Abstract
Background
Epidemiology and outcomes of Japanese patients with advanced chronic kidney disease (CKD)—an estimated glomerular filtration rate (eGFR) < 45 ml/min/1.73 m2—has remained largely unexamined.
Methods
We conducted a nationwide survey to determine the distribution of Japanese CKD patients, and are conducting a cohort study of these patients. A questionnaire eliciting details about facilities and their CKD practices was sent to all clinics/hospitals with nephrologists. Based on the survey results, we recruited 2400 advanced CKD patients receiving nephrologist care from at least 30 representative facilities throughout Japan, selected randomly with stratification by region and facility size. Through patient questionnaires and nephrologist-practice surveys aligned with the international CKD Outcomes and Practice Patterns Study (CKDopps), we shall annually or semi-annually collect patient, physician and clinic data prospectively, detailing CKD practices for 5 years, with a primary outcome of death or renal replacement therapy initiation, and secondary outcomes being decline of eGFR by 30% or 50%, CKD progression to CKD G5, or a cardiovascular event.
Results
Of 790 eligible, responding facilities, 330 (41.8%) treat ≥80 advanced CKD patients in the average 3-month period. Regional distribution of these facilities is similar to that of persons in the general population. Hence, the 30 facilities selected for data collection appear to be geographically representative in Japan.
Conclusions
Our study will enhance understanding of various CKD practices and biological data associated with CKD progression, and allow international comparisons using the CKDopps platform. This will provide evidences to improve the health and quality of life for patients with advanced CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the definition of chronic kidney disease (CKD) was proposed by the kidney disease quality outcomes initiative (KDOQI) in 2002 [1], classification of renal function by estimated glomerular filtration rate (eGFR) has been universally accepted. Furthermore, renal function has been recognized as a risk factor not only for end-stage renal disease (ESRD) but also for cardiovascular, cerebrovascular and peripheral arterial diseases, and for death [2]. There has also been a sharp increase in CKD-associated risks (ESRD, cardiovascular death and all-cause death) and in uremia-associated complications (hypertension, anemia, hyperparathyroidism, hyperphosphatemia and acidosis) among patients with eGFR < 45 ml/min/1.73 m2. These findings in effect led to the 2012 Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines being updated, so CKD stage 3 was divided into eGFR 45–<60 ml/min/1.73 m2 (CKD G3a) and 30–<45 ml/min/1.73 m2 (CKD G3b) [3]. As a result, nephrology specialists have come to consider management of patients with eGFR < 45 ml/min/1.73 m2 (advanced CKD) a key factor in CKD clinical practice.
The prevalence of patients with CKD is reported to be 10–15% worldwide [4], with treated ESRD expected to rise sharply in Asian countries [5], with Japan being among the highest. Because dietary habits and risks of life-threatening conditions associated with CKD—such as coronary artery disease—are quite different in Asian countries from those in the West, there is a clear need for a cohort study focused on advanced CKD patients in Asia. Best practice recommendations for early detection of CKD were proposed in 2011 for Asia [6]. However, to our knowledge, there has never been even a nationwide cohort study in Asia focused on advanced CKD patients—let alone such an Asia-wide study. So we undertook a prospective nationwide cohort study to investigate the care of advanced CKD patients throughout Japan.
The study has been implemented in two steps. The first was a questionnaire-based survey of all Japanese facilities with nephrology specialists to determine their distribution both by region and number of patients treated, and to clarify the state of day-to-day CKD practice in Japan. This was the Reach-J survey. Based on the results of this survey, as the second step, we are now conducting a nationwide cohort study—the Reach-J CKD cohort study—targeting advanced CKD patients in Japan from at least 30 representative facilities throughout the country as determined by a balanced sampling design that accounted for facility size and geographic location. This study will also compare results in Japan with those in other countries through use of the international Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps) platform [7].
