Abstract
Background
The reasons for improved outcomes associated with preemptive kidney transplantation (PKT) are incompletely understood, and post-transplant complications have been scarcely investigated.
Methods
We evaluated the outcomes of PKT in both unmatched (n = 1060) and propensity score matched cohorts (n = 186) of adults who underwent living kidney transplant between 2000 and 2014. Outcomes were estimated glomerular filtration rate (eGFR), biopsy-proven rejection, cytomegalovirus (CMV) infection, post-transplant diabetes mellitus (PTDM), cardiovascular disease (CVD), graft failure (non-censored for death), and malignancy. Primary endpoint was post-transplant renal function assessed with eGFR.
Results
A total of 95 patients (9.0 %) underwent PKT. The 2-week mean eGFR after transplant was comparable between the matched PKT and non-PKT groups (45.2 vs. 46.5 mL/min/1.73 m2, respectively, P = 0.56). Sensitivity analysis using various formulas did not change the results. PKT was not superior to non-PKT in reducing the risk of biopsy-proven rejection, CMV, PTDM, and malignancy, regardless of matching. The risk of graft failure and CVD was significantly reduced in the unmatched PKT group (ARR, −6.2 %; 95 % CI, −8.6 to −0.7; P = 0.03, and ARR, −6.7 %; 95 % CI, −9.6 to −0.7, P = 0.03, respectively); nevertheless, the corresponding ARRs were −3.2 % (95 % CI, −10.0 to 2.9; P = 0.44) and −2.2 % (95 % CI, −9.1 to 4.4; P = 0.72) after matching.
Conclusions
PKT was associated with neither improvement of post-transplant renal function nor a lower rate of common post-transplant complications than non-PKT among patients with end-stage renal disease who underwent living KT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preemptive kidney transplantation (PKT), defined as kidney transplantation (KT) prior to maintenance dialysis, has been associated with improvements in outcomes compared with non-PKT [1–3]. Thus, in the past decade, PKT has become widespread and plays an important role worldwide, especially in living-donor KT.
Several explanations were proposed for this beneficial effect of PKT. To date, the contribution of residual native kidney function often appears to be the most likely hypothesis [4]. However, few studies have compared outcomes when PKT was performed at different levels of estimated glomerular filtration rate (eGFR) [5–7]. In addition, there are no randomized controlled trials (RCTs) that test this hypothesis or RCTs demonstrating that PKT reduces the risk of adverse outcomes [8, 9].
Recent systematic review revealed that all previous studies on PKT had a high risk for selection bias, and it was uncertain whether adjustments to correct for potential compromise in the integrity of the findings were corrected. In addition, patient survival, graft survival, and rejection rate have been reported in many studies, but post-transplant complications have rarely been reported [9]. Thus, the reasons why PKT is associated with improved outcomes are not completely understood. Additionally, data regarding its effect on post-transplant complications remain lacking.
Although RCTs on PKT involving living donors should be conducted, this seems to be challenging in many countries. Thus, we investigated whether PKT in recipients with end-stage renal disease (ESRD) would improve clinical outcomes compared with non-PKT when analyzed differently from other conventional multivariable modeling methods. The objective of this study was to use a matched observational cohort study design to attempt to estimate a more reliable effect of PKT on post-transplant renal function using eGFR and a wide range of major post-transplant adverse events that have not been investigated in previous studies, such as cytomegalovirus (CMV) infection, post-transplant diabetes mellitus (PTDM), cardiovascular disease (CVD), and malignancy.
Materials and methods
Study oversight and population
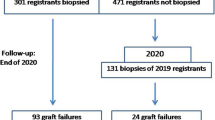
A total of 1060 patients from the Japan Academic Consortium of Kidney Transplantation (JACK) study were included in this study. The JACK study is an ongoing multicenter, observational cohort study in Japan. The objectives and protocol of the JACK have been described in more detail previously [10]. We included adults (≥18 years old) who underwent living KT between 2000 and 2014 and who had completed follow-up at three transplant centers. We excluded patients if they had donor-specific antibody (DSA)-positive results on complement-dependent cytotoxicity assay or flow cytometry crossmatch. All patients were administered optimal immunosuppressive therapy at that time. Our detailed immunosuppressive regimens have been also published previously [11]. We evaluated the effect of PKT on endpoints using a two-cohort approach, an unmatched crude cohort, and a matched cohort using propensity score matching (PSM) [12]. The study flow diagram is shown in Fig. 1.
