Abstract
Background
To compare the efficacy of glucocorticoids in primary focal segmental glomerulosclerosis (pFSGS) patients with moderate proteinuria. Registered at http://www.chictr.org.cn/, study No. ChiCTR-OPN-17012789.
Methods
pFSGS patients with urine protein between 1.0 and 3.5 g/24 h were recruited from 2006 to 2016. No decline in urine protein > 50% was observed after 2 months of run-in angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (ACEI/ARB) treatment. Patients were assigned to study group (glucocorticoids with ACEI/ARB) or control group (ACEI/ARB without glucocorticoids). Variables including 24-h urinary protein, serum albumin and serum creatinine during the trial were recorded. Remission was defined as proteinuria < 0.3 g/24 h or declined > 50%, and our composite end point as > 30% decrease of eGFR or eGFR < 30 ml/min.
Results
A total of 102 patients were enrolled (study group N = 52, control group N = 50), and the median follow-up time was 36 (12–117) months without significant difference between groups. During the 12-month follow-up, the remission rate was significantly higher in study group [73.1 vs 50.0% (P = 0.01)], and the initial median response time was 3 months in the study group while 6 in the control group. The end point was reached by 22.2% cases in study group, and 42.0% in control. The medium survival times were study group 72 months and control 57 (P = 0.03). Minor adverse reactions were observed in 10 patients (study group N = 8, control group N = 2).
Conclusions
Additional glucocorticoids therapy is more efficacious compared to ACEI/ARB alone in the treatment of patients with pFSGS and moderate proteinuria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary focal segmental glomerulosclerosis (pFSGS) has emerged as leading cause of nephrotic syndrome (NS) in adults over the past 30 years, accounting for 20–30% NS cases [1,2,3]. FSGS accounts for 3.3–16% adult patients undergoing renal biopsy in China, and has been one of the most common glomerulonephritis [4]. In previous research of our department, 97.7% of the FSGS patients present with proteinuria at initial onset, 27.7% with NS, 31.4% with hypertension and 38.2% with impaired renal function [estimated glomerular filtration rate (eGFR) < 60 ml/min] [4]. In FSGS patients present with nephrotic range proteinuria, progression to end stage renal disease (ESRD) often occurs over 5–10 years, whereas patients with non-nephrotic proteinuria and those achieving remission have relatively favorable prognosis [5, 6]. The proportion of patients with ESRD attribute to FSGS is reported to be 2.3%, compared with 0.4% for membranous glomerulonephritis and 0.3% for IgA nephropathy [7].
The initial treatment of FSGS patients without NS consists of optimal blood pressure control and the use of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin-receptor blockers (ARB). Although the use of ACEI/ARB in non-nephrotic FSGS patients results in slower progress of renal insufficiency, a few patients still aggravated and progressed to ESRD, especially those with urine protein > 1 g/24h [8]. However, the use of prednisone is associated with significantly increased likelihood of remission [9]. Nevertheless, the KDIGO guidelines recommend that corticosteroids and immunosuppressive therapy be considered only in pFSGS with NS [10].
In this study, we aim to compare the efficacy of glucocorticoids combined with ACEI/ARB vs ACEI/ARB alone in patients with pFSGS and moderate proteinuria prospectively.
Materials and methods
All procedures performed in our study were in accordance with the ethical standards of the Ethics Committee of Ruijin Hospital [ (2017) Clinical Ethics Approval No.154] and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consents were obtained from all participants included in the study.
Inclusion criteria
FSGS patients were recruited in the Department of Nephrology, Ruijin Hospital from 2006 to 2016. The following qualifiers have to be fulfilled prior to the recruitment: (1) aged 17–75 years; (2) renal biopsy acquired; (3) eGFR ≥ 30 ml/min; (4) urine protein between 1.0 and 3.5 g/24 h for at least twice; (5) serum albumin > 30 g/L; (6) decline in urine protein was < 50% after 2 months of ACEI/ARB treatment; (7) no usage of glucocorticoids within 3 months before study; (8) Signed informed consent.
