Abstract
Background
Podocytes play a central role in the formation of the glomerular filtration barrier in the kidney, and their dysfunction has been shown to result in proteinuria. In the present study, we sought to determine the cell-autonomous role of NF-κB, a proinflammatory signaling, within podocytes in proteinuric kidney disease.
Methods
Podocyte-specific IκBΔN transgenic (Pod-IκBΔN) mice, in which NF-κB was inhibited specifically in podocytes, were generated by the Cre-loxP technology, and their phenotype was compared with control mice in adriamycin-induced nephropathy.
Results
Pod-IκBΔN mice were phenotypically normal and did not exhibit proteinuria at the physiological condition. By the intravenous administration of adriamycin, overt proteinuria appeared in Pod-IκBΔN mice, as well as in control mice. However, of interest, the amount of proteinuria was significantly lower in adriamycin-injected Pod-IκBΔN mice (373 ± 122 mg albumin/g creatinine), compared with adriamycin-injected control mice (992 ± 395 mg albumin/g creatinine). Expression of podocyte-selective slit diaphragm-associated proteins, such as nephrin and synaptopodin, was markedly decreased by adriamycin injection in control mice, whereas the reduction was attenuated in Pod-IκBΔN mice. Adriamycin-induced reduction in synaptopodin expression was also seen in cultured podocytes derived from control mice, but not in those from Pod-IκBΔN mice.
Conclusions
Because nephrin and synaptopodin are essential for the maintenance of the slit diaphragm in podocytes, these results suggest that proteinuria in adriamycin-induced nephropathy is caused by the reduction in expression of these proteins. The results also suggest that the NF-κB signalling in podocytes cell-autonomously contributes to proteinuria through the regulation of these proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Podocytes play a central role in the formation of the glomerular filtration barrier in the kidney [1]. They express a repertoire of cell-specific proteins, such as nephrin (Nphs1), podocin (Nphs2), and synaptopodin (Synpo), to retain albumin and other larger proteins in the blood. Dysfunction or loss of podocytes results in proteinuria in humans and in experimental animal models. Because proteinuria is an independent risk factor for progression to end-stage renal failure and for cardiovascular mortality [2], identification of molecular mechanisms and factors controlling podocyte function is likely to be the key to improve the prognosis of patients with proteinuric kidney diseases.
The nuclear factor-κB (NF-κB) family of transcription factors participates in the inflammatory process in a variety of cells [3–5]. In the resting state, NF-κB exists in the cytoplasm as an inactive dimer by association with an inhibitory protein, IκB. In response to inflammatory signals, IκB is phosphorylated on specific serine residues, serines 32 and 36, leading to its ubiquitination and consecutive proteasomal degradation. Released from IκB, NF-κB is free to translocate into the nucleus, engage DNA, and initiate transcription of many genes including cytokines, chemokines, adhesion molecules, and antioxidant proteins. Results of previous studies showed that NF-κB was activated in multiple renal cells including podocytes in various proteinuric kidney diseases, such as lupus nephritis, minimal change disease, and idiopathic membranous nephropathy in humans [6]. NF-κB was also shown to be activated in podocytes in passive Heymann nephritis in rats [7]. Moreover, systemic administration of NF-κB inhibitors, pyrrolidine dithiocarbamate and dehydroxymethylepoxyquinomicin, respectively, ameliorated proteinuria in animal models of proteinuric kidney disease [7, 8]. Results of the aforementioned studies suggest that activated NF-κB in podocytes is a potential target for treating proteinuria, thereby preventing the progression to end-stage renal failure. However, the NF-κB inhibitors described above have also been shown to block other intracellular signaling pathways [1]. In addition, systemic administration of these reagents inhibits the NF-κB activity in the whole body, including host immune responses. It is of critical importance to determine the cell-autonomous role of NF-κB within podocytes to further develop novel anti-proteinuric approaches.
Recently, IκBΔN mice have been developed in our laboratory [9, 10]. The IκBΔN mice contain the human IκBΔN transgene separated from an universal CAG promoter by a floxed STOP sequence. Following the activation of Cre recombinase, they express IκBΔN, which lacks its N-terminal of 54 amino acids including 2 phosphorylation sites at serines 32 and 36, thereby continuously inhibiting NF-κB activation as a super-repressor. In the present study, podocyte-specific IκBΔN transgenic (Pod-IκBΔN) mice were generated by crossing IκBΔN mice with Nphs1-Cre mice, which express Cre recombinase in a podocyte-specific manner [11]. Using these mice, we examined the effect of NF-κB inhibition specifically in podocytes on proteinuria in adriamycin (ADR)-induced nephropathy.
