Abstract
Background
The Oxford Classification is utilized globally, but has not been fully validated. In this study, we conducted a comparative analysis between the Oxford Classification and Japanese Histologic Classification (JHC) to predict renal outcome in Japanese patients with IgA nephropathy (IgAN).
Methods
A retrospective cohort study including 86 adult IgAN patients was conducted. The Oxford Classification and the JHC were evaluated by 7 independent specialists. The JHC, MEST score in the Oxford Classification, and crescents were analyzed in association with renal outcome, defined as a 50% increase in serum creatinine.
Results
In multivariate analysis without the JHC, only the T score was significantly associated with renal outcome. While, a significant association was revealed only in the JHC on multivariate analysis with JHC.
Conclusions
The JHC and T score in the Oxford Classification were associated with renal outcome among Japanese patients with IgAN. Superiority of the JHC as a predictive index should be validated with larger study population and cohort studies in different ethnicities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
IgA nephropathy (IgAN) is one of the most common presentations of glomerulonephritis worldwide, particularly in eastern Asian and Mediterranean countries [1, 2]. Initially, IgAN was thought to follow a benign course. However, it has been affirmed that 40% of patients will progress to end-stage renal disease (ESRD) within 20 years [3, 4]. The amount of proteinuria, hypertension, and renal impairment are well-established clinical prognostic factors [5–10]. A renal biopsy is essential to diagnose IgAN, and histological findings are informative for treatment selection and prognosis prediction [11]. Lee et al. [12] published the first pathological grading system of IgAN; Lee’s scoring system, in 1982, based on Meadow’s Classification of Henoch–Schonlein nephritis [13]. Thereafter, several histological IgAN Classifications have been developed [14–19], and Lee’s scoring system and the Haas Classification [20, 21] are widely used among nephrologists. All of these classification systems have been developed from specialist concepts, each has strengths and limitations in predicting renal prognosis, and none have achieved widespread acceptance as a comprehensive classification [22].
In 2009, the Oxford Classification was developed by the cooperation of the International IgAN Network and the Renal Pathology Society [22, 23] to predict prognosis in IgAN by pathological findings. The working group reproduced the mesangial hypercellularity (M), endocapillary proliferation (E), segmental sclerosis (S), and tubular atrophy/interstitial fibrosis (T) that accurately predict renal outcomes independent of clinical parameters. At present, the Oxford Classification is globally used, although it was originally developed mainly with the Caucasian population other than Korean, and only 20 adult Japanese patients were included [22].
Parallel to the Oxford Classification, an original histologic classification system, the Japanese Histologic Classification (JHC), has been developed in Japan to predict the long-term risk of progression to ESRD [24]. The JHC was brought forth by an IgAN study group of the progressive Renal Disease Study Committee organized by the Ministry of Health, Labor, and Welfare of Japan in 2013 [24], and validated in 2015 [25]. Since then, both the Oxford Classification and JHC have been widely used in Japan, although the clinical value of these two IgAN classifications have not yet been fully analyzed. Therefore, this is the first paper to elucidate the utility of the JHC and the Oxford Classification simultaneously to predict renal outcome in Japanese patients with IgA nephropathy in the same cohort study.
Subjects and methods
Study design and study subjects
This study was a retrospective cohort study in a single center. Study subjects were 122 adult (age ≥18 years) patients with IgAN, who were diagnosed by renal biopsy examination between 2001 and 2009 at Nagoya University Hospital. Clinical and laboratory data at the time of biopsy were available for all patients. One patient with IgA vasculitis, 5 patients with less than 8 glomeruli in renal biopsy specimens, and 30 patients with a follow-up period of less than 12 months were excluded from the study. Thus, a total of 86 patients were analyzed.
Histologic parameters
The renal biopsy specimens were stained with periodic acid-Schiff, hematoxylin and eosin, periodic acid-methenamine silver, and Masson’s trichrome, and were evaluated by 7 independent nephrologists (Y.Y, T.K, T.I, T.N, T.O, M.H, and AB.K) who were blinded from clinical data. Each histologic lesion was evaluated according to the instructions of the JHC 2013 [24] and the Oxford Classification [23]. Coordination meetings among 7 renal specialists were held before histological evaluation, and every inconsistency in JHC and the Oxford Classification was discussed for the final histological diagnoses.