Methods
Objectives
The overarching purpose of the Reach-J CKD cohort study is to create a research platform for advanced CKD patients that will illuminate practice patterns and renal prognoses, and will identify associations between CKD clinical practice and CKD-associated outcomes at the patient, physician, and facility level. The main objectives are to:
-
Examine associations between practice variations (e.g., management of hypertension, anemia, mineral and bone disorders, glycemic control, nutrition) and both renal and non-renal outcomes including CKD progression;
-
Evaluate guideline adherence in terms of practice variations;
-
Identify the best timing for renal replacement therapy—including vascular access placement—to enhance patient outcomes and quality of life;
-
Assess the value of laboratory data and new biomarkers to predict CKD progression and outcomes;
-
Compare practice patterns with those in other countries through the CKDopps platform to pinpoint the best CKD management;
-
Study the cost-effectiveness of different treatment practices including clinical services (e.g., dietitian, social workers, and educational programs) in light of patient outcomes both nationally and internationally.
Our study will provide various important longitudinal follow-up data with biological samples from a large cohort of patients with advanced CKD.
Study design and participants
The Reach-J survey
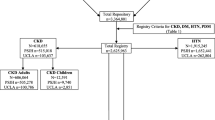
The flowchart of this study is shown in Fig. 1. We identified 2111 hospitals/clinics with board-certified nephrologists from the list of registered nephrologists (n = 4357), and sent a questionnaire asking about the characteristics of each facility and its CKD practice: the number of board-certified nephrologists; the total number of beds in the facility and in the nephrology unit; and the number of outpatients with CKD (eGFR < 60 ml/min/1.73 m2) and advanced CKD (eGFR < 45 ml/min/1.73 m2) treated in the average 3-month period—a timespan in which all advanced CKD patients would come for treatment at least once. Patients who visited multiple times during the 3 months were counted only once. We excluded patients receiving dialysis or transplantation, and also excluded facilities treating only dialysis patients or children, and nephrologists who did not have a clinical practice.
The Reach-J CKD cohort study
The Reach-J CKD cohort study will prospectively enroll outpatients with advanced CKD (CKD G3b-5) who are receiving nephrologist care in Japan. To be eligible, patients must be ≥20-years-old, have an eGFR < 45 ml/min/1.73 m2 at the time of screening, have no history of kidney transplantation or dialysis, and, of course, must have agreed to participate in our study. A key goal is to obtain representative data from 2400 outpatients with advanced CKD. Initially, we have selected 30 nephrologist-staffed facilities, with an enrollment goal at each facility of at least 80 patients with advanced CKD.
In addition to being a nationwide cohort study, this study is also designed to make comparisons internationally. We therefore are closely following the CKDopps platform to allow comparison of the practice patterns we discern throughout Japan with those of other CKDopps countries [7].
Study organization
The Reach-J CKD cohort study is coordinated by the Department of Nephrology, University of Tsukuba, and is funded by the Japan Agency for Medical Research and Development (AMED); the database is managed by the Tsukuba Clinical Research and Development Organization (T-CReDO). Study partners are listed in the acknowledgement.
Selection of facilities
Participant facilities were randomly selected based upon results of the Reach-J survey, which was important for informing sampling stratification according to facility size (the number of advanced CKD outpatients treated in the average 3-month period: small, 80–160; middle, 161–240; or large, ≥241 patients) and geographic location (the regions Hokkaido and Tohoku, Kanto and Tokyo, Chubu, Kinki, or Chugoku and Shikoku and Kyusyu). Data from the Reach-J survey are detailed below in the Results section. If a candidate facility indicates that it cannot participate in our study or cannot collect 80 patients, the next candidate facility in the same cluster of facility size and location is approached for study participation based upon the randomized facility sampling list. The outpatient recruitment phase will last until the patient enrollment goal is reached, typically within 12 months for each facility. Clinical data will be collected for each patient every 6 months for 5 years unless, of course, some event like initiation of dialysis or death intervenes.