Methods
Generation of Pod-IκBΔN mice
Animal protocols were approved by Keio University Animal Care and Use Committee. Nphs1-Cre mice, generously provided by Dr. Taiji Matsusaka (Tokai University, Kanagawa, Japan) [11], were bred with IκBΔN mice [9, 10] to generate Pod-IκBΔN (Nphs1-Cre+/−/IκBΔN +/−) mice and control (Nphs1-Cre+/−/IκBΔN −/−, Nphs1-Cre−/−/IκBΔN +/−, or Nphs1-Cre−/−/IκBΔN −/−) mice. Both mice were on the C57BL/6J background, and littermates were used for all comparisons. Genotyping was performed by PCR as described previously [9, 10]. Blood pressure and heart rate were measured by the tail-cuff method (BP-98E, Softron, Tokyo, Japan).
Podocyte-specific over-expression of IκBΔN was confirmed by the following two approaches. First, total RNA was extracted from the kidney and the liver of Pod-IκBΔN and control mice, and RT-PCR was performed to amplify the fragment of human IκBΔN cDNA by human IκB-specific primers (hIκB-F: 5′-ATTGCTGAGGCACTTCTGGG-3′; and hIκB-R: 5′-GCACCCAAGGACACCAAAAG-3′), which do not amplify mouse IκB cDNA. Second, Nphs1-Cre mice were bred with Rosa LacZ-reporter mice (Jackson Laboratory, Bar Harbor, ME) that carry a loxP-flanked LacZ reporter gene in the Rosa26 locus. LacZ staining was performed in the kidney from Nphs1-Cre+/−/Rosa LacZ-reporter+/− mice.
ADR-induced nephropathy
Male Pod-IκBΔN and control mice at 12 to 14 weeks of age were injected with ADR (18 mg/kg) intravenously [12]. Two weeks after ADR injection, urine was collected for 24 h for quantification of albumin (Albuwell ELISA kit, Exocell, Philadelphia, PA) and creatinine, and the mice were killed under pentobarbital anesthesia. Blood samples were harvested for the measurement of serum concentrations of creatinine and urea nitrogen. Kidneys were divided into multiple pieces for total RNA extraction and histological analyses.
Primary culture of podocytes
Primary culture of murine podocytes was performed, as described previously [13]. Briefly, kidneys were perfused with 20 mL PBS containing 2 × 108 tosyl-activated Dynabeads M450 (Invitrogen, Carlsbad, CA). The kidney cortices were minced into 1 mm3 pieces using a surgical blade, and the tissues were digested in Hanks’ balanced salt solution (HBSS) containing 1 mg/mL collagenase A (Roche, Indianapolis, IN) and 0.2 mg/mL deoxyribonuclease I (Invitrogen) at 37 °C for 30 min. The digested tissues were pressed gently through a sterile 100 μm cell strainer, and the cell strainer was washed with 5 ml HBSS. Glomeruli were removed from the solution by a magnetic particle concentrator, and were grown on type I collagen-coated dishes in RPMI medium containing 10 % fetal bovine serum (Life Technologies, Carlsbad, CA). Subculture was performed after 5 days of the primary culture to remove remaining glomerular cores and phagocytic cells. Twenty-four hours later, cells were treated with 0.25 μg/mL ADR or saline for additional 24 h, and subjected to immunofluorescence studies.
Histology and immunostaining
The kidneys were fixed in 4 % paraformaldehyde and embedded into paraffin. The 5-μm sections were prepared and subjected to periodic acid-Schiff (PAS) staining, immunohistochemistry, and immunofluorescence studies. Histological analyses were performed, as previously described [12, 14, 15]. Immunohistochemistry was performed with antibodies for neutrophil (7/4, Abcam, Cambridge, MA), F4/80 (CI:A3-1, Abcam), and WT-1 (C19, Santa Cruz Biotechnology, Santa Cruz, CA) [15]. Staining was visualized by diaminobenzidine and sections were counterstained by hematoxylin. Immunofluorescence studies were performed with antibodies for nephrin (Progen Biotechnik, Heidelberg, Germany), podocin (Abcam), and synaptopodin (G1D4, Progen) [16]. Nuclear staining was performed with 4′, 6-diamidino-2-phenylindole. For quantification of the intensity of immunofluorescence signals, pictures were taken by Axioskop2 microscope (Carl Zeiss, Oberkochen, Germany). Digital camera exposure times were kept constant. The intensity of immunofluorescence signals was measured by Image-Pro software (Media Cybernetics, Silver Spring, MD). Ten glomeruli per mouse or twenty cells per culture were measured.
