Abstract
Background
Hemodialysis (HD) sessions induce changes in plasma electrolytes that lead to modifications of QT interval, virtually associated with dangerous arrhythmias. It is not known whether such a phenomenon occurs even during peritoneal dialysis (PD). The aim of the study is to analyze the relationship between dialysate and plasma electrolyte modifications and QT interval during a PD exchange.
Methods
In 15 patients, two manual PD 4-h exchanges were performed, using two isotonic solutions with different calcium concentration (Ca++1.25 and Ca1.75++ mmol/L). Dialysate and plasma electrolyte concentration and QT interval (ECG Holter recording) were monitored hourly. A computational model simulating the ventricular action potential during the exchange was also performed.
Results
Dialysis exchange induced a significant plasma alkalizing effect (p < 0.001). Plasma K+ significantly decreased at the third hour (p < 0.05). Plasma Na+ significantly decreased (p < 0.001), while plasma Ca++ slightly increased only when using the Ca 1.75++ mmol/L solution (p < 0.01). The PD exchange did not induce modifications of clinical relevance in the QT interval, while a significant decrease in heart rate (p < 0.001) was observed. The changes in plasma K+ values were significantly inversely correlated to QT interval modifications (p < 0.001), indicating that even small decreases of K+ were consistently paralleled by small QT prolongations. These results were perfectly confirmed by the computational model.
Conclusions
The PD exchange guarantees a greater cardiac electrical stability compared to the HD session and should be preferred in patients with a higher arrhythmic risk. Moreover, our study shows that ventricular repolarization is extremely sensitive to plasma K+ changes, also in normal range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is the leading cause of mortality and morbidity in patients receiving dialysis. Among end-stage renal disease (ESRD) patients on renal replacement therapy, 40% of deaths are due to cardiovascular causes and 60% of these deaths are on arrhythmic basis [1]. In the hemodialysis (HD) patient, the high incidence of arrhythmic events may be partly explained by abrupt changes in plasma electrolyte concentrations due to the HD session [2, 3].
Hemodialysis sessions induce significant changes in plasma electrolyte levels and these alterations are partly dependent on the composition of the dialysis bath [4]. The intradialytic modifications of the electrolytes (in particular K+, Ca++ and HCO3−) are associated with changes in the duration of the ventricular action potential of the cardiomyocytes, represented by the duration of the QT interval as measured by the electrocardiogram (ECG). This association has been demonstrated both clinically with ECG recordings [4,5,6,7,8] and with computational data (in silico models) [9, 10]. A role of electrolyte alterations in the genesis of arrhythmic phenomena during the HD session and in the immediately following hours has been hypothesized [11]. Low potassium and calcium concentrations in the dialysis bath can cause dangerous alterations of ventricular repolarization and are associated with an increased incidence of intradialytic cardiac arrest [4, 12, 13]. Although the incidence of arrhythmic death is higher than in the general population even in patients on peritoneal dialysis (PD) [14], the role of this treatment in itself in determining the phenomenon is less evident. In fact, changes in electrolytes during PD exchanges are less marked and acute than those occurring during HD sessions. However, a prolongation of the QT interval was shown to be associated with greater total mortality and increased cardiac mortality also among patients on PD [15].
To our knowledge, there are no data regarding QT interval and cardiac action potential modifications during a PD exchange. The aim of our study is to analyze, in uremic patients undergoing PD, the relationship between changes in dialysate and plasma electrolyte concentrations and different electrocardiographic parameters (heart rate, RR interval and QT interval) during a 4-h exchange. Moreover, the effects of the exchange performed with two different concentrations of Ca++ in the dialysate (1.25 mmol/L and 1.75 mmol/L) are analyzed.
Methods
Study participants
This is a single-center study (Dialysis and Nephrology Unit, IRCCS Multimedica, Sesto S. Giovanni, Italy). All patients undergoing PD (Continuous Ambulatory Peritoneal Dialysis, CAPD or automated peritoneal dialysis) for at least six months, who had none of the exclusion criteria and who agreed to participate in the study were recruited. The recruitment period lasted 18 months (from September 2016 to February 2018).