The JHC is a lumped system that evaluates the severity of glomerular histologic lesions. Independent histologic lesions that predict renal outcomes were divided into two classes. Early progressors to ESRD were Global Sclerosis (GS), Segmental Sclerosis (SS), and Fibrous Crescent (FC). Late progressors to ESRD were Global Sclerosis (GS), Cellular Crescent (CC), and Fibrocellular Crescent (FCC) [24]. The glomerular lesion percentage score (GLPS) was calculated with the following formula: [(number of glomeruli with cellular/fibrocellular/fibrous crescents, global/segmental sclerosis)/number of total obtained glomeruli × 100 (%)], Histologic grade (HG) was categorized as HG1, HG2, HG3 and HG4, corresponding to GLPs of <25, 25–49.9, 50–74.9, and ≥75%, respectively.
The Oxford Classification assesses several factors, culminating in MEST scores: a mesangial score ≤ or >50% of glomeruli with ≥4 cells/mesangial area not adjacent to the vascular stalk is scored as M0/M1. The absence or presence of endocapillary hypercellularity is categorized as E0/E1, while S0/S1 describes the absence or presence of segmental glomerulosclerosis or tuft adhesion, and T0/T1/T2 describes the degree of tubular atrophy or interstitial fibrosis with <25, 25–50, or >50%, respectively [22]. In addition to the MEST scores, the absence or presence of crescent (C0/1) was evaluated in this study. Correlation between MEST scores and crescents were analyzed with the JHC. To improve the statistical power, patients with T1 and T2, or HG3 and HG4 were combined as a group for correlation and renal outcome studies.
Clinical parameters
The following clinical parameters were collected from clinical records at the time of renal biopsy: age, sex, BMI, comorbid diagnosis of hypertension, systolic and diastolic blood pressure (BP), serum total cholesterol, serum IgA, amount of proteinuria, serum creatinine (sCr), serum uric acid (UA) levels, tonsillectomy, and treatment with drugs including antihypertensives renin–angiotensin system blockers (RAS blockade), corticosteroids, and immunosuppressants. As compared with baseline estimated glomerular filtration rate (eGFR) 71 ml/min/1.73 m2 and proteinuria 1.2 g/day, median eGFR was 62 [45–86] ml/min/1.73 m2, proteinuria was decreased to 0.8 [0.6–1.4] g/day, at the end of follow-up. eGFR was calculated using the Japanese GFR equation [=194 × sCr −1.094 × age −0.287 × 0.739 (if female)] [26], and CKD stages were categorized into G1 to G5 stages according to KDIGO guidelines [27]. A comorbidity of hypertension was defined as BP ≥140/90 mmHg or treatment with an antihypertensive drug. RAS blockade indicated exposure to angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, or both at any dose regardless of discontinuation during follow-up. Treatment with corticosteroids was defined as the prescription of corticosteroids for more than 6 months with a dose of 20 mg/day and above in prednisolone and/or steroid pulse therapy. The observational period ended at the end / of 2015.
Renal outcome study
Renal outcome was defined as a 50% increase in sCr from baseline [1, 28–30]. Kaplan–Meier survival curves analyses free from renal outcome were conducted with M0/1, E0/1, S0/1, T0/1 + 2, crescents (C0/1) and JHC (HG1/2/3 + 4). Associations between individual clinical and histological parameters were analyzed with renal outcome in univariate analyses. Significant variables were further analyzed in multivariate analyses in 2 models, with and without JHC.
Statistical analyses
Parametric and nonparametric variables were expressed as mean (SD), and median (IQR). Categorical variables were expressed in percentages and compared using Spearman’s test. The event of a 50% increase in serum creatinine was analyzed with the Kaplan Meier method, using the time of renal biopsy as the starting point, and log rank test was performed for comparisons of renal survival between the two groups. Survival analysis using Cox regression was performed to test the association between histologic lesions and clinical outcomes. Univariate Cox regression was used to determine factors predicting renal outcomes. Histologic variables associated with outcomes were further studied through multivariate models with clinical variables. Results were expressed as hazard ratios (HR) with 95% confidence intervals (CI). The proportional hazards assumption for covariates was tested using scaled Schoenfeld residuals. P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata/SE (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Clinical and histologic characteristic
The clinical and histologic baseline characteristic of 86 patients at the time of biopsy and treatment during the follow-up period are presented in Table 1. During a median observational period of 6.8 years, 13 (15%) patients reached a renal outcome. A total of 72 patients (84%) were treated with RAS blockade and 57 patients (66%) were treated with corticosteroids. At the end of follow-up, the median eGFR was 62 [IQR: 45–86] ml/min/1.73 m2, and proteinuria was decreased to 0.8 [IQR: 0.6–1.4] g/day.