Exclusion criteria
(1) Secondary FSGS; (2) family history of nephropathy; (3) hepatitis virus carrier, chronic viral hepatitis or abnormal liver function; (4) any active or serious infection; (5) obese patients with body mass index > 28 kg/m2; (6) unwilling to adhere to the study protocol.
Withdrawal criteria
(1) Allergic or intolerable adverse effects to the study medications; (2) double of the initial serum creatinine level or the urine protein elevated to NS level; (3) severe uncontrolled infection; (4) decease or voluntary termination by patients.
Renal biopsy
Renal specimens are processed for light microscopy, immunofluorescence study, and electron microscopy. Snap-frozen sections are stained with fluorescein isothiocyanate-conjugated polyclonal rabbit antibodies specific for IgG, IgA, IgM, C3, C4, C1q, Fg, к, and light chains.
The assessment of glomeruli for FSGS lesion is performed using the Columbia classification system [11]. The degree of glomerulosclerosis is divided into four levels. And level 0–3 were defined as no sclerosis, glomerular sclerosis ratio < 25%, 25–50% or > 50%, respectively. Tubular and renal interstitial lesions were evaluated with reference to tubulointerstitial lesions (TIL) grade [12]. Four levels of lesions were defined according to the range and severity of the disease regarding three indicators: tubulointerstitial atrophy, interstitial inflammatory cell infiltration and interstitial fibrosis. By no, mild (< 25%), moderate (25–50%) and severe (> 50%) lesions were scored as 0, 1, 2, 3, respectively. The TIL grade was decided according to the total scores of the three indicators: grade 0 (scored 0), grade 1 (1–3), grade 2 (4–6), grade 3 (7–9).
Treatment protocol
Patients who met the inclusion criteria were all treated with run-in ACEI/ARB for 2 months, with blood pressure within 90/60–130/80 mmHg. Patients without remarkable decline in urine protein observed (decline < 50%) after 2 months of ACEI/ARB treatment are assigned to two groups. According to the patient’s clinical and pathological conditions, the decision on grouping was made by full consideration of the patients’ personal wish, ensuring that there is no difference between groups on the types of ACEI/ARB drugs patients take before enrollment and the number of double-dosed patients. Patients are treated with ACEI/ARB drugs combined with oral prednisone initiating at 0.4–0.6 mg/kg/day in study group. If patients stay remission for over 1 month, prednisone was tapered by 5 mg every 2 weeks until the dose reached 15 mg/day and maintained over 8 weeks for gradual tapering. Back to initial treatment protocol when relapsing. ACEI or ARB alone is given to patients in control group. ACEI/ARB is given with maximum tolerated dose and blood pressure is controlled within the target range (< 135/85 mmHg). Once enrolled, the types and doses of ACEI/ARB in two groups will not be adjusted unless situations like uncontrolled hypotension. If blood pressure is poorly controlled, other types of antihypertensive drugs such as beta-blockers and calcium channel blockers may be used.
Data collection and monitoring schedule
The baseline characteristics are reviewed after the screening visit. The following data are collected: clinical features, blood pressure, side effects, routine examinations for blood and urine, liver and renal function, plasma electrolyte content, 24-h protein excretion, serum albumin and serum glucose. Treatment is started after the first visit, and subsequent visits are done monthly for the first 3 months, then every 3 months thereafter.
Evaluation of outcome
Complete remission (CR) is defined as proteinuria < 0.3 g/24 h with stable renal function. Partial remission (PR) is defined as 50% decline of the initial proteinuria with stable renal function. Stable renal function is defined as eGFR within 10% of baseline level. No response (NR) is defined as proteinuria reduction < 50% compared with baseline with or without renal function deterioration. We defined eGFR decreased > 30% or eGFR < 30 ml/min as renal function deterioration and our composite end point. Relapse is defined as proteinuria of patients with CR/PR for > 2 months increase to baseline level or renal function deterioration. Estimated GFR was evaluated by Chronic Kidney Disease Epidemiology Collaboration equation (EPI) [13].