Exclusion criteria:
-
Patients experiencing acute cardiovascular events (acute coronary syndrome, stroke) or acute infectious events (peritonitis, pneumonia, sepsis) in the month preceding the study.
-
Presence of any form of atrial fibrillation at the study time.
-
Complete bundle branch block at the baseline electrocardiogram.
-
Therapy with antiarrhythmics drugs of class I and III; therapy with and any other drug that could affect the duration of the QT interval.
-
Electrically stimulated patients (presence of pace-maker).
For each patient clinical data, cause of ESRD and comorbidities (ischemic heart disease, diabetes mellitus, hypertension, dyslipidemia, stroke/transient ischemic attack, TIA) were collected.
In 15 patients, two manual PD 4-h exchanges were performed, using two isotonic solutions (two liters of solution, Physioneal 1.36%, Baxter, USA) with different calcium concentration, at least 15 days apart from each other.
The electrolyte concentrations of the two solutions were:
-
Glucose 13.6 g/L, Na+ 132 mmol/L, K+ 0 mmol/L, HCO3− 25 mmol/L, lactate 15 mmol/L, pH 7.4, Ca++ 1.25 mmol/L;
-
Glucose 13.6 g/L, Na+ 132 mmol/L, K+ 0 mmol/L, HCO3− 25 mmol/L, lactate 15 mmol/L, pH 7.4, Ca++ 1.75 mmol/L.
The order of administration of the two dialysates was random. Patients did not change the dialysis treatment the day before the study (icodextrin or isotonic as prescribed by their treatment plan for patients in CAPD or automated night treatment).
Figure 1 shows the study design.
Before baseline blood samples were taken, the patients remained for an hour at rest in the supine position, with full abdomen (Baseline). Samples for the measurement of electrolyte concentration (pH, bicarbonate, sodium, potassium and calcium) in the plasma and in the dialysate were performed immediately before the beginning of the exchange and at the end of each of the four hours of exchange (T1–T4). Electrolyte concentrations were analyzed using direct ion-selective electrode (D-ISE).
Blood pressure values were monitored at each hour of the exchange.
Electrocardiographic Holter recordings and analysis
A 24 h ECG Holter monitoring was recorded in each subject. All recordings were obtained using portable battery-operated 12-channel Holter recorder (Spyder View recorder). Holter ECG recording was performed from 60 min before the start of the dialysis exchange (Baseline) and then for 240 min until the end of the fourth hour.
The digitized channel ECG signals were processed by the commercially available Synescope Holter analysis software (Microport CRM, Saluggia, Italy), which sampled the recording into templates obtained by 30-s time intervals. To improve the signal-to-noise ratio, one median complex was computed every 6 s from the consecutive sinus beats, then the five median beats within each 30 s template were averaged to obtain a single representative PQRST complex for each of the templates. For each template, an algorithm automatically measured the QT and the RR intervals (ms). For each hour, the program provided the mean of QT intervals corrected for heart rate according to the Bazett formula (QTc). Mean QTc values were analyzed in five one hour periods: Baseline, First hour (T1), Second hour (T2), Third hour (T3), Fourth hour (T4). QTc was considered prolonged when equal to or greater than 450 ms in men, and 460 ms in women [16].
Computational model
The ventricular action potential was simulated using the O’Hara et al. model of human ventricular cell [17]. The O’Hara-Rudy cardiac cell model (ORd) was chosen, as it is, nowadays, the gold standard in the cardiac computational scenario since it was developed and validated with data from over 100 undiseased human hearts.
Model differential equations were implemented in Matlab (Mathworks Inc., Natick, MA, USA).
The computer simulator was used to reproduce the five dialysis conditions (baseline, T1, T2, T3, T4) for each patient. Their plasma Ca++, Na+, K+ concentrations and pH, recorded at each time instance of the protocol, were set as extracellular concentrations in the model. Heart rate recorded at each time step of treatment was used as pacing rate in the simulations. Simulations were performed considering an in silico M cell and lasted until a steady state action potential (AP) was reached (typically 1000 beats). The action potential duration was measured as the interval between the action potential upstroke and the 90% repolarization level of the action potential (APduration90). The APc duration90 was calculated in the same way as the QTc interval.
