Abstract
Background
Glomerular podocyte-derived vascular endothelial growth factor (VEGF) is indispensable for the migration and proliferation of glomerular endothelial cells. In contrast, podocyte-specific Vegf overexpression leads to the collapse of glomerular tufts; however, the mechanisms underlying this outcome have not yet been reported.
Methods
To further clarify the effects of elevated levels of Vegf expression on glomerular cells, we established a dual transgenic mouse line in which Vegf was exclusively and inducibly expressed in podocytes under the control of the “Tet-on system” (Podocin-rtTA/TetO-Vegf164 mice).
Results
Macroscopic and microscopic examination of Podocin-rtTA/TetO-Vegf164 animals following Vegf induction identified the presence of prominent red bloody spots. In addition, the endothelial cell number was increased along with enlargement of the subendothelial spaces. We also observed impaired endothelial fenestrations and aberrant plasmalemmal vesicle-associated protein-1 (PV-1) expression. In contrast, the mesangial cell number markedly decreased, resulting in a glomerular tuft intussusceptive splitting defect. Furthermore, whereas platelet-derived growth factor-B (PDGF-B) expression in the glomerular cells of Podocin-rtTA/TetO-Vegf164 mice was not decreased, phospho-PDGF receptor immunoreactivity in the mesangial cells was significantly decreased when compared to wild-type animals.
Conclusion
Taken together, the results of this study indicated that the upregulation of podocyte VEGF decreased the number of mesangial cells, likely owing to inhibition of PDGF-B-mediated signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular endothelial growth factor (VEGF), an endothelium-specific growth factor, has an essential role in both vasculogenesis and angiogenesis [1, 2]. VEGF is secreted from surrounding pericytes and promotes the proliferation, differentiation, and survival of endothelial cells [3]. VEGF also increases vascular permeability and induces the remodeling of the interstitial matrix [3, 4].
In the kidneys, Vegf is constitutively expressed in podocytes [5] and plays an important role in the development of glomerular endothelial cells and in the maintenance of their integrity, as shown by loss of endothelial cells in mice carrying a podocyte-specific null mutation in Vegf [6]. In addition, bevacizumab, an anti-VEGF antibody, has been reported to cause injury of glomerular endothelial cells, leading to overt proteinuria [7]. Therefore, VEGF in podocytes is essential for the normal development and maintenance of glomerular capillary tufts.
Considering these known functions of VEGF, the mechanism for the observed loss of endothelial cells following VEGF downregulation is well understood. However, how VEGF overexpression affects the development of glomerular cells remains unclear. Podocyte-specific VEGF overexpression is known to lead to collapsing glomerulopathy [6]. However, the details of phenotypic changes in mesangial cells associated with VEGF upregulation have not yet been investigated. In contrast, Veron et al. previously reported that excess VEGF in podocytes during kidney organogenesis [18] or adulthood [8] mainly affects podocytes, e.g., causing foot process effacement, absence of the slit diaphragm, and glomerulomegaly [8]. Although mild mesangial expansion was shown [8], a change in the development of mesangial cells during glomerulogenesis was not directly demonstrated.
Thus, to address this issue, we established transgenic mice with inducible podocyte-specific VEGF overexpression during glomerulogenesis and analyzed the effects of elevated levels of podocyte VEGF on the development of each glomerular cell type (mesangial and endothelial cells).
Methods
Generation of Podocin-rtTA/TetO-Vegf164 transgenic mice
All transgenic animals were maintained in a pathogen-free vivarium according to institutional guidelines.
A 2.8-kb Vegf164 cDNA encoding the entire coding region was obtained by reverse transcription-polymerase chain reaction (RT-PCR) using total mouse kidney RNA and the following primers: 5′-AAACCATGAACTTTCTGCTCTCTTGGGT-3′ and 5′-TCACCGCCTTGGCTTGTCACA-3′. After the sequence was verified, the cDNA fragment was inserted downstream of the bidirectional TetO gene promoter of the vector pBI-G containing the LacZ gene (631004; TaKaRa Bio, Shiga, Japan). Five micrograms of the transgene, which was excised using the restriction enzymes NotI and SalI, was injected into 283 fertilized eggs obtained from mating between C57BL/6 and DBF1 mice, as described previously [9]. Of the resulting offspring, 13 mice were found to carry the transgene upon genotyping of tail DNA. These mice, designated TetO-Vegf164, were crossed with Podocin-rtTA mice (a kind gift from Prof. Jeffrey B Kopp [10]) to obtain dual transgenic mice (Podocin-rtTA/TetO-Vegf164). Genotyping was performed by PCR using primers specific for each transgene as follows: podocin, 5′-CGCACTTCAGTTACTTCAGGT-3′ and 5′-GCTTATGCCTGATGTTGATGA-3′; LacZ, 5′-TCTGCTTCAATCAATCAGCGTGCC-3′ and 5′-GCCGTCTGAATTTGACCTGA-3′. Transgenic mice were maintained by sib mating, and F2–F6 generation animals were used in this study.