Data analyses
Results of continuous variables were presented as mean ± SD or median (range) unless otherwise stated. Analyses are performed using SPSS21.0 software. For statistical analysis, (logarithmic) t test or Mann–Whitney/Wilcoxon tests were used, for normally and non-normally distributed variables, respectively. The χ2 test was used for analysis of frequencies. Outcomes were compared by univariate survival analysis using Kaplan–Meier and log-rank test (Breslow’s test) and multivariate analysis using Cox method. P < 0.05 is considered significant. Statistic charts were drawn by GraphPad Prism7.
Results
Baseline characteristics
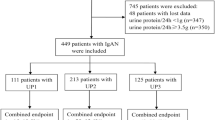
A total of 102 patients with pFSGS were enrolled between 2006 and 2016 (Fig. 1). Baseline clinical and biochemical characteristics of patients are shown in Table 1.
All cases were diagnosed by renal biopsy using standard diagnostic criteria. Patients in cohorts were not secondary to any systemic diseases such as systemic lupus erythematosus, Henoch-Schonlein purpura, or chronic liver disease. FSGS not otherwise specified (NOS) presented in 85.3% and the other patients had perihilar or tip FSGS. TIL grade was grade 1 accounting for 62.7% patients. About vascular lesions, 36.4% patients had no renal arteriolar lesion and 33.9% had only hyaline changes. Immunofluorescence was negative in 71.2% patients. No significant difference between two groups in the distribution of histopathological subtypes, the proportion of glomerular sclerosis, TIL grade and vascular changes was shown (Fig. 2). There was no significant correlation between pathological subtypes and clinical data. Glomerular sclerosis, TIL, and vascular lesions were significantly associated with eGFR (Spearman, P < 0.01). The greater glomerulosclerosis area is, the higher TIL grade is, and the more severe vascular lesion is, the lower eGFR would be. Only the severity of vascular lesions was associated with proteinuria (P < 0.01). There was no significant correlation between other pathological conditions and proteinuria.
Most patients in control group took ARB drugs, and only 4 cases took ACEIs. In the study group, 47 patients took ARBs, 5 took ACEIs. No patient took both ACEI and ARB drugs during the trial.
Outcome of treatment
As shown in Fig. 1, 8 patients in each group dropped out and 86 completed 1 year follow-up. In total, 46 cases (23 each group) completed a prolonged renal function follow-up of 5 years. In the 2nd month, two patients in study group received additional treatment of immunosuppressive agents for elevated proteinuria, and they were withdrawn from the study. 6 months later, two more cases in study group received immunosuppressive agents and withdrawn. Three patients in control group also quitted and started to take steroids due to the occurrence of NS. In the 12th month, three patients received immunosuppressive agents in study group, while five patients began to receive steroids treatment in control group. One patient in study group lost follow-up for unknown reason at 12th month. After being withdrawn, the characteristics of these patients are no longer included in the statistical analysis.
Urine protein excretion After 1 month of treatment, the 24-h urine protein decreased more intensively in study group than control group (33.3 vs 23.6%) (P = 0.07). And it kept decreasing during the 1-year follow-up (Fig. 3a). Although there was no significant difference between groups at the beginning of the trial, the difference became evident after 6 months (P < 0.05, logarithmic t test). After 1 year, urine protein declined by 69.8% in study group, compared to 47.1% in control group (P = 0.01).
Clinical data collected during the follow-up: a The urine medium protein levels of two groups in 12 months; b the remission rate of study group and control group; c the trends of serum albumin level in both groups; (d) The serum creatinine levels during 5 years. “Asterisk” indicates P < 0.05 between two groups. S steroids; C control, CR complete remission; PR partial remission; NR no response to treatment
Proteinuria remission The initial median response time (CR/PR) was 3 months in study group and 6 months in control group. Patients in study group took oral steroids for the median period of 7 months. The comparison of remission between groups was depicted in Fig. 3b. The remission rate of study group was significantly higher than control group after 1 year [73.08 vs 50.00% (P = 0.01)].
