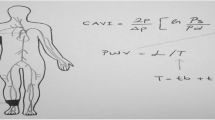
Abstract
Background
Arterial stiffness is an established marker of cardiovascular risk and an independent predictor of cardiovascular disease (CVD) events and mortality in kidney transplant (KT) patients. Adipocyte fatty acid-binding protein (A-FABP), a novel adipokine, is positively associated with atherosclerosis. The present study evaluated the relationship between fasting circulating A-FABP and peripheral arterial stiffness using the cardio-ankle vascular index (CAVI) in KT patients.
Methods
Fasting blood samples were collected from 74 KT patients, and serum A-FABP levels were measured using an enzyme immunoassay. CAVI was calculated using a waveform device (CAVI-VaSera VS-1000). The cutoff values for high and low levels of arterial stiffness were defined by the CAVI values of ≥9 and <9, respectively.
Results
Thirty-four patients (45.9%) were classified into the high arterial stiffness group. Compared with the low arterial stiffness group, the high arterial stiffness group had higher values for age (p = 0.015), systolic blood pressure (p < 0.001), pulse pressure (p < 0.001), duration of kidney transplantation (p = 0.005), serum total cholesterol and triglyceride levels (p = 0.033 and 0.047, respectively), glomerular filtration rate (p = 0.019), fasting glucose levels (p = 0.012), and serum A-FABP levels (p < 0.001). Multivariate forward stepwise linear regression analysis showed that age (p = 0.004), systolic blood pressure (p = 0.001), and serum A-FABP levels (p = 0.003) were independent predictors of CAVI value in KT patients.
Conclusion
Serum fasting A-FABP level is positively associated with peripheral arterial stiffness in KT patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipocyte fatty acid-binding protein (A-FABP; also called fatty acid-binding protein 4) is expressed in the cytoplasm of mature adipocytes and macrophages and is involved in metabolic responses and inflammatory processes [1, 2]. A-FABP affects not only insulin sensitivity, lipid metabolism, and lipolysis but also plays a key role in atherosclerosis [3]. Several studies of human subjects have shown that serum A-FABP levels can predict the development of metabolic syndrome and is independently associated with carotid intima-media thickness [4, 5].
Increased peripheral and central arterial stiffness has been identified at every stage in patients with chronic kidney disease as well as in kidney transplant (KT) patients. Although the impact of arterial stiffness on cardiovascular (CV) prognosis is widely acknowledged, the mechanism of evolving arterial stiffness and clinical applications of remodeling biomarkers after kidney transplantation remain controversial. Currently, carotid–femoral pulse wave velocity (PWV) measurements are proved to improve the prediction of cardiovascular disease (CVD) risk in KT patients [6,7,8].
Cardio-ankle vascular index (CAVI), introduced in 2004, is a noninvasive evaluation of arterial stiffness [9]. In this application, the main advantages of CAVI are the blood pressure (BP) independence of arterial elasticity, extension of the ascending aorta to peripheral arteries, and a combination of simplicity and cost reduction. Studies of the association between serum A-FABP levels and arterial stiffness measured with CAVI in KT patients are still rare despite the wide application of CAVI and A-FABP in various populations.
Materials and methods
Patients
Seventy-four KT patients, including 40 men and 34 women aged 27–75 years, were recruited between February and April 2012 at one medical center in Hualien, Taiwan. Trained staff performed BP measurements in the morning for all participants using standard mercury sphygmomanometers with suitable cuff sizes after the participants had been at rest for at least 10 min. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured at the points of appearance and disappearance of Korotkoff sounds, respectively. SBP and DBP were measured three times at 5-min intervals and were averaged for analysis. In the prevalence survey, hypertension was defined as SBP ≥140 mmHg or DBP ≥90 mmHg, or both, or by the use of antihypertensive medication within the past 2 weeks. Pulse pressure was calculated by subtracting DBP from SBP.