Endpoint and follow-up
The primary endpoint was post-transplant renal function assessed with the eGFR. Secondary endpoints included biopsy-proven rejection, CMV infection, PTDM, CVD, graft failure (non-censored for death), and malignancy. The final follow-up was performed on October 31, 2015; patients whose follow-up periods exceeded 10 years had their data censored at 10 years. Follow-up information on the endpoint was ascertained prospectively. The follow-up duration is shown in Fig. 1.
Definition
PKT was defined as transplant prior to initiation of maintenance dialysis, and we included patients who had received dialysis of 1 week’s duration or less prior to transplantation as preconditioning for operation. The eGFR was calculated using a Japanese-modified equation [13]. For sensitivity analysis, eGFRs using the Modification of Diet in Renal Disease Study (MDRD-4), the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), and the Cockcroft-Gault equations were also calculated [14–16].
The definition of rejection included T cell-mediated rejection or antibody-mediated rejection, acute or chronic, proven from episode or protocol biopsy. Since 2000, our institute and two affiliated centers have used protocol biopsy whenever possible. CMV infection included the presence of CMV in plasma by pp65 antigenemia and clinical signs and symptoms attributable to CMV infection. PTDM was defined as introduction of insulin, oral, or diet therapy. CVD events included heart and blood vessel disorders, including coronary heart disease, cerebrovascular disease, rheumatic heart disease, and related other conditions. Malignancy included cancer of the kidney, bladder, prostate, thyroid, lung, uterus, breast, digestive organs, or other organs. Tacrolimus trough levels were measured using locally available assays.
Statistical analysis
Analyses were performed with SAS system ver. 9.4 software (SAS Institute, Cary, NC, USA). Data are presented as means with standard deviation, medians with interquartile ranges, and frequency (percentage). Student’s t test was used to compare normally distributed continuous variables. The Mann–Whitney U test was used for the variables with skewed distributions. The χ 2 or Fisher’s exact test (when the expected value was <5) was used to compare nominal-scale variables. Propensity scores [1 + exp(−α−β′x)]−1 for the estimated probability of each individual undergoing PKT were calculated using a multiple logistic regression model [12]. This model included 15 variables (14 dummy variables of transplant year and recipient age). Matching procedure was performed with Structured Query Language (a programming language designed for managing data held in a relational database management system). Matched cohort had 92 % power to detect an eGFR mean difference of 5 mL/min/1.73 m2, with α = 0.05 (two-tailed). The between-group comparisons of eGFR were performed with Student’s t test. To identify the measures of effect of PKT on event rate, absolute risk reduction (ARR) with a 95 % confidence interval (CI) based on the Wilson score was applied [17]. Two-tailed P values less than 0.05 were considered to indicate statistically significant differences. All analyses were performed by an independent statistical data center (STATZ Institute, Inc., Tokyo, Japan).
Results
Characteristics
Of the 1060 patients, 95 (9 %) underwent PKT. The characteristics before and after PSM are summarized in Table 1. According to PSM procedures, we were able to match 186 patients (93 patients in each group). There was no appreciable change regarding confounders in patients who underwent PKT between the unmatched (95 patients) and matched population (93 patients). In contrast, substantial changes in covariates were observed in the non-PKT group (decreased from 965 to 93 patients). As a result, the median duration of dialysis in the non-PKT population decreased from 30 to 24 months. In the unmatched cohort, the PKT group was less likely to have glomerulonephritis etiology and more likely to have polycystic kidney disease, hypoplastic kidney, or focal segmental glomerulosclerosis compared with the non-PKT group. In addition, the PKT group was less likely to have preexisting DSA and had a shorter total ischemic time. In immunosuppression regimens, patients in the PKT group were more likely to have once-daily extended-release tacrolimus and lower trough levels and were more often treated with intensive interleukin 2 receptor and chimeric monoclonal antibody regimens. With respect to transplant year, a marked difference was observed between the two groups in the unmatched cohort. More than 80 % of patients in the PKT group had undergone transplantation in the previous 5 years. As a result, the PKT group had a significantly shorter follow-up period than the non-PKT group (Fig. 1). Meanwhile, in the matched cohort, the two groups were well balanced with respect to all potential relevant clinical confounders, and no substantial imbalance was found regarding follow-up duration (Table 1; Fig. 1).
