in patients with advanced CKD [28]. Some potential limitations should be considered when interpreting the results of the present study. First, the observational studies included in this study had their inherent limitations and biases, such as design bias, selection bias, and treatment bias. Although randomized clinical trials can most provide statistically persuasive findings, the small study subjects they enrolled may not represent the real-world population. Hence, this study shed some light on the prognosis in CKD patients. Second, the results of subgroup analyses based on the small number of studies may be not reliable. Third, lacking of individual patient data, the optimal cutoff value of NLR was unavailable for clinical practice, and the results could be subject to confounding and selection bias. Future researches are warranted to investigate the optimal NLR cutoff value. Fourth, detailed information of unknown factors (i.e., life habits, comorbidities, related treatment patterns, and drugs) could affect the NLR value, thus could weaken its actual association with CKD-specific endpoints. Finally, we could not analyze the prognostic value of NLR in different stages of CKD because few studies provide relevant data to calculate.
Despite these limitations, there exist some advantages of our meta-analysis. First, to our best knowledge, this is the first meta-analysis evaluating the prognostic value of NLR in CKD patients, and most of the data were obtained from multivariate analysis of high-quality studies. Furthermore, the heterogeneity across the studies was low, which enhances the reliability of our results. In addition, NLR is an easily available and cheap, which could be a potential useful prognostic marker in the risk stratification of CKD patients.
Conclusion
In conclusion, a high NLR is related to all-cause mortality and cardiovascular mortality in patients with chronic kidney disease. Dialysis patients with high NLR are candidates at high risk of mortality to allow for earlier interventions. Further, large scale and more rigorously designed studies are warranted to confirm the prognostic value of NLR in the different stages of CKD.
References
Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47.
Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–22.
Sarnak MJ, Amann K, Bangalore S, et al. Chronic kidney disease and coronary artery disease: JACC State-of-the-Art review. J Am Coll Cardiol. 2019;74:1823–38.
Ermer T, Eckardt KU, Aronson PS, Knauf F. Oxalate, inflammasome, and progression of kidney disease. Curr Opin Nephrol Hypertens. 2016;25(4):363–71.
Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–304.
Tamhane UU, Aneja S, Montgomery D, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–7.
Tang Y, Zhang F, Huang L, et al. The protective mechanism of fluorofenidone in renal interstitial inflammation and fibrosis. Am J Med Sci. 2015;350(3):195–203.
Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol. 2007;22:2011–22.
Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–51.
Chmielewski PP, Strzelec B. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: a review. Folia Morphol (Warsz). 2018;77(2):171–8.
Hemond CC, Glanz BI, Bakshi R, Chitnis T, Healy BC. The neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios are independently associated with neurological disability and brain atrophy in multiple sclerosis. BMC Neurol. 2019;19(1):23.
Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res. 2019;10(2):137–45.
Gasteiger G, Rudensky AY. Interactions between innate and adaptive lymphocytes. Nat Rev Immunol. 2014;14:631–9.
Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14(5):573–7.
Dentali F, Nigro O, Squizzato A, et al. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: a systematic review and meta-analysis of the literature. Int J Cardiol. 2018;266:31–7.
Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2.
Wang X, Zhang G, Jiang X, et al. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis. 2014;234:206–13.
Yoshitomi R, Nakayama M, Sakoh T, et al. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren Fail. 2019;41:238–43.
Reddan DN, Klassen PS, Szczech LA, et al. White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant. 2003;18(6):1167–73.
Chen T, Yang M. Platelet-to-lymphocyte ratio is associated with cardiovascular disease in continuous ambulatory peritoneal dialysis patients. Int Immunopharmacol. 2020;78:106063.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Wells GA, Shea B, O’Connell D et al. The Newcastle–Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Ottawa: Dept of Epidemiology and Community Medicine, University of Ottawa; https://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed on 10th May 2020
An X, Mao HP, Wei X, et al. Elevated neutrophil to lymphocyte ratio predicts overall and cardiovascular mortality in maintenance peritoneal dialysis patients. Int Urol Nephrol. 2012;44:1521–8.
Solak Y, Yilmaz MI, Sonmez A, et al. Neutrophil to lymphocyte ratio independently predicts cardiovascular events in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(4):532–40.
Abe T, Kato S, Tsuruta Y, et al. Neutrophil/lymphocyte ratio as a predictor of cardiovascular events in incident dialysis patients: a Japanese prospective cohort study. Clin Exp Nephrol. 2015;19(4):718–24.
Neuen BL, Leather N, Greenwood AM, Gunnarsson R, Cho Y, Mantha ML. Neutrophil–lymphocyte ratio predicts cardiovascular and all-cause mortality in hemodialysis patients. Ren Fail. 2016;38(1):70–6.
Ouellet G, Malhotra R, Penne EL, Usvya L, Levin NW, Kotanko P. Neutrophil–lymphocyte ratio as a novel predictor of survival in chronic hemodialysis patients. Clin Nephrol. 2016;85(4):191–8.
