Abstract
The complement activation system plays important roles to maintain homeostasis in the host and to fight foreign invaders to protect the host. Therefore, the complement system is considered a core part of innate immunity which also cross-talks to acquired immunity. In the history of nephrology, the complement system is familiar to us, because complement protein or fragment deposition, including C3, C4, C1q, and/or C4d, is routinely estimated by immunohistochemistry to diagnose renal pathologies. The relationships between pathological mechanisms and complement activation have been investigated for renal diseases such as post-infectious glomerulonephritis, lupus nephritis, and primary membranoproliferative glomerulonephritis, which are usually accompanied by hypocomplementemia. However, unregulated complement activation in local areas might be associated with progression of various renal injuries even in the normocomplementemic patient. Recently, attention has focused on dysfunction of complement regulation in various diseases including renal diseases such as atypical hemolytic uremic syndrome and C3 glomerulopathy. Some mechanisms associated with complement activation in these diseases were clarified. In addition, lots of anti-complement agents were developed and some of the agents have become clinically available. Now, anti-complement therapies represent a realistic choice of therapeutic approaches for complement-related diseases. Research on roles of complement activation is proceeding into new stages in the field of nephrology and in other fields involving both basic and clinical research. We herein summarize relationships between the complement activation and regulation systems, their physiological effects and roles in maintenance of homeostasis in the host, and how dysregulation of the complement system triggers disease, especially renal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Originally, the roles of the complement (C) system have been known as opsonization to scavenge foreign invaders such as microorganisms, fragments of damaged tissues and pathological immune-complexes, chemoattractant effects causing vasodilation and infiltration/accumulation of inflammatory cells, induction of inflammatory cytokines, and cell lysis of foreign and damaged cells through triggering processes of necrosis and apoptosis [1–3]. These roles are positioned to have important roles in innate immunity, the second defender of the host, after breaching of the first defense of epithelial layers such as skin, to protect the host from various physical stresses. In addition, it was recently shown that the C activation system was in close cross-talk with acquired immunity, the third arm of host defense. Now, in addition to roles in innate and acquired immunity, the relationship between the C and coagulation systems is also realized. From these multiple lines of evidence, it is clear that the C system is one of the most important systems to maintain the host homeostasis.
The C system is constructed from three activation pathways, the classical pathway (CP), the alternative pathway (AP), and the lectin pathway (LP), that each form C5 convertase enzymes and initiate the terminal pathway (TP) leading to formation of the lytic membrane attack complex (MAC; C5b-9; terminal complement complex; TCC). Historically, the CP was first recognized, followed by the AP and the LP. Although the CP is present in Reptilia/Aves and Mammalia, C3- and the AP-associated proteins are already expressed in primitive animals such as Echinodermata, Protostomia, and Cnidaria during animal evolution [4]. Indeed, parts of the AP were already constructed 1000 million years ago [4]. The CP is now considered to be the most recently evolved C activation pathway.
C activation has the capacity to damage the host, particularly when occurring through the AP which has limited capacity to distinguish targets from the host. Therefore, there are multiple mechanisms to regulate unexpected activation of the C system mediated by an array of C regulators (CReg). However, it has also been clarified that some of these C regulators have additional roles beyond regulation of C activation.
Here, we briefly summarize C activation and regulation, describe recent knowledge about bridging to acquired immunity, discuss aspects of CRegs, and cross-talk to the coagulation system. We also describe pathological conditions related to uncontrolled activation of C system and renal injuries.
The pathways of C activation, classical roles as a part of innate immunity
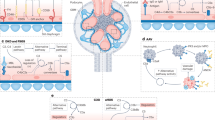
The C system comprises the three activation pathways, the TP, and the regulation system. The three C activation pathways, CP, AP, and LP, consist of enzymatic cascades through to the cleavage of C5, whereas the TP leading to formation of the MAC does not involve enzymatic processes (Fig. 1). The CP is initiated by the binding of C1q to the activating surface. Surface-bound C1q binds antibody attached to particulate antigen but can also bind many other ligands antibody-independently such as components of damaged cells, bacterial lipopolysaccharide, and nucleic acids. C1q is complexed with C1r and C1s; these confer enzymatic activity for cleavage of C4 and C2 to create the C3 convertase, C4b2a and, after addition of C3b, the C5 convertase, C4b2a3b. The AP activates the C pathway in an antibody-independent manner and amplifies C activation on foreign surfaces. An important characteristics of the AP is that it is spontaneously initiated by the phenomenon of “tickover” to generate C3(H2O) which binds factor (F) B to make a C3 convertase, C3(H2O)Bb, in the fluid phase, ensuring that C is always in a “ready-to-fire” situation. The LP differs from the CP only in that mannose-binding lectin (MBL) and its associated serine proteases (MASPs) in an MBL-MASP complex replace the C1qrs-complex. MBL binds to mannose-rich microbial surface and is complexed with MASPs 1, 2, and 3 that collaborate to enzymatically cleave later components [5]. In addition, ficolin-1 (M-ficolin), ficolin-2 (L-ficolin), ficolin-3 (H-ficolin, Hakata antigen), collectin-10 (CL-10, CL-L1), and collectin-11 (CL-11, CL-K1), which also have a collagen-like triple helix structure similar to C1q molecule, can activate LP [6]. They gain enzymatic activity by complexing with MASPs, similar in structure to the C1qrs-complex. Interestingly, it was recently reported that the LP might be related to activation of AP. MASP 1/3 cleaves pro-FD into FD which then activates the AP through cleavage of FB to generate the C3bBb C3 convertase of the AP [7, 8]. The three activation pathways form C3 convertases and subsequently C5 convertase, hence converging in the TP to form MAC on the targeted cell surface.
Pathways of the complement activation system and points of the regulation. Activation of the complement system is three early pathways of the classical pathway (CP), the alternative pathway (AP), and the lectin pathway (LP). These three pathways gather to form C3 convertases following C5 convertases and formation of membrane attack complex [MAC, terminal complement complex (TCC), C5b-9] which following pathway is called as the terminal pathway (TP). C1-INH C1 inhibitor, C4BP C4 binding protein, FH factor H, FI factor I, FB factor B, FD factor D, MBL mannose-binding lectin, MASP MBL-associated serine protease
The MAC comprises a complex of the C proteins C5b to C9; MAC makes holes in the cell membrane and induces targeted cell lysis. This mechanism is very effective to kill foreign invaders such as microorganisms and malignant cells. However, it was recently reported that sublytic MAC worked as a costimulatory molecules to polarize naïve T-cell to Th1-cells [9], to stimulate production of arachidonic acids, IL-1, and TNF-α from renal tubular epithelial cells [10] and to repair/regenerate Schwann cells and glia cells [11]. A detailed 3D MAC structure was recently published, showing the association of C5b, C6, C7, C8α, β, γ, and 18 molecules of C9 in the complex, and that the formed hole size is variable and approximately 240 Å in the targeted cell membrane [12].
During C activation, the anaphylatoxins C3a and C5a are produced. Although C3a and C5a are well known to play roles as strong chemotactic factors [13], C5a acting through C5a receptor (R) was shown to be neuroprotective by inhibiting apoptotic cell death [14], cause regeneration of liver cells, accelerate fracture healing, and reduce post-injury neural damage [3, 15–17]. It was also suggested that C3a and C5a have opposite effects on the disease process in endotoxin shock [18].