Control mice used in this study were the wild-type mice carrying neither Podocin-rtTA nor TetO-Vegf164. To rule out the possibility that the former transgene may affect kidney phenotypes, we compared several histological parameters between wild-type and Podocin-rtTA mice and found no obvious differences (Supplementary Fig. 1), as reported by Shigehara et al. [10]. Thus, Podocin-rtTA was unlikely to have a significant impact on kidney phenotypes observed in Podocin-rtTA/TetO-Vegf164 mice.
To induce exogenous Vegf164 in podocytes during embryonic development, pregnant mice were administered doxycycline at a concentration of 2 mg/L from embryonic day (E) 10.5–E14.5 until birth. The concentration of VEGF-A was measured in urine samples using a Quantikine VEGF ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Histology
Kidneys were routinely processed and subjected to various staining procedures, as described below. Transmission electron microscopy (TEM) was performed as described previously [9].
LacZ staining and western blot analysis
An X-gal (4-Cl-5-Br-3-indolyl-β-galactosidase) assay was performed to assess the expression of the LacZ gene as described previously [9]. Western blot analysis for lacZ was performed according to the method described previously [11] using an anti-β-galactosidase antibody (1:2000, Z3781; Promega, Madison, WI, USA).
Immunohistochemistry
Primary antibodies used for immunohistochemistry were as follows: rabbit anti-Wilm’s tumor (WT)-1 (1:100, sc192; Santa Cruz Biotechnology, Dallas, TX, USA), rat anti-plasmalemmal vesicle-associated protein-1 (PV-1; 1:100, 550563; BD Biosciences, San Diego, CA, USA), rat anti-CD31 (1:100, 550274; BD Biosciences), mouse anti-desmin (1:200, M0760; Dako, Carpinteria, CA, USA), mouse anti-α-smooth muscle actin (αSMA; 1:50, M0851; Dako), rabbit anti- PDGF receptor-β (PDGFR-β; 1:50, ab32570; Abcam, Cambridge, UK), and rabbit anti-phospho-PDGF receptor-β (p-PDGFR-β; 1:500, ab16864; Abcam).
The percentage of marker-positive glomeruli among all glomeruli was measured on the sagittal section with a maximum area from each kidney, and the percentage of phospho-PDGF receptor-β-positive glomeruli among all total PDGF receptor-β-positive glomeruli was assessed in the adjacent sections. Areas positive for αSMA were measured using a computed imaging analyzer (Scion Image). Positivity for each mesangial cell marker was assessed based on the presence of positive staining within the mesangial area, regardless of its intensity.
In situ hybridization
In situ hybridization for Pdgfb was performed according to the method described previously using sections from neonatal mice at birth [11].
Statistical analysis
All values are expressed as medians (ranges). Statistical analysis was performed using Mann–Whitney U tests for single comparisons and univariate analysis to evaluate the relationships of αSMA-positivity with the presence of phenotypes. Differences with p values of less than 0.05 were considered significant.
Results
Macroscopic and microscopic inspection of kidneys from Podocin-rtTA/TetO-Vegf164 mice
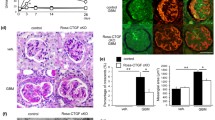
Podocin-rtTA/TetO-Vegf164 mice, but not wild-type mice, expressed LacZ exclusively in podocytes when pregnant mice were administered doxycycline (Fig. 1a, b). Western blot analysis of whole kidney extracts showed that β-galactosidase was present in Podocin-rtTA/TetO-Vegf164 mice upon induction, but not in wild-type mice (Fig. 1c). Podocin-rtTA/TetO-Vegf164 mice exhibited significantly higher concentrations of VEGF (median 48.4 pg/mL, range 44.1–167.8 pg/mL, n = 6) in urine samples at P0 than wild-type mice (median 36.6 pg/mL, range 28.5–53.0 pg/mL, n = 8, p < 0.05; Fig. 1d).