Histologic parameters are shown in Table 1: M0/1: 68/18 (79/21%), E0/1: 51/35 (59/41%), S0/1: 28/58 (33/67%), T0/1/T2: 74/5/7 (86/6/8%) in the Oxford Classification, HG1:42 (49%), HG2:31 (36%), HG3/4:13 (15%) in JHC and crescent negative/positive: 47/39 (55/45%). In the correlation study, a significant but mild correlation was demonstrated only between the T score and JHC (r = 0.28, p = 0.03), but no correlation revealed between MES scores and crescent with JHC.
Renal outcome studies
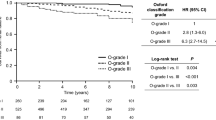
Kaplan–Meier survival curves are shown in Fig. 1. The log-rank test revealed significant differences between T0 and T1+2 (p < 0.001), and among HG1, HG2, and HG3+4 (p < 0.001). Cox regression analyses revealed that the amount of proteinuria, sCr, eGFR, and UA in clinical variables, the T score (T0/1+2) and JHC (HG1/2/3+4) in histologic variables were significantly associated with renal outcome in univariate analyses (Table 2). Multivariate analyses were performed for age, sex, hypertension, amount of proteinuria, eGFR, UA and T score (T0/1+2), with the addition of the JHC in model 2. Although sCr and eGFR were significant variables in univariate analyses, only eGFR was included in multivariate analyses because of theoretical confounding. In model 1, only the T score was a significant variable for renal outcome (HR: 4.44, 95% CI: 1.11–17.73, p = 0.04). In model 2, only the JHC was demonstrated as a significant variable (HR: 3.23, 95% CI: 1.07–9.74, p = 0.04) is shown in Table 2, and Kaplan-Meier survival curves is shown in Fig. 2.
Kaplan–Meier survival curves analyses free from renal outcome were conducted with MEST-scores in the Oxford Classification and crescent. Kaplan–Meier survival curves analysis free from renal outcome a between mesangial hypercellularity [M0 (continuous line) and M1 (dashed line)], b between endocapillary proliferation [E0 (continuous line) and E1 (dashed line)], c between segmental glomerulosclerosis [S0 (continuous line) and S1 (dashed line)], d between tubular atrophy/ interstitial fibrosis [T0 (continuous line) and T1+T2 (dashed line)], and e between crescents [C0 (continuous line) and C1 (dashed line)]. P values by long-rank test were shown
Discussion
In this study, pathological severities in renal biopsy specimens were evaluated according to the Oxford Classification and the JHC and then analyzed for renal outcome defined by a 50% increase in sCr in a retrospective cohort of 86 IgA nephropathy patients at Nagoya University Hospital. Significant associations were demonstrated in the JHC and T score, with regards to renal outcome. However, neither M/E/S scores nor crescents could predict renal outcomes in this study. This was the first known study to analyze both the JHC and Oxford Classifications in regards to renal outcomes. In a good agreement the predictive value of the JHC study in 2013 [24] and the JHC validation study in 2015 [25] were reproduced in current study. Hazard ratios of HG3/4 and HG2 were 8.29 and 2.89, respectively, compared to HG1 (HR: 1) in current study. Furthermore, a mild but significant correlation of JHC with the T score in the Oxford Classification was revealed in present study. Although the JHC was evaluated only by glomerular lesions, it was correlated with tubular atrophy/interstitial fibrosis in renal biopsy specimens, which was a well-established predictive pathological finding in most renal diseases including IgA nephropathy. In multivariate analyses, the JHC was the only significant pathological parameter to predict renal outcomes in the present study, but superiority between the JHC and T score should be analyzed in further studies with a larger study population. In the present study consecutive patients diagnosed as IgA nephropathy between 2001 and 2009 were analyzed. In 1999, Pozzi et al. reported the clinical effect of methyl-prednisolone pulse therapy [31]. Thus, choice of treatment was changed in Nagoya University Hospital around 2000. Therefore, patients diagnosed after 2001 were treated according to the uniformed strategy. Among IgAN patients diagnosed after 2010, no patients met 50% increase in sCr until the end of follow-up.