C regulation
The C system can behave as a double-edged sword, both protective and also harmful in the host. Because of this potential to cause harm, the activation cascade of the C system is controlled by many CRegs to maintain homeostasis in the host. The C activation is mainly regulated at the steps of formation of C3 convertase to control all three pathways of C activation, and during formation of MAC. There are multiple CRegs both in the fluid phase and on surfaces (Table 1). Exceptionally, only C1 inhibitor (C1-INH) inhibits the CP at the level of formation of the C1qrs-complex. Interestingly, FH is an abundant protein in serum as a fluid phase CReg at the C3 converse level, but also plays roles as a CReg on surfaces [19, 20]. FH is a multifunctional CReg, having cofactor activity to work with FI and convertase decay accelerating activity [21]. C4-binding protein (C4BP) which is a ~500 kDa large glycoprotein binds C4b and C3b and inhibits the C activation through decay acceleration of the CP C3 convertase, C4b2a, and as a cofactor for FI cleavage of C4b [19].
CRegs at the C3 convertase level on surfaces include C receptor (CR) 1, CD46, and CD55, together called membrane CRegs; in mouse and rat, Crry is the major membrane regulator of the C3 convertase. CR1 and CD46 are transmembrane molecules. CR1, originally described as a receptor for C3b and C4b, also possesses decay acceleration and FI cofactor activities for C3 convertase regulation. CD46 is an important membrane cofactor of FI cleavage of C3b/C4b; CD46 was also reported as a receptor for several viruses [23]. In human, CD46 is broadly distributed and polymorphic [24, 25]. CD46 has been implicated in recognition of apoptotic and necrotic cells [2], and plays roles in the male reproductive system and sperm maturation—indeed, in rodents, CD46 expression is restricted to testis, on late-phase spermatids and mature sperm [26, 27]. CD55, a glycosyl phosphatidylinositol (GPI)-anchored protein, is a decay accelerator for C3 convertase [28]. A truncated isoform of CD55 was identified in rat genital tissue [29]. In rodents, Crry is broadly expressed and a key CReg at the C3 convertase level, possessing both FI cofactor and decay acceleration activities [30]. C1-INH is also a multifunctional protein, originally discovered to inhibit the CP through binding to C1s and C1r, causing dissociation of C1s and C1r from the C1qrs-complex [31]. C1-INH also works to suppress the LP because of its capacity to bind and dissociate MASPs from the MBL-MASPs complexes [32]. C1-INH has also been associated with effects on acquired immunity by preventing T-cell proliferation and cytotoxic T-cell generation [33]. Moreover, C1-INH plays important bridging roles to the coagulation system (see Sect. 6).
Clusterin (apolipoprotein J) and vitronectin (S-protein) in the fluid phase and CD59 on surfaces inhibit the TP and MAC formation; vitronectin and clusterin bind C5b-7 and prevent its association with membranes [34, 35]. Of note, vitronectin is known to be associated with the coagulation system as we describe in the following Sect. 6. CD59 is a GPI-anchored membrane protein that prevents MAC assembly by binding the C5b-8 complex and preventing C9 insertion into the bilayer [36, 37]. In mouse, CD59 has two isoforms; CD59a which is broadly distributed and CD59b which is expressed only in the male genital tissue [1, 38].
CRegs and microorganisms
The membrane CRegs CD46 and CD55 are multifunctional proteins. Both CD46 and CD55 play roles as receptors for some microorganisms; for instance, CD46 works as a receptor for the measles virus and adenoviruses [39–41], CD55 binds echoviruses, coxsackievirus, and hantavirus [42–44]. Moreover, a CD55 polymorphism was reported to be related to severity of H1N1 influenza A virus infection [45] and to infection of Echoviruses 7 and 11 [46, 47]. Interestingly, it was recently shown that vitronectin might be related to Dengue virus infection because of interaction between vitronectin and dengue virus nonstructural protein 1 [48]. Of note, CRs have also been reported to play roles as microorganism receptors; for example, CR2 works as a receptor of Epstein-Barr virus on B-cells [49].
On the other hand, some microorganisms are able to recruit the host CRegs to escape C attack in the host. For example, CD46 was recruited in paramyxoviruses and mumps virus to escape C-mediated attacks [50], while group A streptococcus binds FH, FH-related protein (FHR) 1, C4BP and CD46 in order to escape from the host C activation system [51]. It was also reported that some other bacteria from Gram positives to Gram negatives, fungus and parasites, including Staphylococcus pneumoniae, Acinetobacter spp., Bordetella pertussis, Pseudomonas aeruginosa, Neisseria meningitidis, Haemophilus influenzae, Onchocera volvulus, Echinococcus granulosus, and Candida albicans, could acquire CRegs to evade C attack in the infected host [51–54].
Roles of C receptors in cross-talk to acquired immunity
During the process of C activation, the products such as C3b and C4b and the degradation products such as iC3b, C3dg, and C3d are generated to target through opsonization invading microorganisms, cell debris, and immune complexes for removal by phagocytes; key phagocyte CRs include CR1 (CD35), CR2 (CD21), CR3 (CD11b/CD18, Mac-1), and/or CD4 (CD11c/CD18) [55–58]. The CRs are expressed on blood cells such as erythrocytes, neutrophils, monocytes, macrophages, T- and B-cells, and follicular dendritic cells (reviewed in [59, 60]). CR1 binds C3b and C4b; CR2 binds iC3b, C3d, and C3dg; CR3 binds iC3b and C4b; and CR4 binds iC3b. The binding interactions enable capture of circulating immune-complexes and foreign cells, resulting in phagocytosis for macrophages, interaction between dendritic cells and B-cells, and differentiation of cellular immunity in the reticuloendothelial system. For example, in the process of opsonization, it was reported that CR2 on B-cell promoted the survival of both mature and transitional B-cells and antibody production [61, 62]. CR2 bound with C3 opsonized immune-complex on the follicular dendritic cell surface might relate to internalization and recycling of immune-complexes to be retained for long period [63]. CD46 is also involved in induction of regulatory T-cells with CD4+ T-cells contributing to immune homeostasis [64, 65]. CR on microglia protects the host through phagocytosis of opsonized amyloid β from cerebral plaques induced by iC3b and CR3, suggesting microglia protection from Alzheimer’s disease [66].
Cross-talking between the C system and the coagulation pathway/fibrinolysis system
It has recently become clear that the C system is closely related to the coagulation system/fibrinolysis system, including thrombi formation and fibrinolysis. It was reported that C5a might be directly generated through activation of the coagulation system [67]. C5a upregulates the coagulation system on endothelial cells through activation of tissue factor [68] and platelet aggregation progresses with interaction between C5a and neutrophils [69]. In our report, interaction between C5a and thrombus formation in glomerulus was demonstrated by the inhibitory effects of C5aR antagonist on thrombus formation in an anti-GBM model [70]. In addition, we also reported that C5a promoted liver injuries in a histone-induced lethal thrombosis model which is a DIC model [71]. In contrast, C5a may promote fibrinolysis with expression of plasminogen activator inhibitor-1 (PAI-1) [72]. Interaction between C3 and fibrinogen in fibrin networks may prevent fibrinolysis and proceed clot formation [73, 74]. Thus, C molecules such as C3, C3a, and C5a appear to play multiple roles during both activation of the coagulation pathways and fibrinolysis system. Carboxypeptidases (N, R) are key protease for inactivation of C5a/C3a to the des-Arg forms, decreasing biological activities of C5a/C3a [75]. Notably, serum carboxypeptidase R was also reported as an inhibitor of fibrinolysis [75] and is alternatively called as thrombin-activatable fibrinolysis inhibitor (TAFI) [75]. In the LP, it was also reported that MBL/MASP complex was related to thrombus formation [76].