Podocyte-specific overexpression of Vegf164. Promoter activity of Tet-O was measured by lacZ staining of the kidneys from wild-type (WT) (a) and Podocin-rtTA/Tet-O-Vegf164 mice (Tg) (b) at P0 following doxycycline administration. c Western blot of kidney lysates showed a clear band for β-galactosidase only in dual transgenic mice administered doxycycline. d Urinary VEGF-A concentrations in wild-type and dual transgenic mice treated with doxycycline. The horizontal bars and rectangles represent the ranges and the interquartile ranges, respectively. The horizontal bars in the rectangles represent the medians. Representative images of macroscopic (e, f) and microscopic (g–l) examination of the kidneys from wild-type (e, g, i) and Podocin-rtTA/Tet-O-Vegf164 mice (f, h, j–l) at P0. Tubules were filled with red blood cells both in the cortex (h) and medulla (j). Microaneurysms were observed in some glomeruli (arrows in k). Magnification: ×10 (e, f); ×200 (g–j); ×400 (a, b, k, l)
Macroscopically, the dual transgenic mice exhibited many bloody spots on the cortical area of the kidney (Fig. 1e, f), a phenotype similar to that previously reported [6]. In addition, light microscopy showed that many tubules were filled with red blood cells both in the cortex (Fig. 1g, h) and medulla (Fig. 1i, j). Microaneurysms had formed (Fig. 1k), and hemorrhages were observed in the Bowman’s space of some glomeruli (Fig. 1l). Obvious morphological abnormalities were not observed in other organs of the transgenic animals.
Overexpression of Vegf164 caused phenotypic changes in endothelial cells
Ultrastructural analysis of Podocin-rtTA/TetO-Vegf164 mice by TEM found that endothelial cells with cuboidal structures increased in number within larger capillary lumens (Fig. 2a, b). In dual transgenic mice, fenestrations of endothelial cells were lacking along the peripheral capillary tufts (Fig. 2d). In addition, the subendothelial space was markedly enlarged (Fig. 2d) when compared with that in wild-type mice (Fig. 2c). PV-1, a structural protein required for the formation of the stromal diaphragms of caveolae, is absent in mature glomerular endothelial cells [12], but present in immature [13] or damaged glomerular endothelial cells [14]. Immunofluorescence analysis of this protein revealed universally positive staining of the glomerulus in Podocin-rtTA/TetO-Vegf164 mice, whereas wild-type glomeruli located in the deep cortical area were almost negative (Fig. 2e, f).
Electron microscopy and PV-1 expression in Podocin-rtTA/TetO-Vegf164 mice. Representative transmission electron microscopic images of glomeruli from wild-type (WT) (a, c) and Podocin-rtTA/TetO-Vegf164 mice (Tg) (b, d). In the dual transgenic mice, the glomerular tufts showed microaneurysms (indicated by asterisk in b) and the endothelial cells increased in number (arrows in b). Endothelial fenestration in controls (arrowheads in c) was lost (d), and dilatation of subendothelial spaces (depicted by double asterisk in d) was observed compared with that in wild-type mice. e, f Immunofluorescence for plasmalemmal vesicle-associated protein-1 (PV-1) (green) and CD31 (red) in glomeruli. f Endothelial cells of Podocin-rtTA/Tet-O-Vegf164 mice were positive for PV-1. DAPI (blue) was used for nuclear staining. Scale bars 10 μm in a, b and 500 nm in c, d. Magnification: ×400 (e, f). EC endothelial cell, fp foot process. White lines depict the Bowman’s capsule of the glomerulus in e, f
Although foot process effacement and the absence of a slit diaphragm were observed to a certain degree in the transgenic mice (Fig. 2d), the cell body of the podocytes appeared to be indistinguishable between the dual transgenic and wild-type mice.