Real-time RT-PCR
Real-time RT-PCR was performed as described previously [17]. Primer sequences were as follows: Nphs1-F: 5′-CTAAGGATGGGCTGCTTCTG-3′; Nphs1-R: 5′-GTGGAACTCACCTTTAGCAC-3′; Nphs2-F: 5′-TGAGGATGGCGGCTGAGAT-3′; Nphs2-R: 5′-GGTTTGGAGGAACTTGGGT-3′; Synpo-F: 5′-TATCAACCAGAACCCGTC-3′; and Synpo-R: 5′-AATCAAGTGTGCCATTCG-3′.
Statistical analyses
Data are presented as mean ± SEM. Statistical analyses were done by SigmaPlot/SigmaStat9 (Systat Software Inc, San Jose, CA). After confirming that the data passed the normality test for parametric analyses, unpaired t test (Fig. 1d–f) or one-way factorial ANOVA with a post hoc Fisher protected least significant difference test (Figs. 2a–c, 3c, d, 6a–d, 7b, c, 8b, c) were performed. When the data failed to pass the normality test, Kruskal–Wallis non-parametric test (Figs. 3b, 4b, d, 7a) was performed. P values < 0.05 were considered significant.
Generation of Pod-IκBΔN mice. a Schematic representation of podocyte-specific overexpression of human IκBΔN transgene is shown. Nphs1-Cre mice were bred with IκBΔN mice to generate Pod-IκBΔN mice. Number 1 represents exon 1 of the Nphs1 gene. Triangles represent the loxP sites. X breeding, neo neomycin resistant gene, poly A polyadenylation signal. b Expression of human IκBΔN transgene in the kidney and the liver of Pod-IκBΔN and control (Ct) mice was examined by RT-PCR. c LacZ expression was examined in the kidneys from Nphs1-Cre+/−/Rosa LacZ-reporter+/− mice. Counterstaining was performed by eosin. Bar 100 μm. d–f Body weight (d), systolic and diastolic blood pressure (e), and heart rate (f) were measured in Pod-IκBΔN and control mice at 12 weeks of age (n = 7–8 for each group)
NF-κB inhibition in podocytes ameliorated proteinuria in ADR-induced nephropathy. Pod-IκBΔN and control mice were injected with ADR intravenously. Serum levels of urea nitrogen (a) and creatinine (b) as well as the ratio of albumin to creatinine in the urine (c) were measured 2 weeks after the injection. n = 5–7 per each group. *p < 0.05 compared with mice before the injection. #p < 0.05 compared with ADR-injected control mice
ADR-induced glomerulosclerosis was attenuated in Pod-IκBΔN mice. Pod-IκBΔN and control mice were injected with ADR intravenously, and renal histology was examined 2 weeks after the injection. n = 5–7 per each group. a Representative pictures of PAS staining are shown. Bar 100 μm. Arrows indicate intratubular casts. Inset: Representative pictures of glomeruli. b Levels of the formation of intratubular casts were scored semi-quantitatively. c The number of nuclei within the glomerulus was measured. Ten glomeruli per mouse were counted. d The severity of glomerulosclerosis was quantified. The PAS-positive area in ten glomeruli per mouse was measured. *p < 0.05 compared with mice before the injection. #p < 0.05 compared with ADR-injected control mice
ADR-induced accumulation of inflammatory cells in the kidneys was not different between Pod-IκBΔN and control mice. Pod-IκBΔN and control mice were injected with ADR intravenously, and the accumulation of neutrophils (a and b) and macrophages (c and d) in the kidneys was examined 2 weeks after the injection. n = 5–7 per each group. a and c Representative pictures of immunohistochemical staining for neutrophils (a) and macrophages (c) are shown. Neutrophils (a) and macrophages (c) were visualized by diaminobenzidine, and sections were counterstained with hematoxylin. Bars 50 μm. b and d The numbers of neutrophils (b) and macrophages (d) per 5 random fields in the kidneys were quantified. *p < 0.05 compared with mice before the injection
Results
Podocyte-specific NF-κB inhibition ameliorated proteinuria in ADR-induced nephropathy in mice
To determine the cell-autonomous role of the NF-κB signaling in podocytes for proteinuria, Pod-IκBΔN mice, in which NF-κB was inhibited specifically in podocytes, were generated by breeding Nphs1-Cre mice and IκBΔN mice (Fig. 1a). Pod-IκBΔN mice were born at the expected Mendelian ratio and were grown to adults without any differences in visible appearance, as compared with controls. As shown in Fig. 1b, expression of human IκBΔN transgene was detectable in the kidney of Pod-IκBΔN mice, but not of control mice, although it was undetectable in the liver of both mice. In addition, LacZ expression was selectively detectable in the glomeruli in the kidney of Nphs1-Cre+/−/Rosa LacZ-reporter+/− mice (Fig. 1c). These results suggest that the NF-κB signaling is specifically blocked in podocytes in Pod-IκBΔN mice. Body weight, systolic blood pressure, diastolic blood pressure, and heart rate at 12 weeks of age were similar between Pod-IκBΔN and control mice (Fig. 1d–f).