Some CRegs are also associated with the coagulation system; C1-INH was originally reported as an inhibitor of the CP to dissociate C1qrs and of the LP to bind MASPs [32]. C1-INH also works to regulate the coagulation system at the levels of factor (F) XIIa [77], thrombin formation, and modification of kallikrein to bradykinin [78]. Vitronectin does not only bind the complex of C5b-7, but also PAI-1 [79] to prevent fibrosis [80].
On the other hand, some of the coagulation factors potentially accelerate the C system. For example, FXa, FXIa, and plasmin may cleave both C5 and C3 accompanied with production of C5a and C3a [81].
Deficiency/mutation and autoantibodies of the C activation pathways and the related various disorders
Dysfunction of the C activation is related to lose of classical roles, opsonization, production of anaphylatoxins, and cell lysis. Deficiencies of the activation components of the C system are associated with autoimmune diseases such as systemic lupus erythematosus (SLE) because of loss of opsonization function leading to accumulation of pathological immune-complexes, and impaired protection from foreign invaders such as microorganisms [82, 83]. Impairment of capacity to form MAC also increases risk of infection; specifically, loss of MAC formation is a strong risk for infection with Neisseria meningitidis [84, 85].
In the LP, an autosomal-recessive disease caused by dysfunction of MASP-1 and MASP-3 has been described; Carnevale, Mingarelli, Malpuech, and Michels (3MC) syndrome presents with multiple abnormalities [86–89].
Autoantibodies against C components are also associated with autoimmune diseases. For example, anti-C1q antibodies are frequent in SLE [90]. Associated with membrane CRegs, development of immune thrombocytopenia was recently linked with production of autoanti CD55 and CD59 [91].
Deficiency/mutation and autoantibodies of CRegs and the related diseases
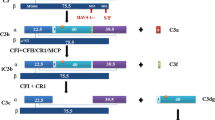
This category of diseases is divided into deficiencies or dysfunction caused by mutations of the molecules and autoantibodies that prevent function of the CRegs or cause excessive C activation [such as nephritic factors (Nef)]. Each can cause dysregulation of the C system leading to development of various diseases. For example, deficiency of C1-INH which causes decreased serum C1-INH leads to increased C activation with increased anaphylatoxin production, and also dysregulation of the kinin pathway with over-production of bradykinin. Together, these lead to type 1 hereditary angioedema (HAE) as the pathological appearance [92]. In contrast, dysfunction of C1-INH causes similar symptoms, termed type 2 HAE but C1-INH levels in serum remain in the normal range [92]. The presence of autoantibodies against C1-INH was also reported as a cause of HAE [93]. Polymorphism in FH was first reported to be related to development of age-related macular degeneration in 2005 [94]. Later, it was shown that the dysfunction of FH and disrupted C regulation was also a cause of atypical hemolytic uremic syndrome (aHUS) and C3 glomerulonephropathy (C3GN) [95–97]. CD46 and FI dysfunctions were also reported as reasons to develop aHUS or C3GN. In addition, production of autoantibodies against FH and FI was also reported as the reasons of dysregulation of C activation associated with aHUS and C3GN [98, 99]. Neurological diseases such as Alzheimer’s disease and multiple sclerosis were also reported to be related to dysregulation of the C system in humans and animal models [100, 101]. Mutation of CFHR1 altered circulating FHR oligomerization and caused C dysregulation in some C3GN patients [102]. In addition, acceleration of C activation can occur even if function of CReg is preserved. Mutations of C3 and FB have been shown to prevent interactions of CRegs with the convertase and induce excessive C activation leading to the development of aHUS and/or C3GN [103–105]. Until now, it was reported that mutation of C3 was more common than those of the other CRegs in Japan, compared with the other centuries [106]. Thus, when deficiencies or polymorphism/mutation of CRegs or proteins affects function this can cause dysregulation of C activation and induce pathological conditions.
There are several ways by which autoantibodies can accelerate C activation: (a) the autoantibodies directly affect the targeted CRegs and prevent C regulatory functions, (b) the autoantibodies block binding sites of the associated CRegs to interfere with binding between the targeted C component and the associated CRegs, and (c) the autoantibodies bind and stabilize the produced C convertases to induce continuous C activation. In the field of nephrology, production of autoantibodies against anti-FH is associated with aHUS and C3GN as an example of (a). Generation of antibodies against C3 and FB to stabilize C3 convertases, accelerate the AP, and induce C3GN as examples of (b). As the example of (c), Nef, for example, C3 Nef which stabilizes C3 convertases C3bBb and C3bBbP [98].
Interestingly, in some aHUS patients with autoantibodies against FH, deficiencies in FHR1 and FHR3 were reported; this is termed deficiency of FHR proteins and autoantibody positive form of HUS (DEAP-HUS) [107].
C activation and regulation are associated with development and progression of renal injuries
As in other tissues, the balance between C activation and regulation is known to be important in kidney to maintain the homeostasis, because the C system has potential to be a double edge sword [1, 82, 108]. In normal human kidney and diseased kidney, membrane CRegs such as CD46, CD55, and CD59 are broadly distributed [109–112]. It was reported that renal injuries were induced and/or accelerated in glomerular and interstitial pathologies when the balance between C activation and regulation was broken in animal models (reviewed in [1, 113, 114]). As another point, it is considered that local (including kidney) production of C components is important for local homeostasis and pathogenesis, although most C components are produced in liver and delivered to systemic circulation [59]. Recently, dysregulation of C caused by genetic abnormalities and autoantibodies of CRegs was demonstrated to be involved in development of renal pathologies such as aHUS and C3GN as we summarized in session 8. However, it was also shown in other renal diseases that most glomerular injuries are related to excessive C activation, although the host did not have genetic abnormalities in or autoantibodies against CRegs. It was also shown that the secondary dysregulation of the membrane CRegs caused by glomerular endothelial damage could induce severe renal injuries accompanied with abundant deposition of C activation products [115]. As supportive evidence, various experimental glomerulonephritidies and interstitial injuries were shown to be dependent on excessive C activation in various types of animal models, including anti-GBM nephritis [116], endothelial injuries caused by MAC formation [117], thrombus formation in glomerular capillaries [70], malarial infection-related nephritis [118], and proteinuria-related interstitial damages (reviewed in [113]). In addition, even if genetic deficiencies and dysfunction of membrane CRegs were absent, dysfunction of membrane CRegs could be secondary consequences. For example, renal injures induced by sea anemone toxins PsTX-T and PsTX-115 were caused by impairment of CRegs’ expression along glomerular capillaries secondary to glomerular endothelial damage, resulting in severe C activation in glomeruli [115]. However, in the recovering phase, the C activation was diminished with recovery of CRegs’ expression along glomerular capillaries [119]. When secondary dysregulation of CRegs was restored, progression of the C activation-dependent renal injuries was prevented.