Data collection
We shall collect anonymized patient, physician, and clinic data relevant to both the Reach-J and CKDopps objectives. The data will be collected electronically and/or manually, and the SS-MIX2 data collection system—developed by the Japan Association for Medical Informatics—will also be used if installed at the facility. Details of the CKDopps study procedures have been reported elsewhere [7]. The steps in our data collection—which are quite similar to those of the CKDopps platform—will consist, briefly, of a medical questionnaire at enrollment, interval summaries collected every 6 months—including monthly patient cares, laboratory measurements and medications—, an annual patient questionnaire and annual nephrologist-practice survey. The contents of these are listed in Table 1. The remaining blood and urine samples collected during study follow-up will be stored at temperatures below −80 °C for further evaluation. Table 2 details the outcomes of our study. As noted above in Methods, the study’s primary endpoint is renal replacement therapy (RRT: hemodialysis, peritoneal dialysis, or kidney transplantation) or death, and the secondary endpoints are 30% eGFR decline, 50% eGFR decline, CKD G5 (eGFR < 15 ml/mon/1.73 m2), or cardiovascular event (acute coronary syndrome, stroke, or amputation). For patients who start RRT, additional interval summaries will be collected at initiation, 6, and 12 months afterward to capture details of their RRT and outcomes.
Sample size calculation
Since we restricted candidate facilities to nephrology-run clinics that treat 80 or more advanced CKD outpatients in an average 3-month period, the possible eligible candidate pool identified by the first-step Reach-J survey comprised 330 facilities. We assumed that about 10% of these would agree to participate in our study, so 30 facilities with 2400 patients became our recruitment goal. That would be a feasible number, and would be sufficient to detect reliable associations in the overall samples: at 80% power, with a 5- year follow-up and 10% loss of follow-up, the estimated minimum detectable hazard ratios with 2400 patients are 1.27, 1.19 and 1.15 for event rates, respectively, of 0.05, 0.10, and 0.20 per year. These event rates are consistent with published estimates of mortality before ESRD in patients with CKD G3 or 4 [8].
Statistical analysis
Data were summarized using proportions and means (±SD) as appropriate. Categorical variables were analyzed with the Chi-squared, Fisher’s exact test, or test for trend analysis, continuous variables compared using the Mann–Whitney U test, or Kruskal–Wallis test. All analyses used Stata® SE version 14.2 (StataCorp, College Station, TX).
Results
The number of advanced CKD outpatients by facility size
Of the 2111 facilities to which we sent the questionnaire, 884 answered (41.9%) and the response rate was very similar in each of the regions in Japan, although it was higher for the larger hospitals (p < 0.001) (Supplementary Table 1). Of the responders, 790 facilities (391 small-scale, 241 mid-scale, and 158 large-scale) met the eligibility criteria for our study. Note that we defined facility scale based on the total number of beds in the hospitals—including beds for non-nephrology inpatients— and not on facility size, which was based on the number of advanced CKD patients. The ranking was: small-scale 0–99; mid-scale 100–499; and large-scale ≧500 beds.
Table 3a and b summarize the number of facilities by scale and by their number of CKD and advanced CKD outpatients. The proportion of facilities taking care of >500 CKD patients in an average 3-month period is 2.1% of the small-scale facilities, 17.4% of the mid-scale, and 54.4% of the large-scale facilities (p < 0.01). Note the inverse relation between facility size and number of CKD patients and note that over half of the small-scale facilities take care of fewer than 50 CKD patients. It therefore, naturally follows that most CKD patients are treated in large-scale hospitals, though more than half (1117/2111) of the facilities with board-certified nephrologists in our study are small sized. Unsurprisingly, facility scale is strongly correlated with the number of advanced CKD patients treated. Thus, the proportion of facilities taking care of >240 advanced CKD outpatients in an average 3-month period is 2.3% of the small-scale, 17.8% of the mid-scale, and 48.7% of the large-scale facilities (p < 0.01) (Table 3b). Over half of the small-scale facilities take care of fewer than 20 advanced CKD patients over 3 months. Of the 790 facilities responding to our questionnaire, 330 (41.8%) were treating >80 advanced CKD patients, and 210 (26.6%) were treating >160 advanced CKD patients. Again unsurprisingly, the proportion of facilities treating both >80 and >160 such patients over 3 months was significantly higher in the large-scale hospitals (test for trend, p < 0.001).
Comparison of distributions by region of facilities taking care of more than 80 advanced CKD outpatients
As shown in Table 3b, 330 facilities (61 small-scale, 131 mid-scale, and 138 large-scale) are taking care of >80 advanced CKD outpatients in an average 3-month period (as noted, these are the candidate facilities for our cohort study) and there is a significant relationship between hospital size and the number of advanced CKD outpatients (p < 0.001) (Supplement Figure 1). Since each facility’s total number of beds includes those for patients with no kidney involvement, we considered the number of advanced CKD outpatients a better index of facility size for purposes of the second step in our study.