Subjects were considered diabetic if fasting plasma glucose levels were 126 mg/dl or more or they were using diabetes medication (oral or insulin) [10]. Patients were excluded if they had malignancy, acute rejection, acute myocardial infarction, pulmonary edema, congestive heart failure, acute infection status at the time of blood sampling, or an arteriovenous shunt or graft in a limb. Those who refused to provide informed consent were also excluded. The study was approved by the Protection of Human Subjects Institutional Review Board of Buddhist Tzu Chi General Hospital in Hualien, Taiwan (IRB No. 100-91).
Anthropometric analysis
Body height was measured to the nearest half-centimeter, and waist circumference was measured to the nearest half-centimeter at the shortest point below the lower rib margin and the iliac crest. Body weight was measured to the nearest half-kilogram with the patient in light clothing and without shoes. Body mass index (BMI) was calculated as weight (kilograms) divided by height squared (meters).
Biochemical determinations
For measuring complete blood cell count (Sysmex K-1000; Bohemia, NY, USA) and other parameters, fasting blood samples of approximately 5 ml were obtained and immediately centrifuged at 3000×g for 10 min after collection. At the same time, the other 5-ml serum samples were stored at 4 °C and used for biochemical analyses within 1 h of collection. Serum levels of blood urea nitrogen (BUN), creatinine (Cre), total cholesterol (TCH), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting glucose, total calcium, and phosphorus were measured using an autoanalyzer (COBAS Integra 800; Roche Diagnostics, Basel, Switzerland). We estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease Study equation. Serum intact parathyroid hormone levels (Diagnostic Systems Laboratories, Webster, TX, USA) were measured using a commercially available enzyme-linked immunosorbent assay [11, 12]. Serum A-FABP levels were measured using a commercially available enzyme immunoassay (SPI-BIO, Montigny le Bretonneux, France) [13,14,15].
CAVI measurements
CAVI measurements were performed in a quiet, temperature-controlled room after patients had been at rest for 10 min as recommended previously. CAVI values were obtained using a waveform device (VaSera VS-1000; Fukuda Denshi Co. Ltd., Tokyo, Japan) according to the manufacturer’s instructions. Briefly, a cuff was applied to the bilateral upper arms and ankles with the participant supine and the head in the midline position. Then, we placed the phonocardiography microphone and electrocardiography electrodes. The device measured BP and PWV, and the CAVI values were calculated automatically. According to the manufacturer’s instructions, a CAVI less than 8.0 is supposed to be normal, whereas a value less than 9.0 but more than (or equal to) 8.0 is considered “borderline”. At the other end of the spectrum, a CAVI equal or more than 9.0 leads to the diagnosis of suspected arteriosclerosis [16]. In this study, the high arterial stiffness group comprised subjects with CAVI values of ≥9, and those with CAVI values of < 9 composed the low arterial stiffness group.
Statistical analysis
Data were tested for normal distribution using the Kolmogorov–Smirnov test. Normally distributed data were expressed as mean ± standard deviation, and comparisons between patient groups were performed using the Student’s independent t test (two tailed). Data that were not normally distributed were expressed as medians and interquartile ranges, and comparisons of parameters (hemoglobin, TG, fasting glucose, BUN, Cre, and iPTH) between patients were performed using the Mann–Whitney U test. Data expressed as the number of patients were analyzed with the χ 2 test. Clinical variables that correlated with CAVI values in KT patients were evaluated with univariate linear regression analyses. Variables that were significantly associated with CAVI values were tested for independence with a multivariate forward stepwise regression analysis. Data were analyzed using SPSS for Windows (version 19.0; SPSS, Inc., Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
Results
The demographics, clinical characteristics, biochemical data, and comorbidities of the 74 participants are shown in Table 1. The mean age was 52.1 years, 54% of the participants were male, the mean dialysis and KT duration were 74.0 and 72.2 months, respectively, and the mean BMI was 23.74 kg/m2. According to the cutoff CAVI values, 45.9% of patients (n = 34) were placed in the high arterial stiffness group (CAVI ≥9) and 54.1% (n = 40) were placed in the low arterial stiffness group (CAVI <9). Values for age, KT duration, pulse pressure, TCH, TG, fasting glucose, eGFR, and serum A-FABP differed significantly between these groups of KT patients.