We know that C deposition in glomeruli occurs in various human renal diseases in both pathogenic immune-complex dependent and independent manner; as references of the former cases, glomeruli in lupus nephritis, post-infectious glomerulonephritis, and membranous proliferative glomerulonephritis (MPGN) have C activation products and accompanying hypocomplementemia, and as references of latter cases, toxin-dependent renal injuries such as Shiga-toxin [120] and the other venoms originated from animals (reviewed in [119]). Part of pathological changes during induction/development of these renal injuries may be caused by the CP, the AP, and/or the LP of the C pathway (Table 2).
Regarding the relationship between anaphylatoxins and kidney, it is known that receptors for C3a and C5a, C3aR and C5aR1 (C5aR), which are G-protein-coupled receptors with seven transmembrane domains [134, 135], and C5aR2 (C5aL2), which is not a coupled G-protein receptor with seven transmembrane domains [136], distribute in kidney [137–139]. Both C3a-C3aR and C5a-C5aR interactions were important in lupus nephritis [138, 140] and interaction between C5a and C5aR is implicated as an amplification loop to induce glomerular injuries in anti-neutrophil cytoplasmic antibodies (ANCA)-associated glomerulonephritis [130, 141]. The other reports were also supported the C activation system to proceed ANCA-associated glomerulonephritis, especially through the AP [142, 143]. In a report using C3aR-deficient mice, onset of renal injuries was accelerated by C3aR knock-out in MRL/lpr mice [144]. Thus, early components can also be important to preserve homeostasis and suppress renal injuries. Indeed, up-regulation of clusterin in mesangial cells was suggested to protect from C-mediated renal injuries [145].
Current therapeutic approaches using anti-C agents and possibilities in future
As an example of the 1st line of anti-C agents in end of the twentieth century, recombinant soluble CR1 (TP10) was tried in phase I and II clinical trials for various diseases in human, because it had been shown to be an efficient CReg to protect from C activation-dependent injuries in various animal models [146]. Following development from TP10, TP20, APT070 (Microcept), and the other CR1 derivatives were developed and studied to show the therapeutic effects [1, 3, 147, 148]. In the present, C1-INH and a neutralizing anti-C5 antibody have reached clinical use as anti-C therapies. In Japan, C1-INH [Berinert P (CSL Behring)] as a C1 inhibitor is available for HAE [92, 149] and eculizumab as a neutralizing C5 antibody [Soliris (Alexion Pharm.)] is now also used for PNH and aHUS [150]. Usefulness of another form of the C5 antibody, pexelizumab, was shown in a clinical trial of acute myocardial infarction [151]. Different from neutralizing monoclonal antibodies, OmCI originated from Ornithodoros moubata was reported as an interesting powerful C5 inhibitor [152]. In the field of nephrology, anti-C therapies have lots of possibilities to prevent renal injuries possibly dependent on excessive activation of C system, such as progression of glomerular and interstitial injuries in glomerulonephritis, ischemia–reperfusion injuries, and antibody-mediated rejections for renal transplantation; usefulness of C5 antibody has been reported for some of them [153–155] in addition to aHUS and C3GN. Of note, patients with some C5 polymorphisms were reported to be refractory for usage of eculizumab in Asian population including Japan, because the polymorphisms prevented to bind between eculizumab and C5 [156].
Despite these advances, C activation remains a double-edged sword playing both protective and damaging roles in the host. Long-term and/or total suppression of the C system may not always be beneficial in the host because of risk of infections and the other C-dependent protective effects. As regards the other aspects, more targeted effective agents, for example, C3 inhibitors such as compstatin and staphylococcal C inhibitor, C5a inhibitors or C5aR antagonists such as PMX-58 and AcePepA, soluble CD59, and clusterin may find roles in therapy [3, 13, 157, 158]. Recently, it was reported that FD inhibition protected in PNH and aHUS [159]. New developing agents including inhibition of FD may be expected to be effective on PNH which is refractory for usage of eculizumab. To develop anti-C agents in future, consideration of these different properties of anti-C therapies may be important to select the right drug, perform appropriate timing, and to regulate.
Conclusion
We are facing to a new stage in understanding of the C system. We understand much more the relevance to disease of dysregulation of the C system. Dysfunction of the C system may be a result of genetic factors and/or anti-antibodies, but excessive C activation may also be secondary to other factors but nevertheless contribute to induction and development of tissue injuries. In recognizing the double-edged sword nature of C activation, we understand that intervention to regulate the C activation may not only show good effects to protect the host from attacks caused by excessive C activation but also have bad effects by eliminating C effects to maintain homeostasis. To develop new therapeutic agents, care must be taken to balance these outcomes, perhaps, by targeting to disease sites, inhibiting the appropriate C pathway, relevant steps of the C pathways, and timing therapy specifically for each pathological condition amenable to anti-C therapies.
References
Baalasubramanian S, Harris CL, Donev RM, Mizuno M, Omidvar N, Song W, et al. CD59a is the primary regulator of membrane attack complex assembly in the mouse. J Immunol. 2004;173:3684–92.
Elward K, Griffiths M, Mizuno M, Harris CL, Neal JW, Morgan BP, et al. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J Biol Chem. 2005;280(43):36342–54.
Cazander G, Jukema GN, Nibbering PH. Complement activation and inhibition in wound healing. Clin Dev Immunol. 2012;2012:534291.
Nonaka M, Kimura A. Genomic view of the evolution of the complement system. Immunogenetics. 2006;58:701–13.
Petersen SV, Thiel S, Jensenius JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol. 2001;38(2–3):133–49.
Garred P, Genster N, Pilely K, Bayarri-Olmos R, Rosbjerg A, Ma YJ, et al. A journey through the lectin pathway of complement-MBL and beyond. Immunol Rev. 2016;274(1):74–97.
Banda NK, Takahashi M, Takahashi K, Stahl GL, Hyatt S, Glogowska M, et al. Mechanisms of mannose-binding lectin-associated serine proteases-1/3 activation of the alternative pathway of complement. Mol Immunol. 2011;49(1–2):281–9.
Banda NK, Acharya S, Scheinman RI, Mehta G, Coulombe M, Takahashi M, et al. Mannan-binding lectin-associated serine protease 1/3 cleavage of pro-factor D into factor D in vivo and attenuation of collagen antibody-induced arthritis through their targeted inhibition by RNA interference-mediated gene silencing. J Immunol. 2016;197(9):3680–94.
Chen Y, Yang C, Jin N, Xie Z, Tang Y, Fei L, et al. Terminal complement complex C5b-9-treated human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization. Eur J Immunol. 2007;37(1):167–76.
David S, et al. Nephrol Dial Tranplant. 1997;12:51–6.
Hila S, Soane L, Koski CL. Sublytic C5b-9-stimulated Schwann cell survival through PI 3-kinase-mediated phosphorylation of BAD. Glia. 2001;36(1):58–67.
Serna M, Giles JL, Morgan BP, Bubeck D. Structural basis of complement membrane attack complex formation. Nat Commun. 2016;7:10587.
Mizuno M, Cole DS. Novel C5a regulators in inflammation. Expert Opin Invest Drugs. 2005;14:807–21.