Next, we focused on the geographic distribution of these facilities by seeing how they were dispersed throughout the 9 regions in Japan. Table 4 shows the number of candidate facilities in each region. The distribution of the candidate facilities by region is very similar to that of the general population, suggesting the representativeness of this survey. The proportion of large facilities was higher in Chubu, lower in Hokkaido, Tohoku, and Kanto; that of mid-size facilities was higher in Kanto; and that of small facilities was higher in Kyushu (Fig. 2; Table 4).

Data of the general population were obtained from 2010 Population Census of Japan (http://www.stat.go.jp/english/data/kokusei/index.htm
Comparison of distributions of facilities taking care of more than 80 advanced CKD outpatients (over 3 months) with the general population
Discussion
As the first step in this study, we conducted a questionnaire survey of all board-certified nephrologists in Japan to determine the distribution of CKD patients by geographical location and facility size. This was the first nationwide survey focused on advanced CKD patients in Japan. We focused on advanced CKD patients since they are known as a high-risk population for all-cause, cardiovascular mortalities, and ESRD [2, 9]. Given the nature of observational studies, measurement of urinary albumin or albumin is not mandatory; nevertheless, we expected that most of the patients would receive urinalysis because they are treated by nephrology specialists. The Reach-J survey found that the great majority of advanced CKD patients were treated at large facilities. This may be due to the fact that there is a national insurance system in Japan, covering everyone, and patients may freely choose which hospital they go to. So patients with high risk for complications—like advanced CKD patients—tend to choose large hospitals, which are perceived to be better equipped to handle complications. Accordingly, we found that only 42% of facilities with nephrologists were caring for >80 advanced CKD patients in an average 3-month period. That proportion was significantly lower in small facilities (fewer than 100 beds) (16%) than it was in mid-size facilities (100–499 beds) (54%) or large facilities (≥500 beds) (87%). We also found that the geographical distribution of these facilities was very similar to that of the Japanese general population, suggesting that regional difference in nephrology-care access may be small in Japan.
The second step of our study, the Reach-J CKD cohort study—which we shall soon conduct—is the first study designed to collect and analyze data from nationally representative facilities and their advanced CKD patients, providing a research platform to identify practice patterns associated with the best outcomes for advanced CKD patients focused on death, transition to ESRD, decline of eGFR, and cardiovascular events. This is not only the first national advanced CKD cohort study with random sampling in Japan, it is also the first study in Asia designed to provide international comparisons of advanced CKD patients using the CKDopps platform. This will permit a greater range of analyses through direct comparisons than did previous studies, including meta-analyses.
The main objective of our study is to determine the “real-world” clinical practice with advanced CKD patients in Japan. It will show associations between practice patterns and outcomes and the actual natural history of advanced CKD patients in Japan, information that is very important for updating our clinical practice guidelines. In addition, the international comparisons may very well lead to major policy changes that can effect better care of advanced CKD patients—just as the DOPPS has influenced policies and guidelines on hemodialysis care in many countries, such as for vascular access, dialysis adequacy, treatment time [10,11,12], and changes in reimbursement for erythropoiesis-stimulating agents in Japan [13].
There have been several milestone cohort studies of CKD management— including meta-analyses—published around the world. For instance, the Chronic Kidney Disease Prognosis Consortium proposed and updated the definition and classifications of CKD [2], demonstrated multiple risks of CKD [4, 14,15,16], and defined the surrogate outcome of ESRD [17]. There also is the Chronic Renal Insufficiency Cohort studies, which have identified elevated fibroblast growth factor 23 as a risk factor for ESRD and mortality, and examined blood pressure control and progression of CKD [18, 19]. Several large cohort studies of CKD have also been published in Japan to examine the prevalence and incidence of ESRD, risk of CKD progression and of left ventricular hypertrophy in CKD patients [20,21,22,23,24,25,26]. These Japanese studies have shown associations between CKD progression and cardiovascular/cerebrovascular events, death, and hospitalization—all of these associations suggesting the importance of management of CKD patients, especially advanced CKD patients. However, the study populations were limited to patients living only in certain geographical locations, or patients taken care of only in large hospitals and their satellite facilities—both of which have limitations—and may have resulted in serious selection bias. So to determine real daily practice with advanced CKD patients in Japan, we saw a clear need to create a nationwide cohort study of advanced CKD patients with random sampling and with analyses on all levels—patient, physician, and clinic. In addition, we thought the study should be designed to facilitate international comparisons that could improve daily practice.