Decreased numbers of mesangial cells in the glomeruli of Podocin-rtTA/TetO-Vegf164 mice
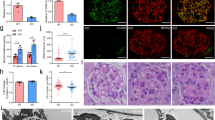
Analysis of the podocyte, endothelial, and mesangial cell populations found that Podocin-rtTA/TetO-Vegf164 mice had similar or slightly fewer positive cells for WT-1, a marker of podocytes, when compared with the normal glomeruli of wild-type mice (Fig. 3a, b). Similarly, the immunoreactivity of CD31, a marker of endothelial cells, was also comparable between dual transgenic and wild-type glomeruli (Fig. 3c, d). In contrast, the intensity of staining with αSMA (Fig. 3e, f), desmin (Fig. 3g, h), and PDGF receptor-β (Fig. 3i, j), all mesangial cell markers, were markedly decreased in the glomeruli of Podocin-rtTA/TetO-Vegf164 mice when compared with that of wild-type mice.
Component cells of glomeruli from wild-type (WT) and Podocin-rtTA/TetO-Vegf164 (Tg) mice. Representative immunostaining for WT-1 (a, b), CD31 (c, d), αSMA (e, f), desmin (g, h), and PDGF receptor-β (i, j). Arrowheads depict marker-negative glomeruli in f, h, j. The percentage of glomeruli positive for αSMA (k), desmin (l), or PDGF receptor-β (m) is shown. Comparison of the αSMA-positive area in glomeruli between phenotype-positive or -negative transgenic mice (n). The horizontal bars and rectangles represent the ranges and the interquartile ranges, respectively. The horizontal bars in the rectangles represent the medians (k–n). White broken lines depict the Bowman’s capsule of the glomerulus in c, d. Magnification: ×200 (a, b, e–j); ×400 (c, d)
Measurement of the percentage of αSMA-positive glomeruli among all glomeruli revealed a significant decrease in Podocin-rtTA/TetO-Vegf164 mice (n = 7, median 51.3%, range 48.2–53.7%) compared with that in wild-type mice (n = 7, median 72.1%, range 63.0–87.5%, p < 0.01; Fig. 3k), and similar results were observed for desmin (n = 7, median 61.7%, range 32.3–86.0% versus n = 7, median 82.4%, range 68.4–88.0%, p < 0.01; Fig. 3l) and for PDGF receptor-β (n = 7, median 67.3%, range 60.4–74.3% versus n = 7, median 80.0%, range 67.3–97.1%, p < 0.01; Fig. 3m).
Next, to examine whether deficiencies in mesangial cells, indeed, affected the formation of glomerular phenotypes, namely, aneurysms and collapsing of the tufts, we evaluated the relationships of αSMA-positive areas with the presence or absence of obvious aneurysms and/or collapsing tufts of the mature glomeruli of transgenic mice. Using univariate analysis, we found that the αSMA-positive area had a significant impact on the formation of phenotypes (odds ratio 90.5; 95% confidence interval 11.9–690.1; p < 0.001). Similarly, the area was significantly lower in phenotype-positive than phenotype-negative glomeruli of transgenic mice (phenotype-negative: median 817 μm2, range 357–2243 μm2, n = 41; phenotype-positive: median 236 μm2, range 8–1151 μm2, n = 38; p < 0.001; Fig. 3n). These results indicated that the glomerular phenotypes were related to a deficiency in mesangial cells in the transgenic mice.
Because defects in mature glomerular basement membrane (GBM), e.g., laminin α5, caused deficiencies in mesangial cells [12], we then examined whether GBM development was impaired in Podocin-rtTA/TetO-Vegf164 mice. Staining for collagen IVα4 (Supplementary Fig. 2a, b), laminin α5 (Supple Fig. 2c, d), and laminin β2 (Supple Fig. 2e, f) revealed that the signals of these mature-type components were compatible between dual transgenic and wild-type mice. However, the structure of the GBM from the Podocin-rtTA/TetO-Vegf164 mice was obviously aberrant (Supple Fig. 2b, d and f), exhibiting a loss of intussusceptive splitting, the process governed by mesangial cells [15].
Expression level of Pdgfb and immunoreactivity of phospho-PDGF receptor-β in glomeruli from Podocin-rtTA/TetO-Vegf164 mice
Because PDGF-B has an essential role in the development of mesangial cells [16], we next evaluated the expression of Pdgfb in the glomerulus by in situ hybridization. The results revealed that the signal was comparable between transgenic (Fig. 4b, d, f) and wild-type mice (Fig. 4a, c, e), indicating that the deficiency in mesangial cells was not attributable to low expression of Pdgfb in transgenic mice.