Chen IC, Yu CC, Wu YH, Chao TH. Elevated Neutrophil-to-lymphocyte ratio predicts intermediate-term outcomes in patients who have advanced chronic kidney disease with peripheral artery disease receiving percutaneous transluminal angioplasty. Acta Cardiol Sin. 2016;32(5):532–41.
Tatar E, Mirili C, Isikyakar T, et al. The association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with clinical outcomes in geriatric patients with stage 3–5 chronic kidney disease. Acta Clin Belg. 2016;71(4):221–6.
Yaprak M, Turan MN, Dayanan R, et al. Platelet-to-lymphocyte ratio predicts mortality better than neutrophil-to-lymphocyte ratio in hemodialysis patients. Int Urol Nephrol. 2016;48(8):1343–8.
Catabay C, Obi Y, Streja E, et al. Lymphocyte cell ratios and mortality among incident hemodialysis patients. Am J Nephrol. 2017;46(5):408–16.
Li H, Lu X, Xiong R, Wang S. High neutrophil-to-lymphocyte ratio predicts cardiovascular mortality in chronic hemodialysis patients. Mediators Inflamm. 2017;2017:9327136.
Diaz-Martinez J, Campa A, Delgado-Enciso I, et al. The relationship of blood neutrophil-to-lymphocyte ratio with nutrition markers and health outcomes in hemodialysis patients. Int Urol Nephrol. 2019;51(7):1239–47.
Yuan Q, Wang J, Peng Z, et al. Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J Transl Med. 2019;17(1):86.
Woziwodzka K, Dziewierz A, Pawica M, et al. Neutrophil-to-lymphocyte ratio predicts long-term all-cause mortality in patients with chronic kidney disease stage 5. Folia Med Cracov. 2019;59(4):55–70.
Tobias R, Thenral S, Patrick E, et al. Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem. 2010;56(6):944–51.
Mocan M, Mocan Hognogi LD, Anton FP, et al. Biomarkers of inflammation in left ventricular diastolic dysfunction. Dis Markers. 2019;2019:7583690.
Nah DY, Rhee MY, et al. The inflammatory response and cardiac repair after myocardial infarction. Korean Circ J. 2009;39(10):393–8.
Maclsaac RJ, Fracp M, Ekinci E, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63(2):S39–S62.
Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–88.
Richard SH, Irene EK. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–50.
Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38(2 Suppl):770–3.
Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2019. https://doi.org/10.1016/j.ajem.2019.10.023.
Balta S, Celik T, Mikhailidis DP, et al. The relation between atherosclerosis and the neutrophil–lymphocyte ratio. Clin Appl Thromb Hemost. 2016;22(5):405–11.
Xu T, Weng Z, Pei C, et al. The relationship between neutrophil-to-lymphocyte ratio and diabetic peripheral neuropathy in type 2 diabetes mellitus. Medicine (Baltimore). 2017;96(45):e8289.
Ozpelit E, Akdeniz B, Ozpelit ME. Prognostic value of neutrophil-to-lymphocyte ratio in pulmonary arterial hypertension. J Int Med Res. 2015;43:661–71.
Uygur F, Tanriverdi H, Aktop Z, et al. The neutrophil-to-lymphocyte ratio in patients with obstructive sleep apnoea syndrome and its relationship with cardiovascular disease. Heart Lung. 2016;45:121–5.
Ye Z, Ai X, Liao Z, You C, Cheng Y. The prognostic values of neutrophil to lymphocyte ratio for outcomes in chronic obstructive pulmonary disease. Medicine (Baltimore). 2019;98(28):e16371.
Qi H, Wang D, Deng X, Pang X. Lymphocyte-to-monocyte ratio is an independent predictor for neurological deterioration and 90-dchenay mortality in spontaneous intracerebral hemorrhage. Med Sci Monit. 2018;24:9282–91.
Erturk M, Cakmak HA, Surgit O, et al. Predictive value of elevated neutrophil to lymphocyte ratio for long-term cardiovascular mortality in peripheral arterial occlusive disease. J Cardiol. 2014;64:371–6.
Faria SS, Fernandes PC Jr, Silva MJ, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702.
Haubitz M, Brunkhorst R. C-reactive protein and chronic chlamydia pneumoniae infection-long-term predictors for cardiovascular disease and survival in patients on peritoneal dialysis. Nephrol Dial Transplant. 2001;16:809–15.
Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35(3):469–76.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and international research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involving human participants and/or animals
For this type of study formal consent is not required. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ao, G., Wang, Y., Qi, X. et al. Association of neutrophil-to-lymphocyte ratio and risk of cardiovascular or all-cause mortality in chronic kidney disease: a meta-analysis. Clin Exp Nephrol 25, 157–165 (2021). https://doi.org/10.1007/s10157-020-01975-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-020-01975-9