Abstract
Background
Severe acute kidney injury (AKI) is associated with chronic kidney disease (CKD), cardiovascular events and increased mortality. However, little is known about the prognosis in hospitalized population suffering from non-severe AKI episodes. The aim of this study is to determine the impact of non-severe AKI episodes in cardiovascular events, mortality and CKD, on short and long term.
Methods
Retrospective cohort study to 360 patients who met the criteria for diagnosis of AKI according ADQI guidelines with full recovery of renal function after the AKI episode, admitted between January 2000 and December 2010 in our hospital. Follow-up was 4 years after the diagnosis of AKI. Covariates included demographic variables, baseline creatinine and diagnosis of comorbidities.
Results
360 AKI survivor patients were included. Twenty five of them (6.7%) had developed CKD after 1-year follow-up. Hypertension (OR 1.62; 95% CI 1.2–2.6, p < 0.05) and serum creatinine >2.6 mg/dL in AKI (OR 1.7; 95% CI 1.2–3.7, p < 0.05) were independent risk factors. After 4-year follow-up, 40 patients (18.3%) had developed CKD; age >66 years was an independent risk factor (OR 1.03, 95% CI 1.03–1.06, p < 0.05). Mortality rate at 4 years was 25.3% and was significantly higher in CKD patients (OR 4.3, 95% CI 1.13–4.90, p < 0.05) and patients >66 years (OR 1.12, 95% CI 1.02–1.06, p < 0.05). The incidence of cardiovascular events also was higher in CKD patients than in non-CKD patients (62.7 vs. 21.7%, p < 0.05).
Conclusion
Even after fully recovered non-severe AKI episodes, some patients develop CKD and those have an increase in the incidence of cardiovascular events and long-term mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Acute kidney injury has an estimated incidence of 14.9–49.6 per 1000 patient years and may affect 13–18% of hospitalized patients. The incidence is increasing, and it is expected to double over the next decade [1,2,3].
AKI has been considered a self-limiting disease, with good prognosis when recovery is noted during admission [4], but several studies demonstrated that survivors of AKI may experience considerable late decline in kidney function. Recent studies suggested that AKI episodes are associated with higher risk of developing chronic kidney disease (CKD) cardiovascular events and overall mortality [5,6,7,8,9,10,11,12,13]. Furthermore, different risk factors for CKD progression have been identified: advanced age, diabetes mellitus (DM), hypertension, heart failure, pre-existing CKD, basal renal function before AKI and severity of AKI episode [8, 9, 14,15,16,17,18]. Most of the studies recruit severe forms of AKI with high incidence of dialysis-requiring AKI, excluding elderly patients or non-severe AKI episodes [6, 14, 19,20,21,22,23], which represent the majority of cases in daily clinical practice. Little information is available concerning renal outcomes and prognosis in this subset. Moreover, the optimal care following AKI in these patients remains controversial, and the transition point of care may be an opportunity to prevent long-term loss of kidney function and its effects [24]. Siew et al. [25] demonstrated that few AKI survivor patients are referred for follow-up nephrology care.
The aim of this study is to determine, in a cohort of patients with non-severe AKI, risk factors for developing CKD, cardiovascular events and mortality, in the short-term (1-year) and long-term (4-year) follow-up.
Methods
Study design and data collection
A retrospective study was designed in which systematic chart review, using hospital’s coding system, identified all patients admitted to the University Hospital del Mar, Barcelona, Spain, between January 2000 and December 2010 with International Classification of Diseases (ICD-9) of AKI or acute tubular necrosis.
Inclusion criteria were age >18 years and full recovery of renal function at discharge. Patients were excluded if they were renal allograft recipients, prior known CKD, cases with complete remission of serum creatinine (SCr) increase before 48 h with fluid reposition without evidence of kidney damage (urine microscopy normal) or post-renal etiologies, and those patients without data of baseline SCr.
We initially identified patients with diagnosis of AKI or acute tubular necrosis by ICD-9 in chart review, and additionally we confirmed the diagnosis of AKI according ADQI criteria [26]. Patients were included if fulfill ADQI criteria as any of the following: increase in SCr by *0.3 mg/dL (*26.5 mmol/L) within 48 h; or increase in SCr to *1.5 times baseline. AKI-patients were assigned to their worst RIFLE category. Briefly, patients were classified into the “risk” category if SCr increased 1.5-fold, or glomerular filtration rate (GFR) decreased 25%; “injury” if SCr increased twofold or GFR decreased 50% and “failure” if SCr increased threefold or GFR decreased 75%. The outcome criteria of loss of renal function and ESRD were defined by the duration of AKI episode. We considered as non-severe AKI those patients classified as Risk, Injury or Failure using ADQI classification.