The original Oxford study, 17 previous validation studies of the Oxford Classification and the present study are summarized in Table 3. Although all MEST scores except crescents could predict renal outcomes in the original Oxford study [22], Katafuchi et al. reported that only S and T scores were significantly associated with end stage renal failure (ESRF) in Japanese IgA nephropathy patients [32]. However, in multivariate analysis including extracapillary proliferation, revealed that only T score was significant independent predictive factor for ESRF. The present study was the second validation study of the Oxford Classification among adult Japanese patients with IgA nephropathy, which demonstrated that the T score rather than the S score was significantly associated with renal outcomes. Crescents showed a tendency to predict renal outcome in the Kaplan-Meier curve without statistical significance, probably due to the limited sample size compared to Katafuchi’s study. No trend associated with renal outcomes was revealed for M, E or S scores in the current study. The negative predictive value of the S score was published in 11 out of 17 (64.7%) validation studies. Reproduction as predictive pathological factors only occurred in 5 (29.4%) studies for the M score and 2 (11.8%) study for the E score. Since a modification of the Oxford classification is in progress, the clinical significance of M, E, S scores and crescents should be fully analyzed in various cohort studies including the Japanese population.
The inconsistency of this study with the original Oxford Classification [22] and VALIGA [33] studies to predict renal outcome may be caused by differences in the clinical and histologic background of patients, such as lower percentages of male (51% vs. 72/73%), lower sCr (0.9 vs. 1.2/1.2 mg/dL) and fewer hypertensive patients (35 vs. 65%). Moreover, histologic parameters of the present study were considerably different from the previous studies. Specifically, patients in the present study exhibited a lower proportion of M1 (21 vs. 78%) and T1/2 (14% vs. 24/21%), but higher proportions of E1 (41% vs. 37/11%) and crescents (45 vs. 41/9%) compared to the original Oxford and VALIGA studies, which would suggest that Japanese patients were diagnosed in earlier and more active phases, because of annual urinalysis screening programs [24]. Due to different inclusion criteria, 11 patients with eGFR less than 30 ml/min/1.73 m2, and 13 patients with proteinuria less than 0.5 g/day were included in this study. In addition, we excluded pediatric patients (<18 years) from our cohort study, while the pediatric population accounted for 9.1% of patients in the 2013 JHC study and 22% of patients in the Oxford Classification. Furthermore, we studied the end point with a 50% increase in sCr but not a 50% decrease in eGFR, ESRD and the rate of renal function decline. Although the proportion of renal outcome was 15% in this study, the median observation period was longer than the original Oxford and VALIGA studies. This is most likely because Japanese IgAN patients were diagnosed and treated with immunosuppressive therapies at earlier phases.
Limitations of the current study are its retrospective study design and the limited number of cases. However, all patients with inclusion criteria were able to be analyzed in this study, and significant results were demonstrated. Also, there have been smaller retrospective cohort studies published from Korea [34] (69 patients) and from USA (54 patients) [35]. The definition of renal outcome in current study was a 50% increase in sCr. Although the defined renal outcome varies among validation studies, several lines of evidence suggest clinical value of using a 50% increase in sCr as the renal outcome definition [1, 28–30].
Conclusion
In conclusion, the JHC and the T score in the Oxford Classification were associated with renal outcome among Japanese patients with IgAN. The superiority of the JHC as a predictive index should be validated with larger study population and cohort studies in different ethnicities.
References
Tatematsu M, Yasuda Y, Morita Y, Sakamoto I, Kurata K, Naruse T, et al. Complete remission within 2 years predicts a good prognosis after methylprednisolone pulse therapy in patients with IgA nephropathy. Clin Exp Nephrol. [Internet]. 2012; Available from: http://www.ncbi.nlm.nih.gov/pubmed/22618296.
Donadio J V, Grande JP. IgA nephropathy. N Engl J Med [Internet]. 2002;347:738–48. Available from: http://www.nejm.org/doi/full/10.1056/NEJMra020109.
Geddes CC, Rauta V, Gronhagen-Riska C, Bartosik LP, Jardine AG, Ibels LS, et al. A tricontinental view of IgA nephropathy. Nephrol Dial Transplant. 2003;18:1541–8.
Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. Prognosis in IgA Nephropathy: 30-Year Analysis of 1, 012 Patients at a Single Center in Japan. 2014;9.
D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. American journal of kidney diseases: the official journal of the National Kidney Foundation [Internet]. 2000;36:227–37. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10922300.
Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses [Internet]. American Journal of Kidney Diseases. 1991. p. 12–9. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-0025821025&partnerID=tZOtx3y1.
Szeto CC, Lai FMM, To KF, Yuk-Hwa Wong T, Chow KM, Cheung-Lung Choi P, et al. The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am J Med. 2001;110:434–7.
Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol JASN. 2011;22:752–61.
Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrology Dialysis. Transplantation. 2009;24:3068–74.
Ikee R, Kobayashi S, Saigusa T, Namikoshi T, Yamada M, Hemmi N, et al. Impact of hypertension and hypertension-related vascular lesions in IgA nephropathy. Hypertens Res Off J Jpn Soc Hypertens [Internet]. 2006;29:15–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16715649.
Bartosik LP, Lajoie G, Sugar L, Cattran DC. Predicting progression in IgA nephropathy. Am J Kidney Dis Off J Natl Kidney Found [Internet]. 2001;38:728–35. Available from: http://www.sciencedirect.com/science/article/pii/S0272638601420440.
Lee SMK, Rao VM, Franklin WA, Schiffer MS, Aronson AJ, Spargo BH, et al. IgA nephropathy: Morphologic predictors of progressive renal disease. Hum Pathol. 1982;13:314–22.
Meadow SR, Glasgow EF, White RHR, Moncrieff MW, Cameron JS, Ogg CS. Schönlein—henoch nephritis. QJM. 1972;41:241–60.
Alamartine E, Sabatier JC, Berthoux FC. Comparison of pathological lesions on repeated renal biopsies in 73 patients with primary IgA glomerulonephritis: value of quantitative scoring and approach to final prognosis. Clin Nephrol [Internet]. 1990;34:45–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2225552.
Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N, Ikeda K, Yanase T, et al. Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. 1998;49:9491278.
Lee HS, Koh HI, Lee HB, Park HC. IgA nephropathy in Korea: a morphological and clinical study. Clin Nephrol. 1987;27:131–40.
Manno C, Strippoli GFM, D’Altri C, Torres D, Rossini M, Schena FP. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis. 2007;49:763–75.
Radford MG, Donadio JV, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. Journal of the American Society of Nephrology: JASN. 1997;8:199–207.
Shlgematsu H. Histological grading and staging of IgA nephropathy. 1997;194–202.
Lee HS, Lee MS, Lee SM, Lee SY, Lee ES, Lee EY, et al. Histological grading of IgA nephropathy predicting renal outcome: revisiting H.S. Lee’s glomerular grading system. Nephrol Dial Transplant. 2005;20:342–8.
Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis Off J National Kidney Found. 1997;29:829–42.
Cattran DC, Coppo R, Cook HT, Feehally J, Roberts ISD, Troyanov S, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–45.
Roberts ISD, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–56.
Kawamura T, Joh K, Okonogi H, Koike K, Utsunomiya Y, Miyazaki Y, et al. A histologic classification of IgA nephropathy for predicting long-term prognosis: Emphasis on end-stage renal disease. J Nephrol. 2013;26:350–7.
Sato R, Joh K, Komatsuda A, Ohtani H, Okuyama S, Togashi M, et al. Validation of the Japanese histologic classification 2013 of immunoglobulin A nephropathy for prediction of long-term prognosis in a Japanese single-center cohort. Clin Exp Nephrol [Internet]. Springer Japan; 2015;19:411–8. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s10157-014-1004-z.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Of OJOS, Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements [Internet]. 2013;3:4–4. Available from: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO CKD-MBD GL KI Suppl 113.pdf%5Cn. http://www.nature.com/doifinder/10.1038/kisup.2012.73%5Cn, http://www.nature.com/doifinder/10.1038/kisup.2012.76.
Peters HPE, Brand JAJ Van Den, Berger SP, Wetzels JFM. Immunosuppressive therapy in patients with IgA nephropathy. 284–9.
J.V. DJ, J.P. G, E.J. B, R.A. D, T.S. L, D.C. S. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. J Am Soc Nephrol [Internet]. 1999;10:1772–7. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed4&NEWS=N&AN=1999272369.
Lee H, Hwang JH, Paik JH, Ryu HJ, Kim DK, Chin HJ, et al. Long-term prognosis of clinically early IgA nephropathy is not always favorable. BMC nephrologyyr [Internet]. 2014;15:94. Available from: /pmc/articles/PMC4070337/?report = abstract.
Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet [Internet]. 1999;353:883–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10093981.
Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H. Validation study of Oxford classification of IgA nephropathy: the significance of extracapillary proliferation. Clin J Am Soc Nephrol. 2011;2806–13.
Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney international [Internet]. 2014;86:828–36. Available from: http://www.nature.com.e.bibl.liu.se/ki/journal/v86/n4/full/ki201463a.html.