Transition to ESRD by patients with advanced CKD is another main problem we had to consider. In 1997, KDOQI guidelines recommended that initiation of dialysis be considered when the arithmetic mean of creatinine clearance and urea clearance fell below 10.5 ml/min/1.73 m2 (except in well-nourished, asymptomatic patients). As a result, the number of early starts of dialysis (especially in elderly patients) increased in the US [27]. Nevertheless, recent studies have shown no benefit from early start of dialysis in either the US or Japan [28,29,30]. Accordingly, the KDIGO 2012 guidelines recommended that initiation of dialysis should be considered only with the appearance of symptoms associated with kidney failure—including progressive deterioration in nutritional status, which often (but not invariably) occurs in the GFR range between 5 and 10 ml/min/1.73 m2 [3]. We previously reported that in terms of the duration of nephrology care before dialysis initiation, 6 months or longer of nephrology care significantly decreased mortality. However, evidences of the benefits of nephrology care over a long period of years for patients with advanced CKD are lacking. Our study will illuminate these important topics.
There are several limitations in this study. First, it is not nationwide, collecting all data from throughout Japan. Although we chose a balanced sampling design that accounted for facility size and geographic location, it is important to keep in mind that while our data may be representative in Japan, these are not a Japanese national database. Second, the target population in this study consists of patients with advanced CKD, so study of our cohort could not assess the proportion of patients with advanced CKD out of those with CKD—or out of the general population, which is beyond the scope of this study. Third, in the nature of questionnaire surveys, there is a bias for facility selection that may overestimate the quality of care in Japan. However, considering the higher response rate in this survey, that selection bias may be minimal.
In summary, the Reach-J CKD cohort study is a national prospective cohort study that will define “real-world” clinical practice and outcomes for patients with advanced CKD in Japan. The random sampling strategies used to recruit facilities and their patients were based on the results of the Reach-J survey, and the study protocols were designed to make use of the CKDopps platform for future international comparisons. Overall, the study will provide evidences to clarify epidemiology, to improve the health and quality of life for patients with advanced CKD, and to facilitate cost analyses in this field.
References
National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266.
Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80:17–28.
Group KDIGOKCW. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81.
Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–82.
Li PK, Chow KM, Matsuo S, Yang CW, Jha V, Becker G, et al. Asian chronic kidney disease best practice recommendations: positional statements for early detection of chronic kidney disease from Asian Forum for Chronic Kidney Disease Initiatives (AFCKDI). Nephrology (Carlton). 2011;16:633–41.
Mariani L, Stengel B, Combe C, Massy ZA, Reichel H, Fliser D, et al. The chronic kidney disease outcomes and practice patterns study (CKDopps): rationale and methods. Am J Kidney Dis. 2016;68:402–13.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305.
Inaguma D, Imai E, Takeuchi A, Ohashi Y, Watanabe T, Nitta K, et al. Risk factors for CKD progression in Japanese patients: findings from the Chronic Kidney Disease Japan Cohort (CKD-JAC) study. Clin Exp Nephrol. 2017;21:446–56.
Fuller DS, Pisoni RL, Bieber BA, Port FK, Robinson BM. The DOPPS practice monitor for US dialysis care: update on trends in anemia management 2 years into the bundle. Am J Kidney Dis. 2013;62:1213–6.
Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, et al. Vascular access use and outcomes: an international perspective from the dialysis outcomes and practice patterns study. Nephrol Dial Transplant. 2008;23:3219–26.
Pisoni RL, Zepel L, Port FK, Robinson BM. Trends in US vascular access use, patient preferences, and related practices: an update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis. 2015;65:905–15.