Expression of Pdgfb in glomeruli and immunostaining for phosphorylated PDGF receptor-β. In situ hybridization analysis for Pdgfb mRNA in glomeruli from wild-type (WT) (a, c, e) and induced Podocin-rtTA/TetO-Vegf164 mice (Tg) (b, d, f) at P0. Broken lines depict the Bowman’s capsule of the glomerulus. Representative glomeruli stained for phosphorylated PDGF receptor-β (g, h), or PDGF receptor-β (i, j) are shown. Images (g, i) or (h, j) are from adjacent sections. Arrowheads in h indicate phosphorylated PDGF receptor-β-negative but PDGF receptor-β-positive (assessed in panels i and j) glomeruli. k Percentage of the phosphorylated PDGF receptor-β-positive glomeruli among all PDGF receptor-β-positive glomeruli in the Podocin-rtTA/Tet-O-Vegf164 mice and wild-type mice. *p < 0.01. Magnification: ×200 (a–d, g–j); ×400 (e, f)
VEGF has been reported to suppress PDGF receptor-β signaling in vascular smooth muscle cells/pericytes, thereby inhibiting vessel maturation and stabilization [17]. Therefore, we next examined the immunoreactivity of phosphorylated PDGF receptor-β-positive glomeruli (Fig. 4g, h) in adjacent sections to those subjected to immunostaining for PDGF receptor-β (Fig. 4i, j). This demonstrated that the percentage of phospho-PDGF receptor-positive glomeruli among all PDGF receptor-β-positive glomeruli was significantly smaller in Podocin-rtTA/TetO-Vegf164 mice (n = 7, median 53.7%, range 42.6–69.2%) than in wild-type mice (n = 7, median 82.1%, range 72.5–85.7%, p < 0.01; Fig. 4k).
Discussion
In this study, we found, for the first time, that Podocin-rtTA/TetO-Vegf164 mice exhibited a deficiency in mesangial cells, in addition to marked hemorrhage, formation of microaneurysms, and endothelial differentiation defects in the glomeruli. These phenotypes during glomerulogenesis resembled those reported by Eremina et al. [6] but were somewhat different from those described by Veron et al. [18]. In a former study, podocyte-specific overexpression of VEGF was driven by a nephrin promoter. Although the expression was not inducible, the induction likely extended over the whole period of glomerulogenesis, and the time-frame of VEGF overexpression was almost comparable to that in the present study [6]. In the latter study, the inducible system used for VEGF overexpression was almost comparable to that in the present study, as well as the time-frame of VEGF induction. Podocytes and their foot processes were predominantly affected in their study, with no direct description of phenotypic changes in mesangial cells [18]. In contrast, the deficiency of mesangial cells was a key feature in the current study, with only minor structural alternations observed in podocytes. These discrepancies may be explained as follows. First, the genetic background of our mice was not the same as that of the previous models [18]. In addition, the exact time-frame and/or the amount of VEGF induction may have differed between the present and previous studies [18].
For the several phenotypes observed in Podocin-rtTA/TetO-Vegf164 mice, red blood cells inside the tubules were prominent. However, the origin of hemorrhage could not be determined, because only a few glomeruli showed hemorrhage in their Bowman’s spaces. Accordingly, the origin of hemorrhage may mainly be the glomerular tufts; however, red blood cells drained into the proximal tubules too rapidly to be detected in Bowman’s spaces in the tissue sections.
PV-1, a key component of caveolar diaphragms, is thought to be important for the formation of endothelial fenestration [19]. However, it is unclear whether PV-1 and/or caveolae are involved in the formation of glomerular endothelial cell fenestrations. Indeed, PV-1 is uniquely absent from glomerular endothelial cell fenestrae [13]. In addition, caveolin-1-deficient mice, which do not form caveolae, nevertheless, had a fully differentiated glomerular endothelium with a fenestrae density similar to that of wild-type controls [20]. There are two possible explanations for linking the aberrant expression of PV-1 and loss of endothelial fenestration in our transgenic mice. First, overexpression of VEGF can attenuate endothelial differentiation. In this regard, endothelial cells in immature glomeruli are characterized by the presence of diaphragmed caveolae positive for PV-1, with fewer fenestrae or transendothelial channels, as reported by Ichimura et al. [14]. Alternatively, glomerular endothelial cells may be damaged by excess VEGF, leading to re-expression of PV-1 in glomerular endothelial cells and loss of fenestrations. Theses phenotypic changes have already been reported in many experimental models and in human proteinuric glomerulopathies, e.g., diabetic nephropathy [15] and transplant glomerulopathy [21].