The KT patients were prone to have higher arterial stiffness if they were older (p = 0.015) and had longer post-transplantation duration (p = 0.005); higher SBP (p < 0.001), pulse pressure (p < 0.001), TCH levels (p = 0.033), TG levels (p = 0.047), and fasting glucose levels (p = 0.012); and lower eGFR (p = 0.019). The mean level of serum fasting A-FABP was 40.24 ng/ml, and compared with A-FABP levels in patients with low arterial stiffness (29.72 ng/ml), those in patients with high arterial stiffness (52.61 ng/ml) were significantly higher (p < 0.001). The linear regression analysis of serum A-FABP and eGFR showed moderately negative correlation (r = −0.349; p = 0.002), and the two-dimensional scattered plots of serum A-FABP levels and eGFR are shown in Fig. 1.
The prevalence of diabetes, hypertension, and smoking was 59.5% (n = 44), 27% (n = 20), and 9.5% (n = 7), respectively. Compared with patients in the low arterial stiffness group, KT patients with hypertension had higher arterial stiffness than those without hypertension (p < 0.001). The CVD events occurred in three patients of which one was myocardial infarction, another was myocardial infarction with heart failure and the other was acute coronary syndrome during the post-KT period, and the incidence of CVD events is 11.23 per 1000 person-years. All three patients with CVD events are within high arterial stiffness group. The immunosuppressive agents in current use by the subjects included tacrolimus (n = 43; 58.1%), mycophenolate mofetil or mycophenolic acid (n = 53; 71.6%), steroids (n = 60; 81.1%), rapamycin (n = 14; 18.9%), and cyclosporine (n = 18; 24.3%). No significant differences were observed between the high and low arterial stiffness groups with respect to sex, donor type of transplantation, CVD events, rejection events, infection events, or use of immunosuppressive agents, statins, or fibrate (Table 2).
The results of univariate linear regression analyses and multivariate stepwise linear regression analysis of CAVI values (adopted factors: hypertension, age, SBP, pulse pressure, post-transplantation duration, TCH, logarithmically transformed TG [log-TG], log-glucose, eGFR, and A-FABP) are shown in Table 3. Hypertension (r = 0.459; p < 0.001), advanced age (r = 0.411; p < 0.001), SBP (r = 0.474; p < 0.001), pulse pressure (r = 0.422; p < 0.001), TCH (r = 0.320; p = 0.005), and A-FABP levels (r = 0.519; p < 0.001) were positively correlated with CAVI values. Finally, the multivariate stepwise linear regression analysis of the factors correlated with CAVI values showed that age (β = 0.282; p = 0.004), SBP (β = 0.332; p = 0.001), and serum A-FABP levels (β = 0.314; p = 0.003) were independent predictors of CAVI values in KT patients.
Discussion
CAVI, an arterial stiffness index, was developed in 2004 by combining the stiffness parameter β and the Bramwell–Hill formula [9, 17, 18]. CAVI is a quantitative measurement of vascular wall stiffness in the aorta and lower limb arteries using heart–femoral PWV and BP. CAVI reportedly correlates with CV risk markers such as intimal-medial thickening and coronary atherosclerosis [19]. Several clinical and experimental studies also showed that CAVI values were independent of BP at the time of measurement [9, 19,20,21]. Therefore, the acute and chronic effects of high BP on arterial stiffness during measurement should be considered differently. Lim et al. [22] described the impact of BP on arterial stiffness measurement under acute physiological and psychological interventions, and both β stiffness index and CAVI were somewhat dependent on BP changes. Ibata et al. [23] found that CAVI is independent of SBP and can indicate early arteriosclerosis in subjects with diabetes who do not have hypertension. However, the results of the multivariate logistics regression analysis in the present study demonstrated that CAVI value was dependent on SBP. Our study population included 20 hypertensive patients (27%), most of whom (19/20) were in the high-CAVI group. This outcome can likely be attributed to the chronic effects of high BP rather than acute psychological stress during examination, and it also agrees with several reports indicating that patients with chronic hypertension have high CAVI values [24, 25].