Relapsing rate Relapse of proteinuria occurred after 6 months of therapy among patients with CR/PR, and the proportion of relapse in control group was higher than study group. 12 months after the initial therapy, only five (13.5%) relived patients were relapsed in study group, while in the control group ten (55.6%) patients presented with proteinuria recurrence (P < 0.01).
Serum albumin During the follow-up, both groups of patients presented with elevated serum albumin (Fig. 3c), which was related to the decrease of urine protein. Although the initial serum albumin level in study group was lower than control group, there was no significant difference (P = 0.14) between groups after 2 months.
Renal function Serum creatinine and eGFR remained stable during 1 year in both groups, and there was no significant difference compared with baseline. During the follow-up of 5 years, creatinine in study group decreased slightly, while the control group showed an upward trend. There was a significant difference between two groups after 30 months (P < 0.05) (Fig. 3d).
Only two patients in control group progressed to ESRD in 39 months during follow-up. The composite end point of > 30% decrease of eGFR or eGFR < 30 ml/min was reached by 11 cases in study group, and 21 in control group. The medium survival time was 72 months in study group while 57 months in control group (P = 0.03) (Fig. 4).
Multivariate analysis
We conducted Cox multivariate regression analysis of the patients’ gender, age, pathological indicators, and clinical indicators. The results showed that only age was an independent risk factor for renal progression (HR per 1 year increase, 1.038; 95% confidence interval, 1.007–1.071; P = 0.02). Other characteristics including serum creatinine showed no statistical significance. Detailed results were shown in Table 2.
Adverse effects
In 1 year, adverse reactions were observed in eight (15.4%) patients in study group, including elevated serum glucose (N = 3), skin acne (N = 2), upper respiratory tract infection (N = 2) and urinary tract infection (N = 1). After adjusting of therapy or symptomatic treatment, all above-mentioned symptoms are alleviated. Major side effects were not observed in patients (Table 3).
Discussion
This is the first single-center prospective open-labeled controlled study focusing on pFSGS patients with moderate proteinuria.
Anti-hypertensive, anti-proteinuria, and dietary approaches aimed at slowing down non-specific mechanisms that contribute to progression of FSGS. Inhibition of renin-angiotensin-aldosterone system (RAAS) is a mainstay therapy for glomerular diseases characterized by podocyte injury, mainly through limiting podocyte apoptosis and detachment [14]. ACEI/ARB have been shown to effectively lower the mild and moderate proteinuria in chronic kidney disease patients with a good safety and tolerance independent of lowering blood pressure [15]. More recently, studies have shown that podocyte number can be increased by RAAS inhibition and that this occurs in the absence of podocyte proliferation [16, 17].
Response to steroids is an important indicator of renal prognosis. The efficacy of steroids in pFSGS patients with NS has been repeatedly confirmed [18, 19]. Chen et al. [20] reported that 56.2% of the patients (N = 143) with FSGS were sensitive to steroids. In our study, 38 (73.08%) cases are sensitive to steroid treatment in study group. Corticosteroids have non-specific immunosuppressive effects on FSGS patients, and may also exert their effect by inducing expansion of myeloid-derived suppressor cells [21]. Pozzi et al. [22] conducted a randomized controlled trial and reported that steroids treatment was shown to reduce proteinuria and preserve renal function in IgA nephropathy patients with urinary protein 1.0–3.5 g/24 h without notable adverse effects. Uzu et al. [23] came to a similar conclusion in their research (study group N = 23; control group N = 22). However, there has been no such trial on pFSGS patients with moderate proteinuria before.