Statistical analysis
Data are expressed as mean (standard deviation) for continuous variables and as number of patients (percentage) for categorical variables. The differences of each hour of dialysis exchange vs. baseline values of hematochemical and electrocardiographic parameters were tested by analysis of variance (one-way ANOVA for repeated measures) followed by Bonferroni’s correction for number of comparisons. Correlations between electrolyte concentration and QT interval were evaluated by univariate and multivariate regression analyses. A value of p < 0.05 was considered significant.
Results
Table 1 shows the clinical features of the study population. Forty-seven percent of patients were male, the mean age was 70.9 (12.8) years. Fourteen patients were in CAPD treatment, only one patient in APD; the mean dialytic age was 27.1 (24.0) months.
The mean ultrafiltration during the exchange was 2013 (190) mL. Several changes in dialysate electrolyte concentrations occurred along the dialysis exchange (Table 2). Both dialysate pH value and HCO3− concentration significantly increased from baseline to T4 (from 7.50 to 7.56 and from 27.1 to 29.6 mmol/L, respectively). The potassium concentration significantly raised during the exchange (from 0.78 to 3.31 mmol/L). The sodium concentration progressively and significantly decreased (from 135.7 to 132.8 mmol/L). The concentration of calcium tended to lightly increase when the exchange was performed with the dialysate concentration of Ca++ 1.25 mmol/L/L and significantly decreased with dialysate concentration of Ca++ 1.75 mmol/L (from 1.60 to 1.41 mmol/L).
Dialysis exchange induced a modest, but significant plasma alkalizing effect, as shown by the progressive increase in pH values and of HCO3− plasma concentration from baseline to T4 (from 7.39 to 7.42 and from 25.05 to 26.54 mmol/L, respectively). Plasma potassium value was unchanged except at the third hour when it decreased significantly compared to baseline (3.96 vs 3.84 mmol/L). Plasma sodium tended to slightly decrease (from 137.0 to 136.2 mmol/L) and plasma calcium did not change, except at T3, when it increased significantly, but only with the dialysate concentration of Ca++ 1.75 mmol/L (1.14 vs 1.16 mmol/L, Table 3).
Dialysis exchange did not result in any blood pressure modifications, but was accompanied by a progressive and significant reduction in heart rate (from 67 to 64 b/min). The mean QT interval at baseline was 433 (25) msec. Two patients (13%) showed higher baseline QTc values than normal. Considering all 30 dialysis sessions, significant changes in QTc were not observed. There was a slight prolongation of ventricular repolarization at the third and fourth hour only with the dialysate concentration of Ca++ 1.25 mmol/L (434 vs 438 ms). The RR interval standard deviation showed a tendency to increase during the dialysis exchange (from 26 to 33 ms), suggesting that the reduction in heart rate could be partially due to an increment in vagal tone (Table 4).
Five patients showed cardiac arrhythmias in baseline condition (n = 3 supraventricular and n = 2 ventricular arrhythmias). The dialysis exchange was not accompanied by an increase in arrhythmic phenomena (rate for hour) compared to baseline.
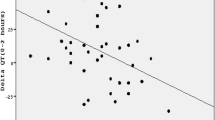
The QTc interval modifications measured hourly during the dialysis exchange (ΔQTc) were strongly and inversely correlated to the changes of plasma potassium values (p < 0.001, black dots in Fig. 2, top panel), indicating that small decreases of potassium are consistently accompanied by small QTc prolongations. A less pronounced correlation was also found between ΔQTc and changes in plasma sodium levels (p < 0.05, black dots in Fig. 2, bottom panel). At multivariate regression analysis only changes in potassium values remained significantly associated to ΔQTc (p < 0.0001). Consistently, model-based simulations, by taking into account PD-induced alterations in extracellular electrolyte concentrations, pH and heart rate, reproduced changes in AP duration in good agreement with the in vivo results on QT interval. Representative traces of patient-specific action potentials at two different stages of the dialysis session are shown in Fig. 3: the [K+]o reduction (T3 [K+]o = 3.65 mM vs baseline [K+]o = 3.96 mM) coincides with an increase in the APduration90 (T3 APduration90 = 339 ms vs baseline APduration90 = 324 ms). The computational analysis confirmed a highly significant inverse correlation between changes in the heart rate-corrected APcduration90, the cellular counterpart of QTc, and the corresponding changes in the extracellular potassium concentrations (p < 0.0001, white dots in Fig. 2, top panel) and also highlighted a slight correlation between ΔAPc duration90 and changes in sodium plasma levels (p < 0.01). Multivariate analysis showed that only changes in potassium plasma levels were significantly correlated to ΔAPc duration90 (p < 0.0001).