Medications for BP control are sophisticated and tailored to individual patients by physicians according to etiology, adherence, and other factors. Currently, the effects of angiotensin-II receptor antagonists are more significant than those of calcium channel blockers in reducing CAVI values [26], but there are no data available on reductions in CAVI values related to the use of other antihypertensive medications such as alpha-blockers, beta-blockers, diuretics, and spironolactone. The design of the present study did not focus on the effects of antihypertensive agents or evaluate the effects of BP control itself on arterial stiffness as determined with CAVI.
The age was an independent factor that predicted CAVI values in the present study. In a large-scale study conducted by Namekata et al. [27] in healthy Japanese individuals, average age-specific baseline CAVI scores were increasing by age advancing in the CV disease risk-free group linearly regardless of gender. In the low arterial stiffness group of the present study, the average age was 49.6 years and the mean CAVI value was 8.02, and these values corresponded to the age-specific baseline scores of the healthy group in the study by Namekata et al. [27]. By comparison, patients in the high arterial stiffness group had an average age of 55.0 years and a mean CAVI value of 10.47. Our results exceeded the upper limit of age-specific baseline scores and also reflected the severity of arterial stiffness in the enrolled patients regardless of age. The clinical threshold of CAVI using a value of 9.0 is based on evidence that CAVI is derived from heart–femoral PWV.
Metabolic syndrome is a cluster of multiple risk factors for atherosclerosis, and some of its components are associated with the severity of arterial stiffness. However, the association between arterial stiffness measured by CAVI and dyslipidemia, which is one component of metabolic syndrome, remains controversial, contrary to associations with fasting glucose levels and blood pressures [19, 28, 29]. Compared with patients without CV risk factors, men older than 30 years and women older than 40 years who have CV risk factors such as hypercholesterolemia and hypertriglyceridemia have significantly higher CAVI values [27]. In the present study, the levels of serum TCH and TG were significantly higher in the high arterial stiffness group than the low arterial stiffness group regardless of statin and fibrate usage. This outcome may be attributable to clinical features of patients in the high arterial stiffness groups, including longer post-transplantation duration with exposure to immunosuppressants (87.18 versus 59.45 months), relatively low eGFR (39.85 versus 54.10 ml/min), and long-term exposure to steroids in most patients (81.1%). Although serum TCH and log-TG values had no significant effects on CAVI in the multivariate logistic regression analysis, we cannot discount the trend of increasing dyslipidemia during long-term post-KT care.