Male Pod-IκBΔN and control mice at 12–14 weeks of age were injected with ADR intravenously. Two weeks after the injection, serum levels of urea nitrogen were unaltered in both Pod-IκBΔN and control mice (Fig. 2a). Serum levels of creatinine were slightly decreased after ADR injection in both mice, compared with those before the injection (Fig. 2b). For the evaluation of proteinuria, the ratio of albumin to creatinine in the urine was measured. As shown in Fig. 2c, ADR injection elicited overt albuminuria in both Pod-IκBΔN and control mice. However, of interest, the amount of albuminuria was significantly lower in ADR-injected Pod-IκBΔN mice (373 ± 122 mg/g creatinine) than ADR-injected control mice (992 ± 395 mg/g creatinine). These results suggest that the NF-κB signaling in podocytes contributes to proteinuria in ADR-induced nephropathy.
ADR-induced reduction in expression of podocyte-selective proteins was attenuated in Pod-IκBΔN mice
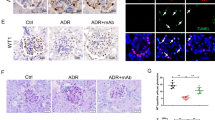
To determine the mechanisms whereby podocyte-specific inhibition of NF-κB ameliorated proteinuria in ADR-induced nephropathy, renal histology was first examined by PAS staining. Results showed that ADR injection induced the formation of intratubular casts in both Pod-IκBΔN and control mice (Fig. 3a). However, the severity of cast formation did not differ between the two groups (Fig. 3b). The number of nuclei within the glomeruli was also not different between Pod-IκBΔN and control mice (Fig. 3c). In contrast, ADR induced glomerulosclerosis more severely in control mice as compared with Pod-IκBΔN mice, although glomerulosclerosis was scarcely detectable in both mice before ADR injection (Fig. 3d). Renal fibrosis, as determined by Masson trichrome staining, was not obvious in any groups examined (data not shown). Infiltration of inflammatory cells in the kidneys was then examined. ADR induced the accumulation of neutrophils and macrophages in the kidneys of Pod-IκBΔN and control mice (Fig. 4). However, the number of the infiltrated cells following ADR injection did not differ between these mice. As such, results suggest that NF-κB inhibition within podocytes ameliorates the severity of glomerulosclerosis, but did not alter the accumulation of inflammatory cells in ADR-induced nephropathy.
Nephrin, podocin, and synaptopodin are selectively expressed in podocytes and are required for the maintenance of the glomerular filtration barrier to prevent proteinuria [1]. Expression of these proteins was examined by immunofluorescence studies. Results showed that expression of nephrin, podocin, and synaptopodin was not different between Pod-IκBΔN and control mice before ADR injection (Figs. 5, 6). However, ADR injection significantly reduced expression of nephrin and synaptopodin, but not podocin in control mice. Of interest, ADR-induced decreases in expression of nephrin and synaptopodin were attenuated in Pod-IκBΔN mice. Indeed, ADR reduced nephrin expression by 26 % in control mice, but by only 7 % in Pod-IκBΔN mice (Figs. 5, 6a). Likewise, ADR-mediated reduction in synaptopodin expression was 76 % in control mice, but only 35 % in Pod-IκBΔN mice (Figs. 5, 6c). Expression of podocin was unaltered by ADR injection in both Pod-IκBΔN and control mice (Figs. 5, 6b). These results were confirmed at mRNA levels by real-time RT-PCR analyses, although ADR-induced reduction in Nphs1 expression did not reach a significant difference (Fig. 7). Expression of WT-1, a nuclear marker of podocytes, was not changed by either ADR injection or NF-κB inhibition (Figs. 5, 6d). These results suggest that the reduction in expression of podocyte-selective proteins, such as nephrin and synaptopodin, at least in part contributes to proteinuria in ADR-induced nephropathy, and that the attenuated reduction of these proteins is likely to be responsible for the amelioration of proteinuria in ADR-injected Pod-IκBΔN mice.