Top: scatter plots and regression lines showing the significant inverse correlation between ΔQTc (black)/ΔAPc duration (white) and ΔK+ (top panel) and ΔNa+ (bottom panel) concentrations computed over each delta interval T(i)-baseline, i = 1,2,3,4 (n = 15 patients, two dialysis sessions with dialysate Ca++ concentrations 1.25 mmol/L and 1.75 mmol/L)
Example of simulated ventricular action potentials before and during a dialysis exchange. Simulations were performed using the following variables as input to the ventricular cell model: measured plasma K+, Ca++, Na+ concentrations and pH value, and pacing at the recorded heart rate (for baseline: K+ = 3.16 mM, Ca++ = 1.27 mM, Na+ = 131.8 mM, pH 7.41; for dialysis exchange: K+ = 2.95 mM, Ca++ = 1.29 mM, Na+ = 130.9 mM, pH 7.44)
Discussion
Our study shows that, during a single PD exchange, the ventricular repolarization does not undergo important modifications and that an increase in arrhythmic phenomena is not observed. On the other hand, in an almost surprising way, all the relationships between cardiac action potential and electrolytes observed during HD sessions are reproduced [4, 9]. These results are particularly robust, as they are perfectly confirmed by the computational model. Moreover, the PD exchange induces a small but significant decrease in heart rate, probably due to a parasympathetic stimulation, as suggested by the increase of RR standard deviation, occurring at the same time [18].
A Chinese study [19], including more than 10,000 subjects from a general population, showed that serum potassium is significantly and inversely associated with QTc interval and the prevalence of QTc prolongation increases significantly with a decrease in serum potassium values even within normal range.
Moreover in this study [19] the lowest plasma potassium quartile was associated with a higher prevalence of QTc prolongation compared to the highest quartile (OR 1.72), more evident in females (OR 2.01). Our results are consistent with these epidemiological data, adding an interesting observation: slight acute modifications of plasma potassium are sufficient to induce small, but significant changes in the duration of the QT interval. The patient on PD, therefore, represents a very interesting “in vivo” model, which demonstrates the ability of the human organism to finely adapt ventricular repolarization to the plasma concentration of potassium. This fact, however, also suggests a clinical consideration. If the QTc interval is so sensitive to changes in plasma potassium, and considering that often patients undergoing PD have low plasma potassium values [20,21,22], it would be advisable, at least in those patients with the lowest plasma potassium values, to use potassium concentrations in the dialysate greater than 0 mmol/L. In fact, it has been shown that a low time-averaged serum potassium level (below 4.0 mmol/L) is associated with higher death risk in PD patients [23]. These data demonstrate that hypokalemia is potentially dangerous not only in HD patients, but also in those undergoing PD. It is interesting to note that the QTc interval slightly, but significantly increased only with the low Ca++ concentration in the dialysate, suggesting that, as occurs during the HD session, even a modest increment of plasma Ca++ is able to counteract the prolongation of ventricular repolarization induced by plasma potassium reduction.
In our study, during the dialysis exchange both dialysate pH value and HCO3− concentration significantly increased and the exchange induced a modest increment of plasma pH and HCO3− values. When evaluating plasma and dialysate electrolyte modifications during a PD exchange performed with isotonic solution, it is important to consider that changes in pH and bicarbonate in the dialysis solution are related to the absence of diffusion of bicarbonate from the dialysate to the plasma, due to the presence of normal plasma level of bicarbonate. The observed increase in plasma pH and bicarbonate is probably due to the absorption of the lactate, which is metabolized to bicarbonate.