A-FABP is a member of the fatty acid-binding protein superfamily that is expressed prominently in adipocytes and macrophages. A-FABP is an abundant cytosolic protein in mature adipocytes, accounting for ~6% of total cellular proteins, and also secreted into the bloodstream [30]. Previous studies in animals and humans demonstrated that A-FABP might play a crucial role in the development of insulin resistance, type 2 diabetes, atherosclerosis, and metabolic syndrome through involvement in metabolic and inflammatory pathways in adipocytes and macrophages [31,32,33]. Several studies, including those with cross-sectional and prospective designs, showed that serum A-FABP levels are positively and significantly correlated with several key components of metabolic syndrome, including abnormal lipid profiles, hyperglycemia, insulin resistance, and hypertension, regardless of gender, age, or adiposity in various ethnic groups [4, 11, 34, 35]. A retrospective study in Japanese patients with end-stage renal disease who were undergoing dialysis showed that the serum A-FABP level was 20 times higher than normal and as a novel predictor of CV mortality in patients at high-risk atherosclerotic events [36]. In the present cross-sectional study of KT patients, serum A-FABP levels in the high arterial stiffness group were significantly higher than those in the low arterial stiffness regardless of gender, presence of diabetes, transplantation model, and immunosuppressant and statin use. We also found that, compared with the low arterial stiffness group, the high arterial stiffness group had a lower eGFR (54.10 versus 39.85 ml/min/1.73 m2; p = 0.019) and longer post-transplantation duration (59.45 versus 87.18 months; p = 0.005). The mean eGFR of patients in both groups remained within the limits of stage 3 chronic kidney disease (CKD) (30–59 ml/min/1.73 m2), and the de novo renal disease developed gradually after transplantation that may meet the current status of post-transplant renal function. Additionally, the negative correlation between serum A-FABP and eGFR was also found in our study. We assumed that the arterial stiffness and CKD might simultaneously contribute to elevation of serum A-FABP level with uncertain proportions. As other adipokines, the serum A-FABP level might be determined by elimination function of kidney and significant elevation in all grades of CKD patients have been proved [36,37,38]. Although ectopic A-FABP expression in endothelial cells and macrophage of glomerulus has been found with association with degree of proteinuria and renal dysfunction in CKD patients, the mechanism and causal relationship between the serum A-FABP level and renal dysfunction have not been well established [39]. In accordance with the study for patients with stable angina pectoris, serum A-FABP level is independently associated with CKD and might have significant role in the interaction between renal dysfunction and development vascular atherosclerosis [40].
A-FABP is secreted into the bloodstream by adipocytes and macrophages, and also found locally in human atherosclerotic plaques. This association with the high-risk phenotype of atherosclerotic plaques demonstrates that the presence of A-FABP is associated with inflammatory and vulnerable plaques [41, 42]. Recently, local production of A-FABP in perivascular fat and macrophages within vascular plaques was found to be associated with coronary atherosclerosis as shown by the results of histopathologic examinations during autopsies and in human studies [43]. In the present study, the average BMIs in the high and low arterial stiffness groups were 24.02 and 23.5 kg/m2, respectively, without overt obesity, but the difference in serum A-FABP levels was significant (52.61 versus 29.72 ng/m, respectively). High serum A-FABP levels might be reflected not only in the greater atherosclerotic change in vessels that results in high CAVI values but also in the aggravated inflammatory activity of macrophages in vascular plaques, which increases CV risk.
The present study had several limitations. First, we enrolled a limited number of KT patients and had no case-matched control group, which may have resulted in selection bias. Second, the study had a cross-sectional design, and the results must be investigated further with a long-term cohort study before a causal correlation between serum A-FABP levels and arterial stiffness in KT patients can be established. Third, the observational design of the study may not allow us to draw conclusions about the mechanism of the statistical association between serum A-FABP levels and CAVI values. Moreover, the relationship between serum A-FABP levels and metabolic/inflammatory activity in arterial stiffness was not observed.
Conclusions
The results of the present study demonstrated that increased serum A-FABP levels quantified with CAVI were significantly associated with greater arterial stiffness in KT patients. Our results indicate that the measurement of serum A-FABP levels will be helpful in evaluating the risk of arterial stiffness in KT patients. The variability of serum A-FABP levels related to renal function after kidney transplantation should be investigated further.
References
Makowski L, Hotamisligil GS. Fatty acid binding proteins—the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004;134(9):2464S–8S.
Krusinova E, Pelikanova T. Fatty acid binding proteins in adipose tissue: a promising link between metabolic syndrome and atherosclerosis? Diabetes Res Clin Pract. 2008;82(Suppl 2):S127–34.
Boord JB, Fazio S, Linton MF. Cytoplasmic fatty acid-binding proteins: emerging roles in metabolism and atherosclerosis. Curr Opin Lipidol. 2002;13(2):141–7.
Xu A, Tso AW, Cheung BM, Wang Y, Wat NM, Fong CH, et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007;115(12):1537–43.
Yeung DC, Xu A, Cheung CW, Wat NM, Yau MH, Fong CH, et al. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(8):1796–802.