Mesangial cells in glomerular capillary tufts share many functions with microvessel pericytes [22]. Pericytes are important for vessel maturation and stabilization [23]. In the absence of pericytes, the microvessels leak, hemorrhage, and form multiple microaneurysms. Furthermore, the lack of pericytes causes overproliferation and defects in the differentiation in endothelial cells [24]. Thus, although excess podocyte VEGF acts on glomerular endothelial cells, we can postulate that the glomerular hemorrhage, microaneurysm formation, and defective endothelial differentiation observed in this study may be attributed, as least in part, to deficiencies in mesangial cells. Indeed, we confirmed that the glomerular phenotypes were related to a deficiency in αSMA-positive (and possibly desmin- and PDGFR-β-positive) mesangial cells in the transgenic mice.
A number of growth factors and extracellular matrix components are known to be important for the development of mesangial cells. For example, a null mutation in Pdgfb or its receptor Pdgfrb causes loss of mesangial cells [16, 24]. In addition, lack of the G domain of laminin α5 of the GBM results in the total absence of mesangial cells and defects in the convolution of capillary tufts [12]. In our study, however, the glomerular expression of Pdgfb did not decrease in Podocin-rtTA/Tet-O-Vegf164 mice when compared with that in wild-type littermates. Similarly, laminin α5 was distinctly present along with other mature-type components of the GBM in Podocin-rtTA/TetO-Vegf164 mice.
Mesangial cells express VEGF receptor-2 (Flk1), as do glomerular endothelial cells [25]. In addition, a recent report demonstrated that the VEGF-mediated activation of VEGF receptor-2 suppresses PDGF receptor-β signaling through the assembly of a receptor complex consisting of PDGF receptor-β and VEGF receptor-2 in vascular smooth muscle cells/pericytes, thereby inhibiting vessel maturation and stabilization [17]. We observed a similar phenomenon in this study; although the degree of Pdgfb expression was not decreased, the immunoreactivity of phosphorylated PDGF receptor-β was significantly lower in total PDGF receptor-β-positive glomeruli from Podocin-rtTA/Tet-O-Vegf164 mice than in wild-type mice. Several possible explanations for the inhibition of PDGF-mediated signaling in mesangial cells from Podocin-rtTA/Tet-O-Vegf164 mice can be considered. First, similar to the occurrence in vascular smooth muscle cells/pericytes [17], excess VEGF may suppress PDGF receptor-β-mediated signaling in glomerular mesangial cells. Second, excess VEGF may modulate other signaling cascade factors, such as Notch2 and bone morphogenic proteins (BMPs), which have been reported to regulate the development of mesangial cells [9, 26]. However, the effects of these cascades on PDGF receptor-β-mediated signaling have not yet been clarified.
In conclusion, the phenotype observed following the upregulation of VEGF in podocytes caused marked hemorrhage, microaneurysm formation, and endothelial differentiation defects in a Podocin-rtTA/TetO-Vegf164 mouse model, all of which could be, at least in part, attributed to a deficiency in mesangial cells. Further studies are needed to clarify the mechanisms through which excess VEGF leads to the loss of mesangial cells during glomerulogenesis, a process that is likely to be associated with the pathogenesis of some glomerular diseases in humans.
References
Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9.
Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–45.
Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8.
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5.
Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999;10:2125–34.
Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–16.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34.
Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 2010;77:989–99.
Ueda H, Miyazaki Y, Matsusaka T, Utsunomiya Y, Kawamura T, Hosoya T, Ichikawa I. Bmp in podocytes is essential for normal glomerular capillary formation. J Am Soc Nephrol. 2008;19:685–94.
Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14:1998–2003.