In our cohort, 24-h-urine protein excretion was 1.67 (1.04–3.26)g/24 h in study group and 1.58 (1.09–3.43)g/24 h in control group, without significant difference between groups. NOS presents in most patients and perihilar or tip FSGS also presents in a few cases. The absence of cellular and collapsing subtypes may attribute to the relatively severe clinical phenotype of these two lesions [11, 24]. Moura et al. [19] reported that the median age in their pFSGS group was 32 years, 45% were males (N = 140). Median age of a Pakistan adult FSGS cohort (N = 124) was 31 (17–85) years, 86 (69%) were male [25]. In our study, the median age is 42 years, which may be because of patients in our cohorts present less severe clinical symptoms, thus the time they visited hospital can be years after the disease onset, resulting in presenting older age.
Hypertension, microscopic hematuria, and renal insufficiency are seen in 30–45% of FSGS cases [5]. Proteinuria quantification is essential during the clinical evaluation of patients with FSGS because it is among the strongest determinants of renal prognosis [26]. Earlier research in our department identified proteinuria > 1.0 g/24 h as risk factor of worse renal prognosis [8]. After 1 month of treatment, urine protein was significantly decreased in both groups. And 1 year later, the urine protein declines by 69.8% in study group and by 47.1% in control group. Plasma albumin level is negatively correlated with urinary protein excretion. The concentration of serum albumin is usually used as a surrogate parameter to assess the risk of thromboembolism. In our study, all patients present with initial albumin > 30 g/l, both groups of patients show elevated serum albumin after treatment. Both therapies can decrease protein excretion and elevate serum albumin, but the addition of low-to-medium doses of glucocorticoids is beneficial to adult pFSGS patients with moderate proteinuria.
It has been consistently shown that remission was an independent predictor of renal survival in NS patients [1, 6, 27], and certainly remission is also essential in FSGS patients with moderate proteinuria. According to our results, remission rate in study group is higher than control group. During 12-months follow-up, 5 of 37 (13.5%) patients relapse in study group, while 10 out of 18 (55.6%) in control group. The addition of low-to-medium dose of steroids can reduce the likelihood of recurrence of moderate proteinuria by more than 70%.
Bagchi et al. [1] reported that 9.5% patients (N = 116) had renal deterioration with a median follow-up of 23.6 months in NS cohort. Earlier research in our department showed 38.2% cases with impaired renal function [4]. Estimated GFR is 72.15 ± 29.68 ml/min in our study, with no significant difference between two groups. It remained stable during 1 year of follow-up in both groups. But during 5-year follow-up, serum creatinine in study group decreased slightly, while control group showed an upward trend. Considering the relatively favorable prognosis of FSGS patients with moderate proteinuria, we defined > 30% decrease of eGFR or eGFR < 30 ml/min as our composite end point referring to the standards used in previous studies [23, 28]. To better assess the patient’s prognosis, the end points we defined were relatively easier to reach than common end points such as death, ESRD, and creatinine doubling. The medium survival time was 72 months in study group, while 57 months in control group (P = 0.03). Although FSGS patients with moderate proteinuria had relatively optimistic prognosis, the patients may still suffer from exacerbated renal function. But if treated them with additional glucocorticoids, those patients can obtain renoprotective benefits.
Progression of renal function still occurred in 11 patients in the study group though steroids were used. Meanwhile, there are also 29 cases presented with good renal prognosis in the control group. To analyze the characteristics of those patients, we conducted multivariate analysis of the patient’s gender, age, pathological indicators, and clinical indicators. Interestingly, the results showed that only age was an independent risk factor for the progression of renal function. Other characteristics including serum creatinine showed no statistical significance. These results indicate that even in patients with already elevated serum creatinine, active treatment can effectively delay the progression of renal function and improve the prognosis.