Delahousse M, Chaignon M, Mesnard L, Boutouyrie P, Safar ME, Lebret T, et al. Aortic stiffness of kidney transplant recipients correlates with donor age. J Am Soc Nephrol. 2008;19(4):798–805.
Kneifel M, Scholze A, Burkert A, Offermann G, Rothermund L, Zidek W, et al. Impaired renal allograft function is associated with increased arterial stiffness in renal transplant recipients. Am J Transplant. 2006;6(7):1624–30.
Hornum M, Clausen P, Idorn T, Hansen JM, Mathiesen ER, Feldt-Rasmussen B. Kidney transplantation improves arterial function measured by pulse wave analysis and endothelium-independent dilatation in uraemic patients despite deterioration of glucose metabolism. Nephrol Dial Transplant. 2011;26(7):2370–7.
Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101–7.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53.
Chen YC, Lee MC, Lee CJ, Ho GJ, Yin WY, Chang YJ, et al. N-terminal pro-B-type natriuretic peptide is associated with arterial stiffness measured using the cardio-ankle vascular index in renal transplant recipients. J Atheroscler Thromb. 2013;20(7):646–53.
Lee CJ, Wang JH, Chen ML, Yang CF, Chen YC, Hsu BG. Serum osteoprotegerin is associated with arterial stiffness assessed according to the cardio-ankle vascular index in hypertensive patients. J Atheroscler Thromb. 2015;22(3):304–12.
Hsu BG, Chen YC, Lee RP, Lee CC, Lee CJ, Wang JH. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ J. 2010;74(2):327–31.
Tsai JP, Liou HH, Liu HM, Lee CJ, Lee RP, Hsu BG. Fasting serum fatty acid-binding protein 4 level positively correlates with metabolic syndrome in hemodialysis patients. Arch Med Res. 2010;41(7):536–40.
Tsai JP, Wang JH, Lee CJ, Chen YC, Hsu BG. Positive correlation of serum adipocyte fatty acid binding protein levels with carotid-femoral pulse wave velocity in geriatric population. BMC Geriatr. 2015;15:88.
Fukuda-Denshi Company, Tokyo, Japan (2017). http://www.fukuda.co.jp/english/products/special_features/vasera/cavi.html. Accessed 20 March 2017.
Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res. 1987;21(9):678–87.
Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc Lond Ser B Contain Pap Biol Character. 1922;93(652):298–306.
Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18(11):924–38.
Shirai K, Song M, Suzuki J, Kurosu T, Oyama T, Nagayama D, et al. Contradictory effects of beta1- and alpha1-aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)–CAVI independent of blood pressure. J Atheroscler Thromb. 2011;18(1):49–55.
Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, et al. Cardio-ankle vascular index is a new noninvasive parameter of arterial stiffness. Circ J. 2007;71(11):1710–4.
Lim J, Pearman ME, Park W, Alkatan M, Machin DR, Tanaka H. Impact of blood pressure perturbations on arterial stiffness. Am J Physiol Regul Integr Comp Physiol. 2015;309(12):R1540–5.
Ibata J, Sasaki H, Kakimoto T, Matsuno S, Nakatani M, Kobayashi M, et al. Cardio-ankle vascular index measures arterial wall stiffness independent of blood pressure. Diabetes Res Clin Pract. 2008;80(2):265–70.
Okura T, Watanabe S, Kurata M, Manabe S, Koresawa M, Irita J, et al. Relationship between cardio-ankle vascular index (CAVI) and carotid atherosclerosis in patients with essential hypertension. Hypertens Res. 2007;30(4):335–40.
Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, et al. Cardio-ankle vascular index is superior to brachial-ankle pulse wave velocity as an index of arterial stiffness. Hypertens Res. 2008;31(7):1347–55.
Bokuda K, Ichihara A, Sakoda M, Mito A, Kinouchi K, Itoh H. Blood pressure-independent effect of candesartan on cardio-ankle vascular index in hypertensive patients with metabolic syndrome. Vasc Health Risk Manag. 2010;6:571–8.