Bénard M, Gonzalez BJ, Schouft MT, et al. Characterization of C3a and C5a receptors in rat cerebellar granule neurons during maturation. Neuroprotective effect of C5a against apoptotic cell death. J Biol Chem. 2004;279:43478–96.
Markiewski MM, DeAngelis RA, Strey CW, Foukas PG, Gerard C, Gerard N, et al. The regulation of liver cell survival by complement. J Immunol. 2009;182(9):5412–8.
Ignatius A, Ehrnthaller C, Brenner RE, Kreja L, Schoengraf P, Lisson P, et al. The anaphylatoxin receptor C5aR is present during fracture healing in rats and mediates osteoblast migration in vitro. J Trauma. 2011;71(4):952–60.
Guo Q, Cheng J, Zhang J, Su B, Bian C, Lin S, et al. Delayed post-injury administration of C5a improves regeneration and functional recovery after spinal cord injury in mice. Clin Exp Immunol. 2013;174(2):318–25.
Hollmann TJ, Mueller-Ortiz SL, Braun MC, Wetsel RA. Disruption of the C5a receptor gene increases resistance to acute Gram-negative bacteremia and endotoxic shock: opposing roles of C3a and C5a. Mol Immunol. 2008;45(7):1907–15.
Seya T, Nakamura K, Masaki T, Ichihara-Itoh C, Matsumoto M, Nagasawa S. Human factor H and C4b-binding protein serve as factor I-cofactors both encompassing inactivation of C3b and C4b. Mol Immunol. 1995;32(5):355–60.
Servais A, Frémeaux-Bacchi V, Lequintrec M, Salomon R, Blouin J, Knebelmann B, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet. 2007;44(3):193–9.
Pechtl IC, Kavanagh D, McIntosh N, Harris CL, Barlow PN. Disease-associated N-terminal complement factor H mutations perturb cofactor and decay-accelerating activities. J Biol Chem. 2011;286(13):11082–90.
Kemper C, Pangburn MK, Fishelson Z. Complement nomenclature 2014. Mol Immunol. 2014;61(2):56–8.
Cardone J, Le Friec G, Kemper C. CD46 in innate and adaptive immunity: an update. Clin Exp Immunol. 2011;164(3):301–11.
Liszewski MK, Atkinson JP. Membrane cofactor protein. Curr Topics Microbiol Immunol. 1992;178:45–60.
Post TW, Liszewski MK, Adams EM, Tedja I, Miller EA, Atkinson JP. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med. 1991;174:93–102.
Mizuno M, Harris CL, Johnson PM, Morgan BP. Rat membrane cofactor protein (MCP; CD46) is expressed only in the acrosome of developing and mature spermatozoa and mediates binding to immobilized activated C3. Biol Reprod. 2004;71(4):1374–83.
Harris CL, Mizuno M, Morgan BP. Spermatogenic cells distal to blood-testis barrier in rats lack C3 convertase regulators and may be at risk of complement-mediated injury. J Reprod Immunol. 2006;69:23–34.
Nicholson-Weller A, Wang CE. Structure and function of decay accelerating factor CD55. J Lab Clin Med. 1994;123(4):485–91.
Mizuno M, Donev RM, Harris CL, Morgan BP. CD55 expression in rat male reproductive tissue: differential expression in testis and expression of a unique truncated isoform on spermatozoa. Mol Immunol. 2007;44:1613–22.
Kim YU, Kinoshita T, Molina H, Hourcade D, Seya T, Wagner LM, et al. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J Exp Med. 1995;181(1):151–9.
Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement System Part I - Molecular Mechanisms of Activation and Regulation. Front Immunol. 2015;6:262.
Hansen CB, Csuka D, Munthe-Fog L, Varga L, Farkas H, Hansen KM, et al. The levels of the lectin pathway serine protease MASP-1 and its complex formation with C1 inhibitor are linked to the severity of hereditary angioedema. J Immunol. 2015;195(8):3596–604.
Nissen MH, Bregenholt S, Nording JA, Claesson MH. C1-esterase inhibitor blocks T lymphocyte proliferation and cytotoxic T lymphocyte generation in vitro. Int Immunol. 1998;10:167–73.
McDonald JF, Nelsestuen GL. Potent inhibition of terminal complement assembly by clusterin: characterization of its impact on C9 polymerization. BioChemistry. 1997;36(24):7464–73.
Bhakdi S, Käflein R, Halstensen TS, Hugo F, Preissner KT, Mollnes TE. Complement S-protein (vitronectin) is associated with cytolytic membrane-bound C5b-9 complexes. Clin Exp Immunol. 1988;74(3):459–64.
Meri S, Morgan BP, Wing M, Jones J, Davies A, Podack E, et al. Human protectin (CD59), an 18-20-kD homologous complement restriction factor, does not restrict perforin-mediated lysis. J Exp Med. 1990;172(1):367–70.
Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, et al. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71(1):1–9.
Harris CL, Hanna SM, Mizuno M, Holt DS, Marchbank KJ, Morgan BP. Characterization of the mouse analogues of CD59 using novel monoclonal antibodies: tissue distribution and functional comparison. Immunology. 2003;109:117–26.
Seya T. CD46, a complement regulatory protein/measles virus receptor, and its relation to hematological disorders. Int J Hematol. 1991;64(2):101–9.
Gaggar A, Shayakhmetov DM, Liszewski MK, Atkinson JP, Lieber A. Localization of regions in CD46 that interact with adenovirus. J Virol. 2005;79:7503–13.
Marttila M, Persson D, Gustafsson D, et al. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J Virol. 2005;79:14429–36.
Pettigrew DM, Williams DT, Kerrigan D, Evans DJ, Lea SM, Bhella D. Structural and functional insights into the interaction of echoviruses and decay-accelerating factor. J Biol Chem. 2006;281:5169–77.
Hafenstein S, Bowman VD, Chipman PR, et al. Interaction of decay-accelerating factor with coxsackievirus B3. J Virol. 2007;81:12927–35.
Krautkrämer E, Zeier M. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J Virol. 2008;82:4257–64.
Zhou J, To KK, Dong H, Cheng ZS, Lau CC, Poon VK, et al. A functional variation in CD55 increases the severity of 2009 pandemic H1N1 influenza A virus infection. J Infect Dis. 2012;206(4):495–503.
Sobo K, Rubbia-Brandt L, Brown TD, Stuart AD, McKee TA. Decay-accelerating factor binding determines the entry route of echovirus 11 in polarized epithelial cells. J Virol. 2011;85(23):12376–86.
Plevka P, Hafenstein S, Harris KG, Cifuente JO, Zhang Y, Bowman VD, et al. Interaction of decay-accelerating factor with echovirus 7. J Virol. 2010;84(24):12665–74.
Conde JN, da Silva EM, Allonso D, Coelho DR, Andrade ID, de Medeiros LN, et al. Inhibition of the membrane attack complex by dengue virus NS1 through Interaction with vitronectin and terminal complement proteins. J Virol. 2016;90(21):9570–81.
Young KA, Chen XS, Holers VM, Hannan JP. Isolating the Epstein–Barr virus gp350/220 binding site on complement receptor type 2 (CR2/CD21). J Biol Chem. 2007;282(50):36614–25.