Potential adverse effects of glucocorticoids are important causes of nephrologists’ conservative attitude towards steroids. We adopt prednisone at 0.4–0.6 mg/kg/day, which is a lower dose than we usually apply in NS. Patients in study group took oral steroids for median period of 7 months. Minor adverse reactions were observed in eight patients in study group and two in control group. The adverse symptoms were mild and improved after adjustment of regime, diet modification or symptomatic treatments. To conclude, low-to-medium dose of glucocorticoids are rather safe though mild side effect may occur.
Our study has certain limitations including the heterogeneity of the disease at presentation since patients were not randomized into groups and its origin from single center. The type and dose of ACEI/ARB used were not uniform.
In conclusion, glucocorticoids combined with ACEI/ARB in adult pFSGS patients with moderate proteinuria might have better efficacy in reducing proteinuria, and protecting renal function than ACEI/ARB alone. Our results recommended consider expanding the application of low-to-medium dose of glucocorticoid in pFSGS patients with moderate proteinuria. Our results still need to be validated by multi-centred, randomized, double-blind studies with prolonged follow-up.
References
Bagchi S, Agarwal S, Kalaivani M, Bhowmik D, Singh G, Mahajan S, et al. Primary FSGS in Nephrotic Adults: Clinical Profile, Response to Immunosuppression and Outcome. Nephron. 2016;132(2):81–5. https://doi.org/10.1159/000442999.
Rathi M, Bhagat RL, Mukhopadhyay P, Kohli HS, Jha V, Gupta KL, et al. Changing histologic spectrum of adult nephrotic syndrome over five decades in north India: a single center experience. Indian J Nephrol. 2014;24(2):86–91. https://doi.org/10.4103/0971-4065.127892.
Polito MG, de Moura LA, Kirsztajn GM. An overview on frequency of renal biopsy diagnosis in Brazil: clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol Dial Transplant. 2010;25(2):490–6. https://doi.org/10.1093/ndt/gfp355.
Xie J, Chen N. Primary glomerulonephritis in mainland China: an overview. Contrib Nephrol. 2013;181:1–11. https://doi.org/10.1159/000348642.
Korbet SM. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23(11):1769–76. https://doi.org/10.1681/ASN.2012040389.
Tang X, Xu F, Chen DM, Zeng CH, Liu ZH. The clinical course and long-term outcome of primary focal segmental glomerulosclerosis in Chinese adults. Clin Nephrol. 2013;80(2):130–9. https://doi.org/10.5414/CN107607.
O’Shaughnessy MM, Montez-Rath ME, Lafayette RA, Winkelmayer WC. Patient characteristics and outcomes by GN subtype in ESRD. Clin J Am Soc Nephrol. 2015;10(7):1170–8. https://doi.org/10.2215/CJN.11261114.
Ren H, Shen P, Li X, Pan X, Zhang Q, Feng X, et al. Treatment and prognosis of primary focal segmental glomerulosclerosis. Contrib Nephrol. 2013;181:109–18. https://doi.org/10.1159/000348468.
Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry G. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16(4):1061–8. https://doi.org/10.1681/ASN.2004070593.
Eckardt KU, Kasiske BL. KDIGO clinical practice guideline for glomerulonephritis foreword. Kidney Int Suppl. 2012;2(2):140. https://doi.org/10.1038/kisup.2012.10.
D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365(25):2398–411. https://doi.org/10.1056/NEJMra1106556.
Prayaga AK, Anuradha SV, Manjusha Y, Uppin M, Rapur R, Dakshina Murthy KV. Morphologic evaluation of renal function using semi-quantitative method in primary nonproliferative glomerular diseases. Indian J Pathol Microbiol. 2011;54(1):42–6. https://doi.org/10.4103/0377-4929.77322.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Wennmann DO, Hsu H-H, Pavenstädt H. The Renin-Angiotensin-Aldosterone system in podocytes. Seminars Nephrol. 2012;32(4):377–***84. https://doi.org/10.1016/j.semnephrol.2012.06.009.