Namekata T, Suzuki K, Ishizuka N, Shirai K. Establishing baseline criteria of cardio-ankle vascular index as a new indicator of arteriosclerosis: a cross-sectional study. BMC Cardiovasc Disord. 2011;11:51.
Soska V, Frantisova M, Dobsak P, Dusek L, Jarkovsky J, Novakova M, et al. Cardio-ankle vascular index in subjects with dyslipidaemia and other cardiovascular risk factors. J Atheroscler Thromb. 2013;20(5):443–51.
Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, Recio-Rodriguez JI, Fernando R, Marti R, et al. Association of metabolic syndrome and its components with arterial stiffness in Caucasian subjects of the MARK study: a cross-sectional trial. Cardiovasc Diabetol. 2016;15(1):148.
Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006;52(3):405–13.
Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, et al. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110(11):1492–8.
Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118(7):2640–50.
Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15(12):1383–91.
Mohlig M, Weickert MO, Ghadamgadai E, Machlitt A, Pfuller B, Arafat AM, et al. Adipocyte fatty acid-binding protein is associated with markers of obesity, but is an unlikely link between obesity, insulin resistance, and hyperandrogenism in polycystic ovary syndrome women. Eur J Endocrinol. 2007;157(2):195–200.
Stejskal D, Karpisek M. Adipocyte fatty acid binding protein in a Caucasian population: a new marker of metabolic syndrome? Eur J Clin Invest. 2006;36(9):621–5.
Furuhashi M, Ishimura S, Ota H, Hayashi M, Nishitani T, Tanaka M, et al. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS One. 2011;6(11):e27356.
Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13(1):134–41.
Ebert T, Hopf LM, Wurst U, Bachmann A, Kralisch S, Lossner U, et al. Circulating adipocyte fatty acid binding protein is increased in chronic and acute renal dysfunction. Nutr Metab Cardiovasc Dis. 2014;24(9):1027–34.
Tanaka M, Furuhashi M, Okazaki Y, Mita T, Fuseya T, Ohno K, et al. Ectopic expression of fatty acid-binding protein 4 in the glomerulus is associated with proteinuria and renal dysfunction. Nephron Clin Pract. 2014;128(3–4):345–51.
Iwamoto M, Miyoshi T, Doi M, Takeda K, Kajiya M, Nosaka K, et al. Elevated serum adipocyte fatty acid-binding protein concentrations are independently associated with renal dysfunction in patients with stable angina pectoris. Cardiovasc Diabetol. 2012;11:26.
Agardh HE, Folkersen L, Ekstrand J, Marcus D, Swedenborg J, Hedin U, et al. Expression of fatty acid-binding protein 4/aP2 is correlated with plaque instability in carotid atherosclerosis. J Intern Med. 2011;269(2):200–10.
Peeters W, de Kleijn DP, Vink A, van de Weg S, Schoneveld AH, Sze SK, et al. Adipocyte fatty acid binding protein in atherosclerotic plaques is associated with local vulnerability and is predictive for the occurrence of adverse cardiovascular events. Eur Heart J. 2011;32(14):1758–68.
Furuhashi M, Fuseya T, Murata M, Hoshina K, Ishimura S, Mita T, et al. Local Production of Fatty Acid-Binding Protein 4 in Epicardial/Perivascular Fat and Macrophages Is Linked to Coronary Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(5):825–34.
Acknowledgements
This study was supported by a Grant from Buddhist Tzu Chi General Hospital, Hualien, Taiwan (TCRD-101-06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number 100-91) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Chen, YC., Hsu, BG., Lee, CJ. et al. Serum adipocyte fatty acid-binding protein level is associated with arterial stiffness quantified with cardio-ankle vascular index in kidney transplant patients. Clin Exp Nephrol 22, 188–195 (2018). https://doi.org/10.1007/s10157-017-1438-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1438-1