Hasegawa T, Bragg-Gresham JL, Pisoni RL, Robinson BM, Fukuhara S, Akiba T, et al. Changes in anemia management and hemoglobin levels following revision of a bundling policy to incorporate recombinant human erythropoietin. Kidney Int. 2011;79:340–6.
Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–40.
Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104.
van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–52.
Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–31.
Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, et al. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med. 2015;162:258–65.
Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–9.
Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, et al. Chronic kidney disease Japan cohort (CKD-JAC) study: design and methods. Hypertens Res. 2008;31:1101–7.
Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Doi Y, Okubo K, et al. Chronic kidney disease and cardiovascular disease in a general Japanese population the Hisayama study. Kidney Int. 2005;68:228–36.
Nakayama M, Sato T, Sato H, Yamaguchi Y, Obara K, Kurihara I, et al. Different clinical outcomes for cardiovascular events and mortality in chronic kidney disease according to underlying renal disease: the Gonryo study. Clin Exp Nephrol. 2010;14:333–9.
Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996;49:800–5.
Iseki K, Asahi K, Moriyama T, Yamagata K, Tsuruya K, Yoshida H, et al. Risk factor profiles based on estimated glomerular filtration rate and dipstick proteinuria among participants of the specific health check and guidance system in Japan 2008. Clin Exp Nephrol. 2012;16:244–9.
Konta T, Hao Z, Abiko H, Ishikawa M, Takahashi T, Ikeda A, et al. Prevalence and risk factor analysis of microalbuminuria in Japanese general population: the Takahata study. Kidney Int. 2006;70:751–6.
Irie F, Iso H, Sairenchi T, Fukasawa N, Yamagishi K, Ikehara S, et al. The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int. 2006;69:1264–71.
Rosansky SJ, Clark WF. Has the yearly increase in the renal replacement therapy population ended? J Am Soc Nephrol. 2013;24:1367–70.
Crews DC, Scialla JJ, Liu J, Guo H, Bandeen-Roche K, Ephraim PL, et al. Predialysis health, dialysis timing, and outcomes among older United States adults. J Am Soc Nephrol. 2014;25:370–9.
Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011;171:396–403.
Yamagata K, Nakai S, Iseki K, Tsubakihara Y. Late dialysis start did not affect long-term outcome in Japanese dialysis patients: long-term prognosis from Japanese Society for [corrected] Dialysis Therapy Registry. Ther Apher Dial. 2012;16:111–20.
Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–38.
Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84.
Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30.
Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The rapid assessment of physical activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118.
Weidmer BA, Cleary PD, Keller S, Evensen C, Hurtado MP, Kosiak B, et al. Development and evaluation of the CAHPS (consumer assessment of healthcare providers and systems) survey for in-center hemodialysis patients. Am J Kidney Dis. 2014;64:753–60.
Acknowledgements
We thank all the nephrology specialists in Japan who responded to our Reach-J survey, Dr. Hiroyuki Hoshimoto, Ms. Yukiko Ito and all the staff members of the Clinical Trial and Research Center, University of Tsukuba (T-CReDO), Mr. Yoshihiro Ishihara and other staff members of Flexible Inc., and Mr. Justin Albert, Mr. Brian Bieber, Mr. Doug Fuller, Ms. Christina Pustulka, and all the staff members of Arbor Research, for their contribution to our work.