ADR reduced the expression of nephrin and synaptopodin in control mice, but the reduction was attenuated in Pod-IκBΔN mice. Pod-IκBΔN and control mice were injected with ADR intravenously, and the expression of podocyte-selective markers in the glomeruli was examined 2 weeks after the injection. n = 5–7 per each group. Rows 1–3 Representative pictures of immunofluorescence studies for nephrin, podocin, and synaptopodin are shown. Nephrin and podocin were detected by Alexa-Fluor 594-conjugated secondary antibodies (Red), whereas synaptopodin was detected by Alexa-Fluor 488-cojugated secondary antibody (Green). Nuclear staining was performed with 4′, 6-diamidino-2-phenylindole (Blue). Row 4 Representative pictures of immunohistochemical staining for WT-1 are shown. WT-1 was visualized by diaminobenzidine, and sections were counterstained with hematoxylin. Bar 50 μm
ADR reduced the expression of nephrin and synaptopodin in control mice, but the reduction was attenuated in Pod-IκBΔN mice. Pod-IκBΔN and control mice were injected with ADR intravenously, and the expression of podocyte-selective markers in the glomeruli was examined 2 weeks after the injection. n = 5–7 per each group. a–c Immunofluorescence signals for nephrin (a), podocin (b), and synaptopodin (c), shown in Fig. 5, were quantified. The signal intensity for ten glomeruli per mouse was measured. d Immunohistochemical staining for WT-1 was performed as shown in Fig. 5, and the number of WT-1 positive cells per glomerulus was quantified. Ten glomeruli per mouse were counted. *p < 0.05 compared with mice before the injection. #p < 0.05 compared with ADR-injected control mice
ADR reduced Synpo expression in control mice, but the reduction was attenuated in Pod-IκBΔN mice. Pod-IκBΔN and control mice were injected with ADR intravenously, and the expression of podocyte-selective markers in the kidneys was examined 2 weeks after the injection. n = 5–7 per each group. Expression of Nphs1 (a), Nphs2 (b), and Synpo (c) was determined by real-time RT-PCR. *p < 0.05 compared with mice before the injection. #p < 0.05 compared with ADR-injected control mice
NF-κB inhibition blunted ADR-induced reduction in synaptopodin expression in cultured podocytes
The cell-autonomous effect of NF-κB inhibition on synaptopodin expression was examined in cultured podocytes, derived from Pod-IκBΔN and control mice, respectively. Expression of synaptopodin, but not nephrin, was examined in the present study, because ADR-induced changes in synaptopodin expression were more significant than nephrin in mice in vivo. Results showed that podocin expression was unaltered by either ADR stimulation or NF-κB inhibition in cultured podocytes (Fig. 8a, b). However, synaptopodin expression was markedly decreased by ADR treatment in podocytes derived from control mice, but not in those from Pod-IκBΔN mice (Fig. 8a, c). These results are consistent with those of in vivo experiments, and suggest that ADR directly decreases synaptopodin expression via the NF-κB pathway in podocytes.