Baseline renal function was defined as the lowest SCr value during the 3 months before admission either in our center or in the relevant basic health area. Recovery of renal function was defined when SCr levels improved to be equal or lower that the value taken as a reference to define baseline renal function.
Following the ADQI group recommendations [26], patients were included in the study 48 h after AKI diagnosis to ensure that patients achieved a correct hemodynamic status, avoiding prerenal causes of AKI; obstructive causes of AKI were excluded with imaging techniques. The etiology of AKI was determined by chart review, classifying AKI-patients in four different categories according to the trigger event: Septic AKI, Nephrotoxic AKI, and Ischemic AKI. The patient was classified as “Others” if the etiology of AKI was multifactorial.
Demographic data were collected, along with past medical history of diabetes mellitus (DM), hypertension, ischemic heart disease and peripheral vascular disease (PVD). The follow-up period after the AKI episode was recorded up to 4 years. Clinical and laboratory data were recorded. The occurrence of CKD was defined according KDIGO guidelines, as a decreased GFR (GFR ≤60 mL/min/1.73 m2) present for 3 months [27]. Cardiovascular events (acute coronary event, heart failure, stroke, transient ischemic attack, acute arterial occlusion or deep venous thrombosis with pharmacological and/or endovascular intervention) and mortality were assessed. Multiple organ failure was defined as dysfunction in an organ or 2 or more organs [28]. New episodes of AKI were defined as any increase in SCr by *0.3 mg/dL or >* 1.5 times the baseline SCr the number of AKI episodes, etiology and severity were recorded.
Statistical analysis
Descriptive analysis included the absolute and relative frequencies for qualitative variable as well as the mean and standard deviation (SD) or median and interquartile range (IQR) for quantitative variable. Bivariate comparisons of subjects who did or did not develop CKD were made using t tests, Chi-squared tests, and Fisher’s exact tests as appropriate. Overall rates of CKD, cardiovascular events and death were compared across patient groups by Chi-squared test.
Multivariable models were constructed by applying backwards elimination to a set of candidate predictors until all predictors remaining had p values less than 0.05. The variables included in the models were selected in basis to clinical expertise and literature review. Data are expressed as odds ratio (ORs) and 95% confidence intervals (CIs).
Statistical analysis was performed using SPSS V 21.0(SPSS Inc., Chicago, IL). A p value of less than 0.05 was considered statistically significant.
Results
Study population
Between January 2000 and December 2010, 3492 patients were identified as AKI or acute tubular necrosis by International Classification of Diseases (IC D-9) in our Hospital. Of these, 456 patients had renal function data prior to the episode of AKI and met the criteria for diagnosis of AKI according ADQI guidelines (Fig. 1) [25, 26]. 360 fulfilled the inclusion criteria: 218 patients (60.6%) were men, the mean age was 64.7 years (SD ± 20 years) and 96.1% were Caucasian. The most frequent comorbid condition was hypertension in 190 patients (52.8%) followed by type 2 DM in 100 patients (24.8%).
Mean baseline SCr was 0.99 mg/dL (SD ± 0.35 mg/dL). Mean SCr during AKI episode was 3.4 mg/dL (p25: 1.90 mg/dL; p50: 2.6 mg/dL p75: 4.20 mg/dL). Most of the patients (64.7%) were included between “injury” or “failure” groups, 120 patients (33.3%) and 113 (31.4%), respectively. The duration of AKI was 35 days (SD ± 72) and thirty-six patients (10%) needed intermittent and/or continuous renal replacement therapy. Among the global cohort, 12 (3.3%) patients developed multiple organ failure. The most frequent etiology of AKI was ischemic (41.7%) followed by septic (31.7%).The baseline characteristics of the study population and first AKI episode are shown in Table 1. The patient flowchart is depicted in Fig. 1.
Risk factors for CKD development 1 year after AKI
Twelve months after first AKI episode, we obtained follow-up data in 264 patients, 25 of them (9.4%) developed CKD. In univariate analysis, the occurrence of CKD was significantly higher in patients with hypertension (37.6 vs. 25.2%; p = 0.018), age >66 years (40.5 vs. 28.6%, p < 0.001) and SCr >2.6 mg/dL in the AKI episode (38.7 vs. 26.7%, p = 0.027). In multivariate analysis by logistic regression analysis, the variables independently associated with CKD development 1 year after an AKI episode, were: hypertension (OR 1.62; 95% CI 1.2–2.6, p < 0.05) and SCr >2.6 mg/dL in AKI (OR 1.7; 95% CI 1.2–3.7, p < 0.05) (Table 2).