Miyazaki Y, Shimizu A, Pastan I, Taguchi K, Naganuma E, Naganuma E, Suzuki T, Hosoya T, Yokoo T, Saito A, Miyata T, Yamamoto M, Matsusaka T. Keap1 inhibition attenuates glomerulosclerosis. Nephrol Dial Transplant. 2014;29:783–91.
Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–59.
Ichimura K, Stan RV, Kurihara H, Sakai T. Glomerular endothelial cells form diaphragms during development and pathologic conditions. J Am Soc Nephrol. 2008;19:1463–71.
Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–60.
Kikkawa Y, Virtanen I, Miner JH. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol. 2003;161:187–96.
Lindahl P, Hellstrom M, Kalen M, Karlsson L, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–22.
Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–13.
Veron D, Reidy K, Marlier A, Bertuccio C, Villegas G, Jimenez J, Kashgarian M, Tufro A. Induction of podocyte VEGF164 overexpression at different stages of development causes congenital nephrosis or steroid-resistant nephrotic syndrome. Am J Pathol. 2010;177:2225–33.
Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296:F947–56.
Sorensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol. 2002;13:2639–47.
Yamamoto I, Horita S, Takahashi T, Tanabe K, Fuchinoue S, Teraoka S, Hattori M, Yamaguchi Y. Glomerular expression of plasmalemmal vesicle-associated protein-1 in patients with transplant glomerulopathy. Am J Transplant. 2007;7:1954–60.
Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J. 1987;1:272–81.
Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5.
Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–87.
Frank S, Stallmeyer B, Kampfer H, Schaffner C, Pfeilschifter J. Differential regulation of vascular endothelial growth factor and its receptor fms-like-tyrosine kinase is mediated by nitric oxide in rat renal mesangial cells. Biochem J. 1999;338(Pt 2):367–74.
Boyle SC, Liu Z, Kopan R. Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development. 2014;141:346–54.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (Grant number: 23591202 to Y. M.) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT). We thank Yamato Kikkawa (Tokyo University of Pharmacy and Life Sciences) for providing the anti-collagen IV antibodies and anti-laminin antibodies. We thank Mitsuko Tamatsukuri and Moeno Ishida for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflicts of interest exist.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (approval number: 24-004).
Informed consent
This section is not applicable to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10157_2017_1450_MOESM1_ESM.pdf
Supplementary material 1 (PDF 1533 kb) Supplementary Fig. 1. Histological comparison of kidney tissues between wild-type and Podocin-rtTA single transgenic mice at P0. (a–h) There were no appreciable differences between wild-type and Podocin-rtTA mice in staining with hematoxylin and eosin (a, b); anti-α-smooth muscle actin (αSMA), a marker for mesangial cells (c, d); CD31 (e, f), a marker for endothelial cells; and anti-Wilm’s tumor (WT-1), a marker for podocytes (g, h). (i–l) Paraformaldehyde-fixed paraffin-embedded specimens were reprocessed for the electron microscopy. Ultrastructures of the basement membrane (depicted by * in k, l), epithelial foot process (fp), and endothelial fenestration (black arrowhead) were intact in both mouse lines. Magnification: 200 × (a–h), scale bars = 10 μm in (i, j) and 500 nm in (k, l)
10157_2017_1450_MOESM2_ESM.pdf
Supplementary material 2 (PDF 369 kb) Supplementary Fig. 2. Component structures of the glomerular basement membrane from wild-type (WT) and Podocin-rtTA/TetO-Vegf164 (Tg) mice. Representative immunostaining for collagen IVα4 (a, b), laminin α5 (c, d), and laminin β2 (e, f) of WT (a, c, and e) and Tg (b, d, and f) mice at P0. Magnification: 400×. Rabbit anti-collagen IVα4 and rabbit anti-laminin α5 and β2 antibodies were kindly provided by Mr. Yamato Kikkawa (School of Pharmacy, Tokyo University of Pharmacy and Life Science, Tokyo, Japan) [15]
About this article
Cite this article
Suyama, M., Miyazaki, Y., Matsusaka, T. et al. Forced expression of vascular endothelial growth factor-A in podocytes decreases mesangial cell numbers and attenuates endothelial cell differentiation in the mouse glomerulus. Clin Exp Nephrol 22, 266–274 (2018). https://doi.org/10.1007/s10157-017-1450-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1450-5