Li X, Chen XD, Li ZX. The efficacy and safety of high-dose irbesartan in treatment of clinical proteinuria in patients with chronic kidney disease. Zhonghua Nei Ke Za Zhi. 2011;50 (12):1034–8.
Zhang J, Yanez D, Floege A, Lichtnekert J, Krofft RD, Liu ZH, et al. ACE-inhibition increases podocyte number in experimental glomerular disease independent of proliferation. J Renin Angiotensin Aldosterone Syst. 2015;16(2):234–48. https://doi.org/10.1177/1470320314543910.
Lichtnekert J, Kaverina NV, Eng DG, Gross KW, Kutz JN, Pippin JW, et al. Renin-Angiotensin-Aldosterone system inhibition increases podocyte derivation from cells of renin lineage. J Am Soc Nephrol. 2016;27(12):3611–27.
Korbet SM. Angiotensin antagonists and steroids in the treatment of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23(2):219–28. https://doi.org/10.1053/snep.2003.50020.
Moura LR, Franco MF, Kirsztajn GM. Minimal change disease and focal segmental glomerulosclerosis in adults: response to steroids and risk of renal failure. J Bras Nefrol. 2015;37(4):475–80. https://doi.org/10.5935/0101-2800.20150075.
Chen J, Chen M. A control study of the response from chinese patients with minimal change disease and focal segmental glomerulosclerosis to steroid therapy. Int J Clin Exp Med. 2017;10(4):6902–6.
Li L, Zhang T, Diao W, Jin F, Shi L, Meng J, et al. Role of myeloid-derived suppressor cells in glucocorticoid-mediated amelioration of FSGS. J Am Soc Nephrol. 2015;26(9):2183–97. https://doi.org/10.1681/ASN.2014050468.
Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet. 1999;353(9156):883–7.
Uzu T, Harada T, Ko M, Yamato M, Takahara K, Yamauchi A. Effect of corticosteroid therapy on the progression of IgA nephropathy with moderate proteinuria. Clin Exp Nephrol. 2003;7(3):210–4.
Mungan S, Turkmen E, Aydin MC, Saglam AE, Baydar DE. Tip lesion variant of primary focal and segmental glomerulosclerosis: clinicopathological analysis of 20 cases. Ren Fail. 2015;37(5):858–65. https://doi.org/10.3109/0886022X.2015.1033635 (Epub 2015 Apr 10).
Jafry N, Ahmed E, Mubarak M, Kazi J, Akhter F. Raised serum creatinine at presentation does not adversely affect steroid response in primary focal segmental glomerulosclerosis in adults. Nephrol Dial Transpl. 2012;27(3):1101–6. https://doi.org/10.1093/ndt/gfr430.
Hogan MC, Reich HN, Nelson PJ, Adler SG, Cattran DC, Appel GB, et al. The relatively poor correlation between random and 24-hour urine protein excretion in patients with biopsy-proven glomerular diseases. Kidney Int. 2016;90(5):1080–9. https://doi.org/10.1016/j.kint.2016.06.020.
Swarnalatha G, Ram R, Ismal KM, Vali S, Sahay M, Dakshinamurty KV. Focal and segmental glomerulosclerosis: does prognosis vary with the variants? Saudi J Kidney Dis Transpl. 2015;26(1):173–81.
Coppo R, Peruzzi L, Amore A, Piccoli A, Cochat P, Stone R, et al. IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol. 2007;18(6):1880–8.
Acknowledgements
This work was supported by National Key Research and Development Program of China (2016YFC0904100), the National Natural Science Foundation of China (No. 81570598), and Shanghai Natural Science Foundation (15ZR1426300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared no competing interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Ruijin Hospital [(2017) Clinical Ethics Approval No. (154)] and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Huang, J., Lin, L., Xie, J. et al. Glucocorticoids in the treatment of patients with primary focal segmental glomerulosclerosis and moderate proteinuria. Clin Exp Nephrol 22, 1315–1323 (2018). https://doi.org/10.1007/s10157-018-1585-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-018-1585-z