Risk factors for CKD development 4 years after AKI
After 4 years of follow-up, data from 219 (60.8%) patients were obtained, 40 (18.3%) of them developed CKD. CKD at 4 years was significantly higher in patients with age >66 years (65.1 vs. 30.2%, p < 0.05), hypertension (48.1 vs. 29.8%; p < 0.05) and peripheral vascular disease (54.4 vs. 36.3%, p < 0.05). By logistic regression analysis, age >66 years was an independent risk factor for CKD development (OR 1.12, 95% CI 1.02–1.06, p < 0.05).
Mortality
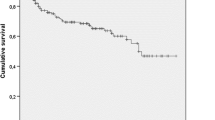
At 12 months we had information about survival status in 264 patients. Thirty-three patients (12.5%) had died at 12 months and 91 (25.3%) at end of follow-up. The most common cause of death was a cardiovascular event (n = 30, 33.0%), followed by infections (n = 22, 24.2%) and malignancies (n = 19, 20.9%).We noted significantly higher mortality risk at 12 months in patients who had developed CKD (OR 2.68, 95% CI 1.26–5.7, p < 0.05), as well as in patients with SCr >2.6 mg/dL (OR 1.26, 95% CI 1.06–1.58, p < 0.05). At the end of follow-up, mortality was significantly higher in males (55.7 vs. 44.3% in females, p = 0.024), patients >66 years (40.2 vs. 24.8%; p < 0.001), those with ischemic heart disease(39.7 vs. 26.4%, p = 0.053), PVD (38.4 vs. 25.4%, p = 0.04), as well as in patients with variables related with severe forms of AKI during the first episode, such as SCr >2.6 mg/dL (36.3 vs. 23.0%, p = 0.016) and dialysis requirement (32.0 vs. 12.8%, p = 0.014). By logistic regression analysis, independent mortality risk factors were: CKD development after AKI (OR 4.3, 95% CI 1.13–4.90, p < 0.05), and age >66 years (OR 1.12, 95% CI 1.02–1.06, p < 0.05) (Table 2). These results were not altered after excluding those patients with death by malignancies causes (data not shown).
Cardiovascular events
Eighty-three cardiovascular events in 80 patients (22.6%) during the 4-year follow-up period were recorded. Congestive heart failure (27.7%), acute coronary events (26.5%), acute arterial occlusions or deep venous thrombosis (20.5%), stroke (16.9%) and transient ischemic attacks (8.4%) were the most frequent ones. In univariate analysis, higher risk of cardiovascular events we found in patients with >1 episode of AKI (OR 1.58, 95% CI 1.04–2.39), history of hypertension (OR 4.1, 95% CI 2.32–7.24), type 2 DM (OR 3.22, 95% CI 1.93–5.38), ischemic heart disease (OR 5.51; 95% CI 3.02–10.06), peripheral vascular disease (OR 3.53, 95% CI 2.05–6.07), age >66 years (OR 1.06, 95% CI 1.04–1.08) as well as patients who developed CKD (OR 2.57, 95% CI 1.48–4.47).The multivariate analysis showed statistic significant relation with recurrent AKI (OR 3.33, 95% CI 2.10–5.62, p = 0.01).
Additionally, we observed higher rate of cardiovascular events in the subset of patients with CKD at 4 years compared by non-CKD patients (62.7 vs. 21.7%, p < 0.05). Table 3 shows the distribution of cardiovascular events, Table 4 shows the results for univariate and multivariate analysis by risk factor related with cardiovascular events.
Discussion
The study describes the prognosis of a group of patients with complete recovered non-severe AKI to short and long term. We described that some of these patients develop CKD both short as well as long term and those presented higher mortality and cardiovascular event that non-CKD patients.
Our results show that the severity of the AKI episodes has impact on CKD development and mortality at short term, but not at long term, highlighting the relevance of classical risk factors such as age, diabetes mellitus, hypertension, and peripheral vascular diseases.
We observed a rate of 22% of cardiovascular events and 33% of mortality related with cardiovascular events, those was higher in patients with a history of DM, hypertension, cardiovascular diseases and those who develop CKD. This has been well described both in general population [29,30,31,32] and in AKI patients [33].