Johnson JB, Grant K, Parks GD. The paramyxoviruses simian virus 5 and mumps virus recruit host cell CD46 to evade complement-mediated neutralization. J Virol. 2009;83(15):7602–11.
Oliver MA, Rojo JM, Rodríguez de Córdoba S, Alberti S. Binding of complement regulatory proteins to group A Streptococcus. Vaccine. 2008;26(Suppl 8):175–8.
Hallström T, Singh B, Kraiczy P, Hammerschmidt S, Skerka C, Zipfel PF, et al. conserved patterns of microbial immune escape: pathogenic microbes of diverse origin target the human terminal complement inhibitor vitronectin via a single common motif. PLoS One. 2016;11(1):e0147709.
Díaz A, Ferreira A, Sim RB. Complement evasion by Echinococcus granulosus: sequestration of host factor H in the hydatid cyst wall. J Immunol. 1997;158(8):3779–86.
Poltermann S, Kuner A, von der Heide M, Eck R, Hartmann A, Zipfel PF. Gpm1p is a factor H-, FHL-1-, and plasminogen-binding surface protein of Candida albicans. J Biol Chem. 2007;282(52):37537–44.
van Beek J, Elward K, Gasque P. Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann N Y Acad Sci. 2003;992:56–71.
Bonifati DMK U. Role of complement in neurodegeneration and neuroinflammation. Mol Immunol. 2007;44(5):999–1010.
Carroll MC. A protective role for innate immunity in systemic lupus erythematosus. Nat Rev Immunol. 2004;4(10):825–31.
Hannan JP. The structure-function relationships of complement receptor type 2 (CR2; CD21). Curr Protein Pept Sci. 2016;17(5):463–87.
Morgan BP, Gasque P. Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol. 1997;107:1–7.
Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37(2):199–207.
Molnár E, Erdei A, Prechl J. Novel roles for murine complement receptors type 1 and 2 I. Regulation of B cell survival and proliferation by CR1/2. Immunol Lett. 2008;116:156–62.
Shimizu I, Kawahara T, Haspot F, Bardwell PD, Carroll MC, Sykes M. B-cell extrinsic CR1/CR2 promotes natural antibody production and tolerance induction of anti-alphaGAL-producing B-1 cells. Blood. 2007;109:1773–81.
Heesters BA, Chatterjee P, Kim YA, Gonzalez SF, Kuligowski MP, Kirchhausen T, et al. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013;38(6):1164–75.
Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–66.
Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92.
Fu H, Liu B, Frost JL, Hong S, Jin M, Ostaszewski B, et al. Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Aβ by microglia. Glia. 2012;60(6):993–1003.
Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–7.
Ikeda K, Nagasawa K, Horiuchi T, al. e. C5a induces tissue factpr activity on endothelial cells. Thromb Haemast. 1997;77:394–8.
Ferrer-Lopez P, Renesto P, Schattner M, et al. Activation of human platelets by C5a-stimulated neutrophils: a role for cathepson G. Am J Physiol. 1990;258:C1100–C7.
Kondo C, Mizuno M, Nishikawa K, Yuzawa Y, Hotta N, Matsuo S. The role of C5a in the development of thrombotic glomerulonephritis in rats. Clin Exp Immunol. 2001;124:323–9.
Mizuno T, Yoshioka K, Mizuno M, Shimizu M, Nagano F, Okuda T, et al. Complement component 5 promotes lethal thrombosis. Sci Rep. 2017;7:42714.
Kastl SP, Speidl WS, Kaun C, Rega G, Assadian A, Weiss TW, et al. The complement component C5a induces the expression of plasminogen activator inhibitor-1 in human macrophages via NF-kappaB activation. J Thromb Haemost. 2006;4(8):1790–7.
Hess K, Alzahrani SH, Price JF, Strachan MW, Oxley N, King R, et al. Hypofibrinolysis in type 2 diabetes: the role of the inflammatory pathway and complement C3. Diabetologia. 2014;57(8):1737–41.
King R, Tiede C, Simmons K, Fishwick C, Tomlinson D, Ajjan R. Inhibition of complement C3 and fibrinogen interaction: a potential novel therapeutic target to reduce cardiovascular disease in diabetes. Lancet. 2015;385(Suppl 1):S57.
Campbell W, Okada N, Okada H. Carboxypeptidase R is an inactivator of complement-derived inflammatory peptides and an inhibitor of fibrinolysis. Immunol Rev. 2001;180:162–7.
La Bonte LR, Pavlov VI, Tan YS, Takahashi K, Takahashi M, Banda NK, et al. Mannose-binding lectin-associated serine protease-1 is a significant contributor to coagulation in a murine model of occlusive thrombosis. J Immunol. 2012;188(2):885–91.
Madsen DE, Sidelmann JJ, Biltoft D, Gram J, Hansen S. Ca-inhibitor plymers activate the FXII-dependent kallikrein-kinin system: Implication for a role in hereditary angioedema. Biochim Biophys Acta. 2015;1850(6):1336–42.
Ravindran S, Schapira M, Patston PA. Modulation of C1-inhibitor and plasma kallikrein activities by thpe IV collagen. Int J Biomater. 2012;2012:212417.
Zhou A, Huntington JA, Pannu NS, Carrell RW, Read RJ. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat Struct Biol. 2003;10(7):541–4.
Zhong J, Yang HC, Kon V, Fogo AB, Lawrence DA, Ma J. Vitonectin-binding PAI-1 protects against the development of cardiac fibrosis through interaction with fibrosis. Lab Invest. 2014;94(6):633–44.
Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, et al. Interaction between the coagulation and complement system. Adv Exp Med Biol. 2008;632:71–9.
Mizuno M, Morgan BP. An update on the roles of the complement system in autoimmune diseases and the therapeutic possibilities of anti-complement agents. Curr Drug Therapy. 2011;6:35–50.
Soto K, Wu YL, Ortiz A, Aparício SR, Yu CY. Familial C4B deficiency and immune complex glomerulonephritis. Clin Immunol. 2010;137(1):166–75.
Fijen CA, Kuijper EJ, te Bulte MT, Daha MR, Dankert J. Assessment of complement deficiency in patients with meningococcal disease in The Netherlands. Clin Infect Dis. 1999;28(1):98–105.
Drogari-Apiranthitou M, Kuijper EJ, Dekker N, Dankert J. Complement activation and formation of the membrane attack complex on serogroup B Neisseria meningitidis in the presence or absence of serum bactericidal activity. Infect Immun. 2002;70(7):3752–8.
Rooryck C, Diaz-Font A, Osborn DPS, Chabchoub E, Hernandez-Hernandez V, Shamseldin H, et al. Mutations in the lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrom. Nat Gent. 2011; 43(3):197–203.
Yongqing T, Wilmann PG, Reeve SB, Coetzer TH, Smith AI, Whisstock JC, et al. The X-ray crystal structure of mannose-binding lectin-associated serine proteinase-3 reveals the structural basis for enzyme inactivity associated with the Carnevale, Mingarelli, Malpuech, and Michels (3MC) syndrome. J Biol Chem. 2013;288(31):22399–407.
Urquhart J, Roberts R, de Silva D, Shalev S, Chervinsky E, Nampoothiri S, et al. Exploring the genetic basis of 3MC syndrome: findings in 12 further families. Am J Med Genet A. 2016;170A(5):1216–24.