During the exchange, the dialysate potassium concentration significantly raised, while plasma potassium tended to decrease. The increase of the potassium in the dialysis solution is clearly related to the diffusion of potassium due to the concentration gradient, leading to a slight reduction of plasma potassium level.
The sodium concentration in the dialysis solution significantly decreased, as did the plasma sodium level. The reduction of sodium concentration in the dialysate at the first hour is likely due to a sodium dialysate value higher than expected, probably caused by an incomplete drainage of the evening exchange (residual volume). Plasma sodium is slightly reduced during the exchange because, with an isotonic solution, the sodium diffusion is modest and slow.
Calcium concentration in the dialysis solution changed in different ways according to the gradient between plasma and dialysate, gradient due to different calcium concentration in the dialysis solution. With calcium 1.25 mmol/L, there was an increase in calcium dialysate concentration due to the shift of calcium from plasma to peritoneal fluid. Instead, with calcium 1.75 mmol/L there was a reduction of calcium in the peritoneal fluid during the exchange due to the calcium shift from peritoneal fluid to plasma. As a result, plasma calcium was not modified with Ca++ 1.25 mmol/L dialysate, while it slightly increased with Ca++ 1.75 mmol/L.
It should be noted that in PD patients, the presence of a modest alkalosis and an hypokalemia is favored by the presence of residual renal function; moreover, the possibility that an inadequate diet could cause a reduced intake of acids and potassium should not be underestimated. We, therefore, believe that the use of peritoneal solutions with higher potassium content (K+ 2 mmol/L) could be reasonable.
In this study, the computational analysis was performed by separately simulating the specific experimental conditions of each patient (electrolyte extracellular concentrations, pH and heart rate) with a mathematical model of the human ventricular AP duration, and the APcduration90 was used as the QTc equivalent. This combined approach, using experimental and in silico data, has been frequently and successfully applied to assess the acute effects of HD-induced electrolyte modifications on cardiomyocyte electrophysiology and ECG parameters [9, 24, 25]. In this study, for the first time, the model was applied to analyze the impact of PD on cardiac electrophysiology. Computational results strongly support the hypothesis that the observed QTc alterations can be fully ascribed to the variations in electrolyte balance rather than to other dialysis-related factors, which are not included in the model. In particular, a causal and highly sensitive link to potassium was pointed out, with very small changes in potassium concentration able to induce detectable QTc changes. Notably, we found a quantitative, not only qualitative, match between simulation results and QTc clinical data, both in terms of ΔAPc duration vs ΔQTc values (reported on the same scale in Fig. 2), and in terms of their correlation with ΔK+ (regression slopes, Fig. 2).
A limitation of the study is that hourly measurements of serum magnesium were not available. However, we measured serum magnesium in 8 patients at the beginning and at the end of the 4-h exchange. Basal plasma magnesium values were in a low-normal range [0.72 (0.18) mmol/L (normal values 0.66–1.07 mmol/L)] and did not change at the end of dialysis exchange [0.73 (0.17) mmol/L].
In conclusion, PD is a dialysis treatment that guarantees a greater hemodynamic and cardiac electrical stability compared to HD and should, therefore, be preferred in patients with a higher arrhythmic risk. Our study also demonstrates that the ventricular repolarization is extremely sensitive to changes in plasma electrolyte concentration, even when they are small and in normal range, and, therefore, suggests to pay attention to PD patients who have low plasma potassium values, in whom the dialysis exchange could induce significant alterations of the duration of the QTc interval.
References
United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; 2016.
Genovesi S, Valsecchi MG, Rossi E, Pogliani D, Acquistapace I, De Cristofaro V, Stella A, Vincenti A. Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24:2529–36.
Jadoul M, Thumma J, Fuller DS, et al. Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol. 2012;7:765–74.
Genovesi S, Dossi C, Galbiati E, Prolo F, Stella A, Stramba-Badiale M. Electrolyte concentration during haemodialysis and QT interval prolongation in uraemic patients. Europace. 2008;10:771–7.