Primary endpoint
Table 2 shows pre- and post-transplant eGFR as renal function. In the unmatched cohort, the pre-transplant mean eGFR was significantly higher in the PKT group than in the non-PKT group (7.2 vs. 4.7 mL/min/1.73 m2, respectively), and the mean difference was 2.5 (P < 0.001). In the matched cohort, the corresponding mean eGFR was 7.3 and 4.9 mL/min/1.73 m2, respectively (mean difference, 2.4; P < 0.001). In the matched cohort, the mean eGFR at 2 weeks after transplant was similar in both groups (45.2 vs. 46.5 mL/min/1.73 m2 in the PKT and non-PKT groups, respectively; P = 0.56). We also performed sensitivity analysis using another estimate formula in the matched cohort. The mean eGFR at 2 weeks after transplant in the PKT and non-PKT groups was 58.5 ± 21.9 vs. 60.5 ± 18.1 (P = 0.50) in the MDRD-4 equation, 70.3 ± 34.0 vs. 69.2 ± 24.3 (P = 0.80) in the CKD-EPI equation, and 53.2 ± 16.1 vs. 52.0 ± 13.1 mL/min/1.73 m2 (P = 0.62) in the Cockcroft–Gault equation (mL/min), respectively. Significant improvement of post-transplant renal function in patients with PKT was not observed in any equation.
Secondary endpoints
Figure 2 shows the number (percentage) of events and ARR of each outcome. In the unmatched population, 82 (7.7 %) of the 1060 patients lost their graft during the follow-up period. In both unmatched and matched cohorts, PKT was not superior to non-PKT in reducing the risk of biopsy-proven rejection, CMV infection, PTDM, or malignancy. There was significant reduction in the risk of graft failure (ARR −6.2 %; 95 % CI −8.6 to −0.7; P = 0.03) and CVD events (ARR −6.7 %; 95 % CI −9.6 to −0.7; P = 0.03) for patients who received PKT as compared with those in the non-PKT group. Nevertheless, after PSM, this reduction in graft failure (ARR −3.2 %; 95 % CI −10.0 to 2.9; P = 0.44) and CVD events (ARR −2.2 %; 95 % CI −9.1 to 4.4; P = 0.72) was not observed in the PKT group.
Outcomes before and after matching. Values are given as numbers (%). aThe absolute risk reduction in outcomes between the two groups was derived by subtracting the value of the non-PKT group from the value for the PKT group. b95 % confidence interval was calculated using the Newcombe method. cP values were calculated using Fisher’s exact test. dPost-transplant diabetes mellitus included preexisting diabetes
Discussion
In a propensity score matched cohort study involving adult kidney transplant recipients from living donors, we found post-transplant mean eGFR levels in the PKT group to be similar to those in the non-PKT group. Additionally, we were not able to show a significant definitive beneficial effect on PKT in terms of biopsy-proven rejection, CMV, PTDM, CVD, graft failure and malignancy.