Degn SE, Jensenius JC, Thiel S. Disease-causing mutations in genes of the complement system. Am J Hum Genet. 2011;88(6):689–705.
Stojan G, Petri M. Anti-C1q in systemic lupus erythematosus. Lupus. 2016;25(8):873–7.
Unterberger U, Eichelberger B, Ulz A, Panzer S. Antibodies against complement-regulatory proteins on platelets in immune thrombocytopenia. Platelets. 2016;13:1–5.
Horiuchi T, Ohi H, Ohsawa I, Fujita T, Matsushita M, Okada N, et al. Guideline for hereditary angioedema (HAE) 2010 by the Japanese association for complement research. Allergol Int. 2012;61(4):559–62.
Varga L, Széplaki G, Visy B, Füst G, Harmat G, Miklós K, et al. C1-inhibitor (C1-INH) autoantibodies in hereditary angioedema. Strong correlation with the severity of disease in C1-INH concentrate naïve patients. Mol Immunol. 2007;44(6):1454–60.
Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–21.
Ito N, Ohashi R, Nagata M. C3 glomerulopathy and current dilemmas. Clin Exp Nephrol. 2016. doi:10.1007/s10157-016-1358-5.
Kato H, Nangaku M, Hataya H, Sawai T, Ashida A, Fujimaru R, et al. Joint Committee for the Revision of Clinical Guides of Atypical Hemolytic Uremic Syndrome in Japan. Clinical guides for atypical hemolytic uremic syndrome in Japan. Clin Exp Nephrol. 2016;20(4):536–43.
Sawai T, Nangaku M, Ashida A, Fujimaru R, Hataya H, Hidaka Y, et al. Joint Committee of the Japanese Society of Nephrology and the Japan Pediatric Society. Diagnostic criteria for atypical hemolytic uremic syndrome proposed by the Joint Committee of the Japanese Society of Nephrology and the Japan Pediatric Society. Clin Exp Nephrol. 2014;18(1):4–9.
Paixão-Cavalcante D, López-Trascasa M, Skattum L, Giclas PC, Goodship TH, de Córdoba SR, et al. Sensitive and specific assays for C3 nephritic factors clarify mechanisms underlying complement dysregulation. Kidney Int. 2012;82(10):1084–92.
Goodship TH, Pappworth IY, Toth T, Denton M, Houlberg K, McCormick F, et al. Factor H autoantibodies in membranoproliferative glomerulonephritis. Mol Immunol. 2012;52(2–3):200–6.
Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at GLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1094–9.
Ingram G, Loveless S, Howell OW, Hakobyan S, Dancey B, Harris CL, et al. Complement activation in multiple sclerosis plaques: an immunohistochemical analysis. Acta Neuropathol Commun. 2014;2:53.
Tortajada A, Yébenes H, Abarrategui-Garrido C, Anter J, García-Fernández JM, Martínez-Barricarte R, et al. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest. 2013;123(6):2434–46.
Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, Vazquez-Martul E, Torreira E, Montes T, et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120(10):3702–12.
Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2007;104(1):240–5.
Martínez-Barricarte R, Heurich M, López-Perrote A, Tortajada A, Pinto S, López-Trascasa M, et al. The molecular and structural bases for the association of complement C3 mutations with atypical hemolytic uremic syndrome. Mol Immunol. 2015;66(2):263–73.
Yoshida Y, Miyata T, Matsumoto M, Shirotani-Ikejima H, Uchida Y, Ohyama Y, et al. A novel quantitative hemolytic assay coupled with restriction fragment length polymorphisms analysis enabled early diagnosis of atypical hemolytic uremic syndrome and identified unique predisposing mutations in Japan. PLoS One. 2015;10:e0124655.
Zipfel PF MC, Müller D, Licht C, Wigger M, Skerka C; European DEAP-HUS Study Group. DEAP-HUS: deficiency of CFHR plasma proteins and autoantibody-positive form of hemolytic uremic syndrome. Pediatr Nephrol. 2010;25(10):2009–19.
Mizuno M. A review of current knowledge of the complement system and the therapeutic opportunities in inflammatory arthritis. Curr Med Chem. 2006;13:1707–17.
Cosio FG, Sedmak DD, Mahan JD, Nahman NSJ. Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int. 1989;36:100–7.
Tamai H, Matsuo S, Fukatsu A, Nishikawa K, Sakamoto N, Yoshioka K, et al. Localization of 2O-kD homologous restriction factor (HRF20) in diseased human glomeruli. An immunofluorescence study. Clin Exp Immunol. 1991;84:256–62.
Endoh M, Yamashina M, Ohi H, Funahashi K, Ikuno T, Yasugi T, et al. Immunohistochemical demonstration of membrane cofactor protein (MCP) of complement in normal and diseased kidney tissues. Clin Exp Immunol. 1993;94:183–8.
Ichida S, Yuzawa Y, Okada H, Yoshioka K, .S. M.. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 1994;46:89–96.
Matsuo S, Morita Y, Mizuno M, Nishikawa K, Yuzawa Y. Complement mediated renal injury: Its mechanisms and role of membrane regulators of complement. Clin Exp Nephrol. 1998;2:276–81.
Hanafusa N, Sogabe H, Yamada K, Wada T, Fujita T, Nangaku M. Contribution of genetically engineered animals to the analyses of complement in the pathogenesis of nephritis. Nephrol Dial Transplant. 2002;17(Suppl 9):34–6.
Mizuno M, Nozaki M, Morine N, Suzuki N, Nishikawa K, Morgan BP, et al. A protein toxin from the sea anemone Phyllodiscus semoni targets the kidney and causes a severe renal Injury with predominant glomerular endothelial damage. Am J Pathol. 2007;171(2):402–14.
Hatanaka Y, Yuzawa Y, Nishikawa K, Fukatsu A, Okada N, Okada H, et al. Role of a rat membrane inhibitor of complement in anti-basement membrane antibody-induced renal injury. Kidney Int. 1995;48:1728–37.
Nangaku M, Alpers CE, Pippin J, Shankland SJ, Kurokawa K, Adler S, et al. Renal microvascular injury induced by antibody to glomerular endothelial cells is mediated by C5b-9. Kidney Int. 1997;52:1570–8.
Yashima A, Mizuno M, Yuzawa Y, Shimada K, Suzuki N, Tawada H, et al. Mesangial proliferative glomerulonephritis in murine malaria parasite, Plasmodium chabaudi AS, infected NC mice. Clin Exp Nephrol. 2016 (in press)
Mizuno M, Ito Y, Morgan BP. Exploiting the nephrotoxic effects of venom from the sea anemone, Phyllodiscus semoni, to create a hemolytic uremic syndrome model in the rat. Mar Drugs. 2012;10(7):1582–604.
Yamamoto ET, Mizuno M, Nishikawa K, Miyazawa S, Zhang L, Matsuo S, et al. Shiga toxin-1 causes direct renal injury in rats. Infect Immun. 2005;73:7099–106.
Sethi S, Nasr SH, De Vriese AS, Fervenza FC. C4d as a diagnostic tool in proliferative GN. J Am Soc Nephrol. 2015;26(11):2852–9.
Sethi S, Nester CM, Smith RJ. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 2012;81(5):434–41.