Previous studies developed in AKI patients describe similar rates of cardiovascular events. Chawla et al. [33] described a rate of 23.0% of cardiovascular events in patients with AKI and myocardial infarct and Omotoso et al. reported 29.9% of major cardiovascular events in non-dialysis AKI patients [34]. We also observed a clear increase of cardiovascular events between patients who developed CKD compared with those without CKD, this finding is in concordance with the concurrent literature. Weiner et al. [36] described a rate of 17.6% of myocardial infarction, fatal cardiac heart disease and/or stroke in patients with GRF between 15 and 59 mL/min/m2 vs. 8.1% in patients with GFR ≥60 mL/min/m2, Meisinger et al. [37] reported an increase of 48% of risk of major cardiovascular events in males with CKD compared by without CKD.
The association between AKI, development of CKD, cardiovascular events and mortality, has been widely documented in several studies. In most of them, the design compared patients with AKI and patients without AKI, usually in the intensive care or cardiac surgery settings, enrolling patients with severe forms of AKI, high rates of dialysis requirement and multiples comorbidities [5,6,7,8,9, 15, 20,21,22,23, 35]. Milder cases and those developing in the non-surgical setting are underrepresented in those studies. Few studies have evaluated the prognosis of patients after an episode of mild–moderate AKI.
Some studies describe an increase in the risk of CKD development regardless of the severity of AKI [16, 35,36,37]. In our population, we found a rate of CKD development after AKI of 9% at 1 year and 18% at 4 years, similar to those reported in other studies performed in patients with reversible AKI. Jones et al. [38] found 15% of CKD development after 2.5 years of follow-up, and Thakar et al. [9] 13.6% at 5 years follow-up.
We have recorded lower mortality than which is reported in other studies [15, 38,39,40]. This lower mortality rate is probably related to the higher prevalence of patients with milder forms and more benign causes of AKI compared with other studies. In our study, the mortality in patients with CKD at 4 years was fourfold higher than in patients without CKD, a similar ratio to that previously observed [15].
As showed in a systematic review [18], the renal function pre-AKI and post-AKI are strong predictors of poor prognosis in AKI patients. These authors found a twofold increased risk of mortality and a fourfold increased risk of developing advanced chronic kidney disease in CKD pre-AKI patients compared by patients with AKI and pre-AKI normal kidney function. For this reason we included only patients with normal renal function before the AKI renal episode aiming to avoid bias at the time of outcome analyses.
The strengths of our study include the long duration of follow-up, the detailed participant clinical data, accurate diagnosis of AKI through ADQI guidelines, checked baseline creatinine before the episode for increasing the specificity than with the use of ICD-9 alone, and a very strict definition of recovery after AKI, even stricter than the usual recommendation in some studies or guidelines [38, 41]. In this way, we avoided the inclusion of patients with underdiagnosed CKD.
Our study has limitations, especially the retrospective nature of the assessment and the relatively small sample size. Thus, the comparison between different subsets of patients and a better evaluation between some relevant variables is difficult and a cause-and-effect association cannot be established. Additionally, for the methodology used for the selection of the patients included in the final analysis could be the risk of selection bias.
Our study shows how these episodes of AKI considered initially harmless, can lead to cardiovascular and renal poor outcomes. This is probably related to a systemic damage secondary to inflammatory process during the AKI episode [23, 42,43,44]. CKD development and some predisposing factors (old age, diabetes, hypertension, etc.) making it necessary a tight monitoring of patients surviving after an episode of AKI independently of the severity itself. Regrettably, adequate preventive surveillance strategies in this area have not been widely implemented in most units. Our proposal is fostering those strategies in patients with advanced age, hypertension, diabetes, and especially those with a previous AKI episode regardless its severity. During the following years after the first AKI episode the assessment of yearly kidney function, albuminuria and tight control of the underlying conditions will likely attenuate the development of CKD and decrease mortality. Prospective studies are needed to confirm the effectiveness of these measures.
References
UK Renal Registry. 16th Annual report: appendix F additional data tables for 2012 new and existing patients. Nephron Clin Pract. 2013;125(1–4):331–50.
Ftouh S, Lewington A. Prevention, detection and management of acute kidney injury: concise guideline. Clin Med. 2014;14(1):61–5.
Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292–8.
Star RA. Treatment of acute renal failure. Kidney Int. 1998;54(6):1817–31.
Loef BG, Epema AH, Smilde TD, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16(1):195–200.
Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–73.
Finkenstaedt JT, Merrill JP. Renal function after recovery from acute renal failure. N Engl J Med. 1956;254(22):1023–6.
Lowe KG. The late prognosis in acute tubular necrosis; an interim follow-up report on 14 patients. Lancet. 1952;1(6718):1086–8.
Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6(11):2567–72.
Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol. 2007;293(1):F269–78.
Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72(2):151–6.
Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005;12(4):353–65.
Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281(5):F887–99.
Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82(5):516–24.
Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81(5):477–85.
Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79(12):1361–9.
Browner WS, Li J, Mangano DT. In-hospital and long-term mortality in male veterans following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA. 1992;268(2):228–32.
Sawhney S, Mitchell M, Marks A, et al. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. 2015;5:e006497.
Briggs JD, Kennedy AC, Young LN, Luke RG, Gray M. Renal function after acute tubular necrosis. Br Med J. 1967;3(5564):513–6.
Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275(19):1489–94.
Bates DW, Su L, Yu DT, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis. 2001;32(5):686–93.
Gottlieb SS, Abraham W, Butler J, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8(3):136–41.
Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597–605.
Goldstein SL, Jaber BL, Faubel S, Chawla LS. Acute Kidney Injury Advisory Group of American Society of Nephrology: AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol. 2013;8:476–83.
Siew ED, Parr SK, Abdel-Kader K, et al. Predictors of recurrent AKI. J Am Soc Nephrol. 2016;27(4):1190–200.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12.
Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KIDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2011;2012(2):1–138.
Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40(9):912–8.
National Institute of Diabetes and Digestive and Kidney Diseases (U.S.), USRDS Coordinating Center. U.S. renal data system annual data report, researcher’s guide, reference tables, ADR slides. Ann Arbor: National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Renal Data System Coordinating Center. 2014
Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–8.
Chhabra A, Aronow WS, Ahn C, et al. Incidence of new cardiovascular events in patients with and without peripheral arterial disease seen in a vascular surgery clinic. Med Sci Monit. 2012;18(3):CR131–4.
Ridolfi RL, Hutchins GM. The relationship between coronary artery lesions and myocardial infarcts: ulceration of atherosclerotic plaques precipitating coronary thrombosis. Am Heart J. 1977;93(4):468–86.
Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9(3):448–56.
Omotoso BA, Abdel-Rahman EM, Xin W, Ma JZ, Scully KW, Arogundade FA, et al. Dialysis requirement, long-term major adverse cardiovascular events (MACE) and all-cause mortality in hospital acquired acute kidney injury (AKI): a propensity-matched cohort study. J Nephrol. 2016;29(6):847–55.
Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis. 2003;42(4):677–84.
Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–15.
Meisinger C, Doring A, Lowel H. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006;27(10):1245–50.
Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis. 2012;60(3):402–8.
Pannu N, James M, Hemmelgarn B, Klarenbach S. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8(2):194–202.
USRDS Annual Report. Chronic Kidney Disease (CKD) in the United States. Volume 1, Chapter 5; 2014. http://www.usrds.org/2014/view/v1_05.aspx. Accessed 14 Feb 2015.
Lewington A, Kanagasundaram S. Renal Association Clinical Practice Guidelines on acute kidney injury. Nephron Clin Pract. 2011;118(Suppl 1):c349–90.
Zager RA, Johnson AC, Lund S, Hanson S. Acute renal failure: determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol. 2006;291(3):F546–56.
Meldrum KK, Meldrum DR, Meng X, Ao L, Harken AH. TNF-alpha-dependent bilateral renal injury is induced by unilateral renal ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2002;282(2):H540–6.
Meldrum KK, Hile K, Meldrum DR, Crone JA, Gearhart JP, Burnett AL. Simulated ischemia induces renal tubular cell apoptosis through a nuclear factor-kappa B dependent mechanism. J Urol. 2002;168(1):248–52.
Acknowledgements
This work was made by Carlos Arias as part of his thesis project in the Department of Medicine, Universitat Autonoma of Barcelona.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Julio Pascual is supported by FIS ISCIII-FEDER PI13/00598, PI16/00617, and RedinRen RD16/0009/0013. María José Soler is supported by FIS ISCIII-FEDER PI14/00557.
Financial disclosure
No external funding.
Conflict of interest
The authors declare that there is no conflict of interests regarding publication of this paper.
Ethics
This study is adhered to the Principles of Helsinki Declaration and was approved by the hospital’s Ethics Committee (CEIC-IMAS) with IRB number 201/3777/I. Written informed consent was not required because of the non-intervention and retrospective chart review design.
Human and animal rights statement
This article does not contain any studies with animals performed by any of the authors.
About this article
Cite this article
Arias-Cabrales, C., Rodríguez, E., Bermejo, S. et al. Short- and long-term outcomes after non-severe acute kidney injury. Clin Exp Nephrol 22, 61–67 (2018). https://doi.org/10.1007/s10157-017-1420-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1420-y