ADR decreased expression of synaptopodin in cultured podocytes derived from control mice, but not from Pod-IκBΔN mice. Primary cultures of podocytes were performed using Pod-IκBΔN mice and control mice, respectively, and they were treated with ADR or saline (Ct) for 24 h. a Expression of podocin (Red) and synaptopodin (Green) was examined by immunofluorescence studies. Nuclear staining was performed with 4′, 6-diamidino-2-phenylindole (Blue). Bar 50 μm. b, c Immunofluorescence signals for podocin (b) and synaptopodin (c) were quantified. The signal intensity for twenty cells per culture was measured. *p < 0.05 compared with control podocytes treated with saline. #p < 0.05 compared with control podocytes treated with ADR
Discussion
In the present study, we showed that podocyte-specific inhibition of the NF-κB signaling ameliorated proteinuria in ADR-induced nephropathy in mice. Indeed, the administration of ADR induced massive proteinuria in both Pod-IκBΔN and control mice, but the amount of proteinuria was significantly lower in Pod-IκBΔN mice than control mice. In addition, we showed that expression of podocyte-selective slit diaphragm-associated proteins, such as nephrin and synaptopodin, was decreased by ADR injection in control mice, and that ADR-induced decreases in expression of these proteins were attenuated in Pod-IκBΔN mice. The inverse relationship between proteinuria and expression of nephrin and synaptopodin suggests that these proteins regulate proteinuria in ADR-induced nephropathy. We also showed that ADR decreased synaptopodin expression in primary cultures of podocytes derived from control mice, but not from Pod-IκBΔN mice, suggesting that ADR-induced reduction in synaptopodin expression is cell-autonomously mediated by NF-κB. As such, results of the present study provide evidence that the NF-κB signaling in podocytes contributes to proteinuria through the regulation of podocyte-selective molecules including nephrin and synaptopodin in a mouse model of proteinuric kidney disease.
In addition to the results of our present study, previous studies have also demonstrated that NF-κB contributes to proteinuria in experimental animal models [7, 8, 18]. Indeed, up-regulation of NF-κB in transgenic mice has been shown to lead to albuminuria and glomerulosclerosis [18]. On the contrary, a NF-κB inhibitor, pyrrolidine dithiocarbamate, significantly reduced albuminuria in passive Heymann nephritis [7]. Intraperitoneal injection of dehydroxymethylepoxyquinomicin, another NF-κB inhibitor, also ameliorated proteinuria in puromycin aminonucleoside-induced nephrosis in mice [8]. Although results of the preceding studies provide evidence that the activation of NF-κB is critical for proteinuria, they did not address the cell types where NF-κB plays a role. In fact, the NF-κB activity in various cell types contributes to multiple renal diseases. For example, fibroblast-specific inhibition of NF-κB by IκBΔN transgene attenuated renal fibrosis in an unilateral ureteral obstruction model [9]. Endothelial cell-specific over-expression of IκBΔN attenuated hypertension-induced albuminuria and renal damage in mice [19]. Adenovirus-mediated over-expression of IκBΔN in renal tubular cells prevented tubulointerstitial injury in protein-overloaded rats [20]. Regarding the podocytes, Brähler et al. [21] showed that podocyte-specific knockout of NF-κB essential modulator (NEMO) reduced proteinuria in nephrotoxic sheep serum-induced glomerulonephritis in mice. NEMO is a subunit of an IκB kinase complex required for phosphorylation and proteasomal degradation of IκB. They showed that NEMO deletion abrogated activation of NF-κB in podocytes and ameliorated glomerular function following disease induction [21]. These findings are consistent with those of our present study, but the same group later raised a possibility that the attenuation of proteinuria in podocyte-specific NEMO knockout mice might be caused in part by NF-κB independent mechanisms, such as small GTPases and MAP kinases [22]. Nevertheless, based on the studies by ourselves and theirs, it is reasonable to conclude that NF-κB in podocytes contributes to proteinuria in multiple kidney diseases.
In the present study, we did not provide direct evidence that the NF-κB activity was inhibited specifically in podocytes in Pod-IκBΔN mice, because of the following reasons. First, the anti-phosphorylated p65 antibody, which we previously used [9] and was able to detect the activated NF-κB, is unavailable at present. Second, although the electrophoretic mobility shift assay using the whole kidney homogenates was utilized to measure the NF-κB activity in many previous studies [7, 19, 20], this approach is not suitable to examine the podocyte-specific inhibition of the NF-κB activity because the population of podocytes is very tiny in the kidney. Alternatively, we showed that human IκBΔN transgene was selectively detectable in the kidney of Pod-IκBΔN mice, but not of control mice, and that LacZ was specifically expressed in the glomeruli in the kidney of Nphs1-Cre+/−/Rosa LacZ-reporter+/− mice. Although these results are indirect evidence, they suggest the podocyte-specific inhibition of the NF-κB signaling in Pod-IκBΔN mice.