Sheerin NS, Springall T, Carroll MC, Hartley B, Sacks SH. Protection against anti-glomerular basement membrane (GBM)-mediated nephritis in C3- and C4-deficient mice. Clin Exp Immunol. 1997;110(3):403–9.
Thanei S, Vanhecke D, Trendelenburg M. Anti-C1q autoantibodies from systemic lupus erythematosus patients activate the complement system via both the classical and lectin pathways. Clin Immunol. 2015;160(2):180–7.
Kim MK, Maeng YI, Lee SJ, Lee IH, Bae J, Kang YN, et al. Pathogenesis and significance of glomerular C4d deposition in lupus nephritis: activation of classical and lectin pathways. Int J Clin Exp Pathol. 2013;15(6):2157–67.
Segawa Y, Hisano S, Matsushita M, Fujita T, Hirose S, Takeshita M, et al. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr Nephrol. 2010;25(6):1091–9.
Kawa S. The immunobiology of immunoglobulin G4 and complement activation pathways in IgG4-related disease. Curr Top Microbiol Immunol. 2017;401:61–73.
Ma R, Cui Z, Hu SY, Jia XY, Yang R, Zheng X, et al. The alternative pathway of complement activation may be involved in the renal damage of human anti-glomerular basement membrane disease. PLoS One. 2016;9(3):e92150.
Daha MR, van Kooten C. Role of complement in IgA nephropathy. J Nephrol. 2016;29(1):1–4.
Yuan J, Chen M, Zhao MH. Complement in antineutrophil cytoplasmic antibody-associated vasculitis. Clin Exp Nephrol. 2013;17(5):642–5.
Kallenberg CG, Heeringa P. Complement system activation in ANCA vasculitis: a translational success story? Mol Immunol. 2015;68(1):53–6.
Borza DB. Alternative pathway dysregulation and the conundrum of complement activation by IgG4 immune complexes in membranous nephropathy. Front Immunol. 2016;7:157.
Farrar CA, Tran D, Li K, Wu W, Peng Q, Schwaeble W, et al. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J Clin Invest. 2016;126(5):1911–25.
Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–7.
Chao TH, Ember JA, Wang M, Bayon Y, Hugli TE, Ye RD. Role of the second extracellular loop of human C3a receptor in agonist binding and receptor function. J Biol Chem. 1999;274:9721–8.
Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN. The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol Immunol. 2009;46(6):1149–62.
Braun MC, Reins RY, Li TB, et al. Renal expression of the C3a receptor and functional responses of primary human proximal tubular epithelial cells. J Immunol. 2004;173:4190–6.
Mizuno M, Blanchin S, Gasque P, Nishikawa K, Matsuo S. High levels of complement C3a receptor in the glomeruli in lupus nephritis. Am J Kidney Dis. 2007;49:598–606.
Kiafard Z TT, Schweyer S, Bley A, Neumann D, Zwirner J. Use of monoclonal antibodies to assess expression of anaphylatoxin receptors in tubular epithelial cells of human, murine and rat kidneys. Immunobiology. 2007;212:129–39.
Bao L, Osawe I, Haas M, Quigg RJ. Signaling through up-regulated C3a receptor is key to the development of experimental lupus nephritis. J Immunol. 2005;175(3):1947–55.
Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediated neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–98.
Huugen D, van Esch A, Xiao H, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, et al. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71(7):646–54.
Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170(1):52–64.
Wenderfer SE, Wang H, Ke B, Wetsel RA, Braun MC. C3a receptor deficiency accelerates the onset of renal injury in the MRL/lpr mouse. Mol Immunol. 2009;46:1397–404.
Yamada K, Hori Y, Hanafusa N, Okuda T, Nagano N, Choi-Miura NH, et al. Clusterin is up-regulated in glomerular mesangial cells in complement-mediated injury. Kidney Int. 2001;59(1):137–46.
Rioux P. TP-10 (AVANT Immunotherapeutics). Curr Opin Investig Drugs. 2001;2(3):364–71.
Schmid RA, Hillinger S, Hamacher J, Stammberger U. TP20 is superior to TP10 in reducing ischemia/reperfusion injury in rat lung grafts. Transplant Proc. 2001;33(1–2):948–9.
Yazdanbakhsh K, Scaradavou A. CR1-based inhibitors for prevention of complement-mediated immune hemolysis. Drug News Perspect. 2004;17(5):314–20.
Bowen T CM, Farkas H, et al. Canadian 2003 internatinal consensus alogrithm for the diagnosis, therapy, and management of hereditary angioedema. J Allergy Clin Immunol. 2003;114:629–937.
Luzzatto L, Gianfaldoni G. Recent advances in biological and clinical aspects of paroxysmal nocturnal hemoglobinuria. Int J Hematol. 2006;84:104–12.
Mahaffey KW, Granger CB, Nicolau JC, et al. Effect of pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to fibrinolysis in acute myocardial infarction: the COMPlement inhibition in myocardial infarction treated with thromboLYtics (COMPLY) trial. Circulation. 2003;108(10):1176–83.
Nunn MA, Sharma A, Paesen GC, et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J Immunol. 2005;174:2084–91.
Ring T, Pedersen BB, Salkus G, Goodship TH. Use of eculizumab in crescentic IgA nephropathy: proof of principle and conundrum? Clin Kidney J. 2015;8(5):489–91.
Pickering MC, Ismajli M, Condon MB, McKenna N, Hall AE, Lightstone L, et al. Eculizumab as rescue therapy in severe resistant lupus nephritis. Rheumatology (Oxford). 2015;54(12):2286–8.
Jordan SC, Choi J, Kahwaji J, Vo A. Complement inhibition for prevention and treatment of antibody-mediated rejection in renal allograft recipients. Transplant Proc. 2016;48(3):806–8.
Nishimura J, Yamamoto M, Hayashi S, et al. Genetic variants in C5 and poor response to eculizumab. N Engl J Med. 2014;370(7):632–9.
Fraser DA, Harris CL, Williams AS, Mizuno M, Sean Gallagher S, Smith RAG, et al. Generation of a recombinant, membrane-targeted form of the complement regulator CD59. J Biol Chem. 2003;278(49):48921–7.
Franco DA, Truran S, Burciu C, Gutterman DD, Maltagliati A, Weissig V, et al. Protective role of clusterin in preserving endothelial function in AL amyloidosis. Atherosclerosis. 2012;225(1):220–3.
Yuan X, Gavriilaki E, Thanassi JA, Yang G, Baines AC, Podos SD, et al. Small-molecule Factor D inhibitors selectively block the althernative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical uremic syndrome. Haematologica. 2017;102(3):466–75.
Acknowledgements
The authors profoundly thank Prof B. Paul Morgan for editing this manuscript. This work was in part supported in part by the Ministry of Education, Culture, Sports, Science and Technology in Japan (Grants-in-Aid 24591227). Mizuno M, Suzuki Y and Ito Y worked in the Department of Renal Replacement Therapy as positions endowed by Baxter Japan at Nagoya University Graduate School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
About this article
Cite this article
Mizuno, M., Suzuki, Y. & Ito, Y. Complement regulation and kidney diseases: recent knowledge of the double-edged roles of complement activation in nephrology. Clin Exp Nephrol 22, 3–14 (2018). https://doi.org/10.1007/s10157-017-1405-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1405-x