In addition to clinical outcome benefits, a number of studies have suggested that PKT improves various outcomes, quality of life, return-to-work interval, and cost-effectiveness [1–3, 8, 18]. Given these excellent reports, the worldwide trend appears to be a promotion of PKT [7–9]. Although PKT varies widely across transplant centers in Japan [19], the number of PKT is growing steadily. According to the domestic 2014 report, the percentage of PKT from living donors was 24.5 % and the pre-transplant average eGFR in PKT was 8.4 mL/min/1.73 m2. The proportion of PKT of current study in 2014 was 19 %. We should be aware of the variance of PKT definition across the studies. For instance, whereas Goto et al. included transplantation with <1 month of dialysis period as PKT in their report [19], our definition of PKT was dialysis of 1 week’s duration or less prior to transplantation. PKTs from deceased donors have been hardly performed due to the legal framework in Japan.
Our results showed that post-transplant mean eGFR are almost equivalent within the 2 weeks after transplant between the groups regardless of matching. Sensitivity analysis using other formulas did not change the results. A possible explanation of this finding was the marked rapid reduction in renal blood flow to the native kidneys following successful living KT [20]. Although contribution from residual native kidney function or lead time bias may explain the better clinical outcomes in PKT, this hypothesis is unlikely to prove that PKT is superior to non-PKT in decreasing the risk of post-transplant complications in our study population.
Our results differ from those of previous studies that showed that PKT is associated with improved outcomes [1–3, 19, 21]. In contrast, our results are similar to those of studies [5–7, 22]. Ishani et al. and Akkina et al. [5, 6]. found no correlation between pre- and post-transplant eGFR. Gill et al. [22]. hypothesized that the apparent graft survival advantage of PKT is a consequence of patient selection. More recently, Grams et al. [7], who used multiple analytic methods to minimize bias including propensity score adjustment (not matching), concluded that PKT may be unlikely to improve clinical outcomes and that performing PKT too early may waste native kidney function and prematurely expose donors and recipients to the operative and immunosuppressive risks associated with KT. The IDEAL study, an RCT, suggested that planned early initiation of dialysis in patients with stage 5 chronic kidney disease was not associated with an improvement in survival or clinical outcomes [23].
In the present study, there were substantial imbalances before PSM, and PKT was associated with a reduction of graft failure and CVD. A confounder to outcomes in an unmatched population with PKT appears to be more favorable than non-PKT [24–28]. Selection bias in an observational study is completely independent of study sample size [29], and this is frequently the most troublesome aspect of an observational therapeutic study. Most authors who have conducted large-scale studies have recognized this limitation [2, 3, 7, 8].
The foremost challenging consideration in this study was a marked difference in transplant year rather than advantages with a plausible biological basis of PKT population. During the preparation of this manuscript, we found an earlier or more rapid decline in the number of patients at risk, using the Kaplan–Meier method, in the PKT group than in the non-PKT group in past studies, despite the fact that those studies demonstrated better outcomes of PKT. This appearance contradicts with the mathematical theory of time-to-event analysis. Indeed, we were able to develop a well-matched cohort with only two covariates: transplant year and recipient age. These findings suggest that observational therapeutic studies of PKT were potentially limited because of biases due to the different KT periods. This is suspected to be the essential drawback, causing other apparent crucial imbalance including immunosuppressive regimens. Since PKT has been increasing across many countries lately, this bias appears to be a considerable modifying factor of the outcomes. Meanwhile, there were no differences in eGFR even before PSM. This result may not have been affected by this bias because it was derived from between-group comparisons over the same visit time period.
We should be aware that the inability of the significant effects of PKT on graft failure and CVD was also associated with sample size and event number. We sacrificed statistical power to ensure maximum internal validity in this study. The matched cohort had more than 90 % power to detect difference in mean eGFR, but had inadequate power of 67 % to detect reduction of graft failure and CVD. Japan has a low death rate from CVD, which is the most frequent cause of death with functioning graft, and the mortality rates of patients with ESRD are lower [30, 31]. However, in general, unlike P values and confidence intervals, the point estimate of ARR as “measures of effect” does not change with sample size [32]. Of particular note is that the beneficial effects of PKT in all endpoints declined by half in the well-balanced matched cohort when compared to those in imbalanced unmatched cohorts. This finding suggests that inadequate adjustment of confounding can lead to risk of overestimated efficacy of PKT.