Covic A, Diaconita M, Gusbeth-Tatomir P, Covic M, Botezan A, Ungureanu G, Goldsmith DJ. Haemodialysis increases QT(c) interval but not QT(c) dispersion in ESRD patients without manifest cardiac disease. Nephrol Dial Transplant. 2002;17:2170–7.
Severi S, Pogliani D, Fantini G, et al. Alterations of atrial electrophysiology induced by electrolyte variations: combined computational and P-wave analysis. Europace. 2010;12:842–9.
Di Iorio B, Torraca S, Piscopo C, et al. Dialysate bath and QTc interval in patients on chronic maintenance hemodialysis: pilot study of single dialysis effects. J Nephrol. 2012;25:653–60.
Vincenti A, Passini E, Fabbrini P, Luise MC, Severi S, Genovesi S. Recurrent intradialytic paroxysmal atrial fibrillation: hypotheses on onset mechanisms based on clinical data and computational analysis. Europace. 2014;16:396–404.
Severi S, Grandi E, Pes C, Badiali F, Grandi F, Santoro A. Calcium and potassium changes during haemodialysis alter ventricular repolarization duration: in vivo and in silico analysis. Nephrol Dial Transplant. 2008;23:1378–86.
Krueger MW, Severi S, Rhode K, et al. Alterations of atrial electrophysiology related to hemodialysis session: insights from a multiscale computer model. J Electrocardiol. 2011;44:176–83.
Santoro A, Mancini E, London G, et al. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol Dial Transplant. 2008;23:1415–21.
Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79:218–27.
Pun PH, Horton JR, Middleton JP. Dialysate calcium concentration and the risk of sudden cardiac arrest in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:797–803.
Genovesi S, Porcu L, Luise MC, et al. Sudden death in end stage renal disease: comparing hemodialysis versus peritoneal dialysis. Blood Purif. 2017;44:77–88.
Kuo HL, Liu YL, Liang CC, et al. Prolonged QT interval is linked to all-cause and cardiac mortality in chronic peritoneal dialysis patients. Nephrology. 2017;22:436–40.
Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:982–91.
O'Hara T, Virág L, Varró A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol. 2011. https://doi.org/10.1371/journal.pcbi.1002061.
Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use task force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Circulation. 1996;93:1043–65.
Chen Y, Guo X, Sun G, Li Z, Zheng L, Sun Y. Effect of serum electrolytes within normal ranges on QTc prolongation: a cross-sectional study in a Chinese rural general population. BMC Cardiovasc Disord. 2018;18:175.
Szeto CC, Chow KM, Kwan BC, Leung CB, Chung KY, Law MC, Li PK. Hypokalemia in Chinese peritoneal dialysis patients: prevalence and prognostic implication. Am J Kidney Dis. 2005;46:128–35.
Torlén K, Kalantar-Zadeh K, Molnar MZ, Vashistha T, Mehrotra R. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol. 2012;7:1272–84.
Yu HL, Lu XH, Su CY, Tang W, Wang T. Potassium metabolism in continuous ambulatory peritoneal dialysis patients. Ren Fail. 2014;36:748–54.
Li SH, Xie JT, Long HB, et al. Time-averaged serum potassium levels and its fluctuation associate with 5-year survival of peritoneal dialysis patients: two-center based study. Sci Rep. 2015. https://doi.org/10.1038/srep15743.
Passini E, Genovesi S, Severi S. Human atrial cell models to analyse haemodialysis-related effects on cardiac electrophysiology: work in progress. Comput Math Methods Med. 2014. https://doi.org/10.1155/2014/291598.
Corsi C, Cortesi M, Callisesi G, De Bie J, Napolitano C, Santoro A, Mortara D, Severi S. Noninvasive quantification of blood potassium concentration from ECG in hemodialysis patients. Sci Rep. 2017. https://doi.org/10.1038/srep42492.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Human rights and participants
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the local ethical committee (Studio VESPA—268.2016).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Genovesi, S., Nava, E., Bartolucci, C. et al. Acute effect of a peritoneal dialysis exchange on electrolyte concentration and QT interval in uraemic patients. Clin Exp Nephrol 23, 1315–1322 (2019). https://doi.org/10.1007/s10157-019-01773-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01773-y