Results of the present study showed that ADR-induced increase in proteinuria was accompanied by the decrease in expression of slit diaphragm-associated proteins, such as nephrin and synaptopodin, and that these changes were blunted in Pod-IκBΔN mice. The decrease in expression of nephrin and synaptopodin has also been demonstrated in multiple proteinuric kidney diseases in animal models and in humans. For example, expression of Nphs1 mRNA as well as nephrin protein has been shown to be decreased in multiple animal models including puromycin aminonucleoside nephrosis in rats [23], passive Heymann nephritis in rats [23], and ADR-induced nephropathy in rats [24]. The reduction in synaptopodin expression has also been shown in ADR-induced nephropathy in mice [25, 26]. Likewise, numerous studies demonstrated that expression of nephrin and synaptopodin was decreased in multiple proteinuric kidney diseases including minimal change disease, membranous nephropathy, and focal segmental glomerulosclerosis in humans [27–29]. These results are consistent with those of our current study, and provide evidence that these slit diaphragm-associated proteins play a central role in regulating the glomerular barrier function in podocytes. However, a limitation of these studies is that they only showed an inverse correlation between proteinuria and expression of podocyte-selective proteins, but did not address the underlying mechanisms. In this regard, the present study provides one of the mechanisms whereby the activation of NF-κB in podocytes regulates expression of nephrin and synaptopodin, thereby inducing proteinuria in an animal model. It is of interest to determine if podocyte-specific inhibition of the NF-κB signaling prevents proteinuria in proteinuric kidney diseases in humans.
In contrast to marked downregulation of nephrin and synaptopodin, results of the present study showed that expression of podocin was unaltered in ADR-induced nephropathy, in spite of its importance for proteinuria. In this regard, there is a report showing that expression of nephrin and synaptopodin, but not podocin, was correlated in acquired proteinruic kidney diseases in humans [30]. It is likely that the regulation of podocin is different from that of nephrin and synaptopodin. Further studies are required to determine how these proteins are regulated in disease conditions.
In summary, results of the present study provide evidence that the NF-κB signaling in podocytes contributes to proteinuria in an animal model of proteinuric kidney diseases. Further studies are needed to develop anti-proteinuric reagents which selectively target NF-κB in podocytes.
References
Reiser J, Gupta V, Kistler AD. Toward the development of podocyte-specific drugs. Kidney Int. 2010;77:662–8.
Komenda P, Rigatto C, Tangri N. Estimated glomerular filtration rate and albuminuria: diagnosis, staging, and prognosis. Curr Opin Nephrol Hypertens. 2014;23:251–7.
Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaría B, Ruiz-Ortega M, Egido J, Ortiz A. NF-κB in renal inflammation. J Am Soc Nephrol. 2010;21:1254–62.
Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–8.
Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–83.
Zheng L, Sinniah R, Hsu SI. In situ glomerular expression of activated NF-κB in human lupus nephritis and other non-proliferative proteinuric glomerulopathy. Virchows Arch. 2006;448:172–83.
Mudge SJ, Paizis K, Auwardt RB, Thomas RJ, Power DA. Activation of nuclear factor-κB by podocytes in the autologous phase of passive Heymann nephritis. Kidney Int. 2001;59:923–31.
Shimo T, Adachi Y, Yamanouchi S, Tsuji S, Kimata T, Umezawa K, Okigaki M, Takaya J, Ikehara S, Kaneko K. A novel nuclear factor κB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates puromycin aminonucleoside-induced nephrosis in mice. Am J Nephrol. 2013;37:302–9.
Inoue T, Takenaka T, Hayashi M, Monkawa T, Yoshino J, Shimoda K, Neilson EG, Suzuki H, Okada H. Fibroblast expression of an IκB dominant-negative transgene attenuates renal fibrosis. J Am Soc Nephrol. 2010;21:2047–52.
Yoshida T, Yamashita M, Horimai C, Hayashi M. Smooth muscle-selective inhibition of nuclear factor-κB attenuates smooth muscle phenotypic switching and neointima formation following vascular injury. J Am Heart Assoc. 2013;2:e000230.
Asano T, Niimura F, Pastan I, Fogo AB, Ichikawa I, Matsusaka T. Permanent genetic tagging of podocytes: fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol. 2005;16:2257–62.
Sakamaki Y, Sasamura H, Hayashi K, Ishiguro K, Takaishi H, Okada Y, D’Armiento JM, Saruta T, Itoh H. Absence of gelatinase (MMP-9) or collagenase (MMP-13) attenuates adriamycin-induced albuminuria and glomerulosclerosis. Nephron Exp Nephrol. 2010;115:e22–32.
Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805.
Homma K, Yoshida T, Yamashita M, Hayashida K, Hayashi M, Hori S. Inhalation of hydrogen gas is beneficial for preventing contrast-induced acute kidney injury in rats. Nephron Exp Nephrol. 2014;128:116–22.
Yoshida T, Yamashita M, Iwai M, Hayashi M. Endothelial Krüppel-like factor 4 mediates the protective effect of statins against ischemic AKI. J Am Soc Nephrol 2016 (in press).
Yoshida T, Sinha S, Dandré F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res. 2003;92:856–64.
Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Krüppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–57.
Hussain S, Romio L, Saleem M, Mathieson P, Serrano M, Moscat J, Diaz-Meco M, Scambler P, Koziell A. Nephrin deficiency activates NF-κB and promotes glomerular injury. J Am Soc Nephrol. 2009;20:1733–43.
Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell-specific NF-κB suppression attenuates hypertension-induced renal damage. Circ Res. 2007;101:268–76.
Takase O, Hirahashi J, Takayanagi A, Chikaraishi A, Marumo T, Ozawa Y, Hayashi M, Shimizu N, Saruta T. Gene transfer of truncated IκBα prevents tubulointerstitial injury. Kidney Int. 2003;63:501–13.
Brähler S, Ising C, Hagmann H, Rasmus M, Hoehne M, Kurschat C, Kisner T, Goebel H, Shankland S, Addicks K, Thaiss F, Schermer B, Pasparakis M, Benzing T, Brinkkoetter PT. Intrinsic proinflammatory signaling in podocytes contributes to podocyte damage and prolonged proteinuria. Am J Physiol Renal Physiol. 2012;303:F1473–85.
Brähler S, Ising C, Aranda BB, Höhne M, Schermer B, Benzing T, Brinkkoetter PT. The NF-κB essential modulator (NEMO) controls podocyte cytoskeletal dynamics independently of NF-κB. Am J Physiol Renal Physiol. 2015;309:F617–26.
Luimula P, Ahola H, Wang SX, Solin ML, Aaltonen P, Tikkanen I, Kerjaschki D, Holthöfer H. Nephrin in experimental glomerular disease. Kidney Int. 2000;58:1461–8.
Nakhoul F, Ramadan R, Khankin E, Yaccob A, Kositch Z, Lewin M, Assady S, Abassi Z. Glomerular abundance of nephrin and podocin in experimental nephrotic syndrome: different effects of antiproteinuric therapies. Am J Physiol Renal Physiol. 2005;289:F880–90.
Zhang X, Qu X, Sun YB, Caruana G, Bertram JF, Nikolic-Paterson DJ, Li J. Resolvin D1 protects podocytes in adriamycin-induced nephropathy through modulation of 14-3-3β acetylation. PLoS One. 2013;8:e67471.
Bao H, Ge Y, Peng A, Gong R. Fine-tuning of NFκB by glycogen synthase kinase 3β directs the fate of glomerular podocytes upon injury. Kidney Int. 2015;87:1176–90.
Furness PN, Hall LL, Shaw JA, Pringle JH. Glomerular expression of nephrin is decreased in acquired human nephrotic syndrome. Nephrol Dial Transplant. 1999;14:1234–7.
Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723–31.
Huh W, Kim DJ, Kim MK, Kim YG, Oh HY, Ruotsalainen V, Tryggvason K. Expression of nephrin in acquired human glomerular disease. Nephrol Dial Transplant. 2002;17:478–84.
Schmid H, Henger A, Cohen CD, Frach K, Gröne HJ, Schlöndorff D, Kretzler M. Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol. 2003;14:2958–66.
Acknowledgments
This study was supported by a Grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan to M.H. and Grants from the Japanese Association of Dialysis Physicians (JADP Grant 2015-6) to T.Y. and the Kidney Foundation, Japan (JKFB15-7) to T.Y.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflicts of interest exists.
About this article
Cite this article
Yamashita, M., Yoshida, T., Suzuki, S. et al. Podocyte-specific NF-κB inhibition ameliorates proteinuria in adriamycin-induced nephropathy in mice. Clin Exp Nephrol 21, 16–26 (2017). https://doi.org/10.1007/s10157-016-1268-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1268-6