We should refer to several limitations. First, our results were derived from an observational study. We used PSM to eliminate potential confounders, and a similar distribution of confounders was observed between the two groups; however, this compatibility was limited to available data. Our study cannot ensure internal validity on unmeasured confounders, unlike in an RCT.
Second, the present study population consisted of recipients with ESRD (mean eGFR <10 mL/min/1.73 m2). To date, PKT is usually considered when renal function is better preserved [7]. Thus, these may not be appropriate study conditions to clarify the contribution from “residual native kidney function” in patients with PKT. In the present study, we cannot refer to the optimal timing of PKT at all.
Finally, PKT varies widely by country and within country by region, and it is influenced by major non-clinical factors. The usefulness of PKT cannot be determined only for adults or in certain clinical outcomes. Pediatric PKT, social aspects of recipients, legal framework, health insurance, and policies in each country are beyond the scope of this article [8, 33–39].
Despite these limitations, our findings present new insights into PKT. To our knowledge, this is the first comparative study to assess the effect of PKT after demonstrating definitive well-balanced covariates between groups. Furthermore, the strength of our study is that it assessed endpoints that have been rarely investigated [9].
In conclusion, PKT was associated with neither improvement of post-transplant renal function nor a lower rate of common post-transplant complications than non-PKT among patients with ESRD who underwent living KT. The contribution from residual native kidney function is unlikely to prove that PKT is superior to non-PKT in decreasing the risk of post-transplant complications.
References
Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58(3):1311–7.
Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344(10):726–31.
Kasiske BL, Snyder JJ, Matas AJ, et al. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol. 2002;13(5):1358–64.
Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726–32.
Ishani A, Ibrahim HN, Gilbertson D, et al. The impact of residual renal function on graft and patient survival rates in recipients of preemptive renal transplants. Am J Kidney Dis. 2003;42(6):1275–82.
Akkina SK, Connaire JJ, Snyder JJ, et al. Earlier is not necessarily better in preemptive kidney transplantation. Am J Transplant. 2008;8(10):2071–6.
Grams ME, Massie AB, Coresh J, et al. Trends in the timing of pre-emptive kidney transplantation. J Am Soc Nephrol. 2011;22(9):1615–20.
Huang Y, Samaniego M. Preemptive kidney transplantation: has it come of age? Nephrol Ther. 2012;8(6):428–32.
Abramowicz D, Hazzan M, Maggiore U, et al. Does pre-emptive transplantation versus post start of dialysis transplantation with a kidney from a living donor improve outcomes after transplantation? A systematic literature review and position statement by the Descartes Working Group and ERBP. Nephrol Dial Transplant. 2016;31(5):691–7.
Okumi M, Unagami K, Tachibana H, et al. Association between body mass index and outcomes in Japanese living kidney transplant recipients: The role of sex differences. Int J Urol. 2016;23(9):776–8.
Okumi M, Toki D, Nozaki T, et al. ABO-incompatible living kidney transplants: evolution of outcomes and immunosuppressive management. Am J Transpl. 2016;16(3):886–96.
Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52(1):249–64.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92.
Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873–90.
Friedewald JJ, Reese PP. The kidney-first initiative: what is the current status of preemptive transplantation? Adv Chronic Kidney Dis. 2012;19(4):252–6.
Goto N, Okada M, Yamamoto T, et al. Association of dialysis duration with outcomes after transplantation in a japanese cohort. Clin J Am Soc Nephrol. 2016;11(3):497–504.
D’Cunha PT, Parasuraman R, Venkat KK. Rapid resolution of proteinuria of native kidney origin following live donor renal transplantation. Am J Transplant. 2005;5(2):351–5.
Becker BN, Rush SH, Dykstra DM, et al. Preemptive transplantation for patients with diabetes-related kidney disease. Arch Intern Med. 2006;166(1):44–8.
Gill JS, Tonelli M, Johnson N, et al. Why do preemptive kidney transplant recipients have an allograft survival advantage? Transplantation. 2004;78(6):873–9.
Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–19.
Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21(8):1398–406.
Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562–75.