Lee H, Yi SH, Seo MS, Hyun JN, Jeon JS, Noh H, et al. Validation of the oxford classification of IgA nephropathy: A single-center study in Korean adults. Korean J Intern Med. 2012;27:293–300.
Yau T, Korbet SM, Schwartz MM, Cimbaluk DJ. The Oxford Classification of IgA Nephropathy: a Retrospective Analysis. American Journal of Nephrology. 2011;435–44.
Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol [Internet]. 2010;5:425–30. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20089495.
Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT, et al. The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney international [Internet]. 2010;77:921–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20200498.
Herzenberg AM, Fogo AB, Reich HN, Troyanov S, Bavbek N, Massat AE, et al. Validation of the Oxford classification of IgA nephropathy. Kidney International [Internet]. 2011;80:310–7. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0085253815550266.
Shi SF, Wang SX, Jiang L, Ji-Cheng L, Liu LJ, Chen YQ, et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the Oxford classification. Clin J Am Soc Nephrol. 2011;6:2175–84.
Alamartine E, Sauron C, Laurent B, Sury A, Seffert A, Mariat C. The use of the oxford classification of IgA nephropathy to predict renal survival. Clin J Am Soc Nephrol. 2011;6:2384–8.
Nasri H, Mortazavi M, Ghorbani A, Shahbazian H, Kheiri S, Baradaran A, et al. Oxford-MEST classification in IgA nephropathy patients: a report from Iran. J Nephropathol. 2012;1:31–42.
Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, et al. A multicenter application and evaluation of the oxford classification of iga nephropathy in adult Chinese patients. Am J Kidney Dis. 2012;60:812–20.
Shima Y, Nakanishi K, Hama T, Mukaiyama H, Togawa H, Hashimura Y, et al. Validity of the Oxford classification of IgA nephropathy in children. Pediatr Nephrol. 2012;27:783–92.
Kang SH, Choi SR, Park HS, Lee JY, Sun IO, Hwang HS, et al. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrol Dial Transplant. 2012;27:252–8.
Le W, Zeng C-H, Liu Z, Liu D, Yang Q, Lin R-X, et al. Validation of the Oxford classification of IgA nephropathy for pediatric patients from China. BMC nephrology [Internet]. 2012;13:158. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3519602&tool=pmcentrez&rendertype=abstract.
Kfoury H, Alsuwaida A, Hussain S, Aloudah N, Alhejaili F, Alsaad K, et al. External validation of the Oxford classification of IgA nephropathy: a retrospective study of 70 patients from Saudi Arabia. Hong Kong J Nephrol. 2014;16:29–33.
Serriello I, Polci R, Feriozzi S, Gigante A, Giulio SDI, Rosa M, et al. Extracapillary proliferation is an independent predictive factor in Immunoglobulin A nephropathy Correspondence: Background: Oxford classification of Immunoglobulin A Nephropathy. 2015;20:654–9.
Ştefan G, Ismail G, Stancu S, Zugravu A, Andronesi A, Mandache E, et al. Validation study of Oxford Classification of IgA Nephropathy: the significance of extracapillary hypercellularity and mesangial IgG immunostaining. Pathol Int. 2016;66:453–9.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Progressive Renal Diseases Research, Research on Rare and Intractable Disease, from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Department of Nephrology, Nagoya University Graduate School of Medicine received research promotion grants from Astellas, Alexion, Otsuka, Kyowa Hakko Kirin, Daiichi Sankyo, Dainippon Sumitomo, Takeda, Torii, Pfizer, and Mochida. Department of CKD Initiatives, Nagoya University Graduate School of Medicine received donation from MSD, Dainippon Sumitomo, Kyowa Hakko Kirin, Kowa, Chugai, Boehringer Ingelheim, Nihon Medi-Physics Co.,Ltd. and research promotion grants from Daiichi Sankyo, Takeda, Torii, Astellas, and Shionogi.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB approval number 1135/2015-0386) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The ethical committee approved this retrospective cohort study without written informed consent, but informed consent was obtained from almost all study participants at the time of renal biopsy.
About this article
Cite this article
Kaihan, A.B., Yasuda, Y., Katsuno, T. et al. The Japanese Histologic Classification and T-score in the Oxford Classification system could predict renal outcome in Japanese IgA nephropathy patients. Clin Exp Nephrol 21, 986–994 (2017). https://doi.org/10.1007/s10157-017-1393-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1393-x