The Reach-J CKD collaborators
Dr. Tomoya Hirayama, Kitasaito hospital, Hokkaido; Dr. Nobuhiko Togashi, JR Sapporo hospital, Hokkaido; Dr. Akira Sugiura, Osaki Citizen hospital, Miyagi; Dr. Kunihiro Yamagata, University of Tsukuba, Ibaraki; Dr. Tatsuo Shiigai, Shiigai clinic, Ibaraki; Dr. Kazue Ueki, Toho hospital, Gunma; Dr. Ken Kikkawa, Kikkawa Naika clinic, Saitama; Dr. Tsukasa Nakamura, Shinmatudo Central General Hospital, Chiba; Dr. Hideki Matsukuma, Funabashi Futawa hospital, Chiba; Dr. Shinsuke Harasawa, Nihon University hospital, Tokyo; Dr. Yuko Shibuya, NTT Medical Center Tokyo, Tokyo; Dr. Hitoshi Tagawa, Kichijoji Asahi Hospital; Dr. Shuzo Kobayashi, Shonan-Kamakura General Hospital, Kanagawa; Dr. Masaki Nagasawa, Shinonoi General Hospital, Nagano; Dr. Minako Wakasugi, Niigata University Hospital, Niigata; Dr. Hajime Yamazaki, Nagaoka Red Cross Hospital, Niigata; Dr. Michio Matsumoto, Toyama Saiseikai Toyama Hospital, Toyama; Dr. Shoichi Maruyama, Nagoya University Hospital, Aichi; Dr. Norihiro Suga, Nagoya City West Medical Center, Aichi; Dr. Keiichi Tamagaki, Kyoto Prefectural University of Medicine Hospital, Kyoto; Dr. Taiko Kimura, Nantan Hospital, Kyoto; Dr. Tsutomu Tabata, Inoue Hospital, Osaka; Dr. Shinichi Nishi, Kobe University Hospital, Hyogo; Dr. Yuriko Yonekura, Akashi Medical Center, Hyogo; Dr. Hitoshi Sugiyama, Okayama University Hospital, Okayama; Dr. Naoki Kashihara, Kawasaki Medical School Hospital, Okayama; Dr. Tadashi Sofue, Kagawa University hospital, Kagawa; Dr. Kazuhiko Tsuruya, Kyushu University Hospital, Fukuoka; Dr. Masato Tadokoro, Nagasaki Harbor Medical Center City Hospital, Nagasaki; Dr. Shoichi Fujimoto, University of Miyazaki Hospital, Miyazaki; and Dr. Kiyoyuki Tokuyama, Tokuyama Clinic, Okinawa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Okada is funded by Takeda Pharm., Chugai Pharm., Kyowa-Hakko Kirin, Pfizer, Boehinger Ingelheim, Astellas Pharm., Otsuka Pharm., MSD, Shionogi, Novaltis, Sumitomo Dainippon Pharm., Mitsubishi Tanabe Pharm., and Daiichi Sankyo Co. Dr. Robinson and Dr. Pisoni are members of Clinical Research for the non-profit organization Arbor Research Collaborative for health, which has designed and carried out the Dialysis Outcomes and Practice Pattern Study (DOPPS) Program. The DOPPS program is supported by Amgen, Kyowa Hakko Kirin, AbbVie Inc., Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma Ltd. Additional support for specific projects and countries is also provided in Canada by Amgen, BHC Medical, Janssen, Takeda, Kidney Foundation of Canada (for logistics support); in Germany by Hexal, DGfN, Shire, WiNe Institute; and for the Peritoneal-DOPPS in Japan by the Japanese Society for Peritoneal Dialysis (JSPD). The DOPPS.org website lists the full details. JDOPPS was administered by the Arbor Research Collaborative for Health, Ann Arbor, MI, USA, and supported by Kyowa Hakko Kirin Co. Ltd. All support is provided without restrictions on publications. All other authors have no conflict disclosure.
Human and animal rights
The study’s protocol was approved by the Tsukuba institutional review board (IRB) (H27-199) and the review board of the Japanese Society of Nephrology (No. 29), and has been or will be approved by the IRB of each participating facility. The study procedures fully adhered to the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement, and was registered with the University Hospital Medical Information Network (UMIN000022145).
Informed consent
Informed consent was and will be obtained from all individual participants included in the study.
Funding source
The study was supported by a Grant-in-Aid for Research on Advanced Chronic Kidney Disease, Practical Research Project for Renal Diseases from the Japan Agency for Medical Research and Development, AMED. Preparation of the manuscript was supported in part by JH’s grant from Grants-in-Aid for Scientific Research (JSPS KAKENHI) Grant Number 15K08719, and the Okinaka Memorial Institute.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hoshino, J., Nagai, K., Kai, H. et al. A nationwide prospective cohort study of patients with advanced chronic kidney disease in Japan: The Reach-J CKD cohort study. Clin Exp Nephrol 22, 309–317 (2018). https://doi.org/10.1007/s10157-017-1453-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1453-2