Webster AC, Playford EG, Higgins G, et al. Interleukin 2 receptor antagonists for renal transplant recipients: a meta-analysis of randomized trials. Transplantation. 2004;77(2):166–76.
Rostaing L, Bunnapradist S, Grinyo JM, et al. Novel once-daily extended-release tacrolimus versus twice-daily tacrolimus in de novo kidney transplant recipients: two-year results of phase 3, double-blind, randomized trial. Am J Kidney Dis. 2016;67(4):648–59.
Kutner NG, Zhang R, Huang Y, et al. Impact of race on predialysis discussions and kidney transplant preemptive wait-listing. Am J Nephrol. 2012;35(4):305–11.
Tripepi G, Jager KJ, Dekker FW, et al. Bias in clinical research. Kidney Int. 2008;73(2):148–53.
Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 Update: a Report From the American Heart Association. Circulation. 2016;133(4):e38–360.
Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol. 2003;14(12):3270–7.
Tripepi G, Jager KJ, Dekker FW, et al. Measures of effect: relative risks, odds ratios, risk difference, and ‘number needed to treat’. Kidney Int. 2007;72(7):789–91.
Bock ME, Cohn RA. Pre-emptive kidney transplantation—just do it! Pediatr Transplant. 2010;14(5):561–4.
Weng FL, Mange KC. A comparison of persons who present for preemptive and nonpreemptive kidney transplantation. Am J Kidney Dis. 2003;42(5):1050–7.
Ferrari P, Weimar W, Johnson RJ, et al. Kidney paired donation: principles, protocols and programs. Nephrol Dial Transplant. 2015;30(8):1276–85.
Knoll G, Cockfield S, Blydt-Hansen T, et al. Canadian Society of Transplantation: consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173(10):S1–25.
Maggiore U, Oberbauer R, Pascual J, et al. Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol Dial Transplant. 2015;30(2):217–22.
Andrews PA, Burnapp L, Manas D, et al. Summary of the British Transplantation Society/Renal Association U.K. guidelines for living donor kidney transplantation. Transplantation. 2012;93(7):666–73.
Jay CL, Dean PG, Helmick RA, et al. Reassessing preemptive kidney transplantation in the United States: are we making progress? Transplantation. 2016;100(5):1120–7.
Acknowledgments
We appreciate the support provided by Katsunori Shimada, PhD (STATZ Institute, Inc., Tokyo, Japan), who provided expert assistance with statistical analysis. Astellas Pharma Inc. (Tokyo, Japan) supported this study with a grant. The sponsor was not involved in the study design, patient enrollment, data collection, analysis, interpretation, or preparation of the manuscript.
JACK Investigators
Department of Urology, Tokyo Women’s Medical University: Kazunari Tanabe (principal investigator), Hideki Ishida, Masashi Inui, Tomokazu Shimizu, Masayoshi Okumi, Toshihito Hirai, and Daisuke Toki. Department of Transplant Surgery, Kidney Center, Toda Chuo General Hospital: Hiroshi Toma and Kazuya Omoto. Department of Urology, Ohkubo Hospital: Hiroki Shirakawa.
Transplant Coordinator
Department of Urology, Tokyo Women’s Medical University: Miyuki Furusawa
Clinical Research Coordinator
Department of Urology, Tokyo Women’s Medical University: Makiko Fujiwara and Kayo Kusubayashi.
Biostatistics and Data Center
Department of Biostatistics, STATZ Institute, Inc., Tokyo, Japan: Katsunori Shimada.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The study was carried out according to the principles of the Declaration of Helsinki. This study protocol was approved by the research ethics committee (Approval No.: 3366-R). Information for the JACK study has been provided in the registration with the University Hospital Medical Information Network (UMIN000018327).
Conflict of interest
None of the authors have any of conflict of interest to declare with regard to the content of this article.
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Okumi, M., Sato, Y., Unagami, K. et al. Preemptive kidney transplantation: a propensity score matched cohort study. Clin Exp Nephrol 21, 1105–1112 (2017). https://doi.org/10.1007/s10157-016-1345-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1345-x