Abstract
Background
The timing for initiating dialysis in chronic kidney disease is often determined by the clinical symptoms and estimated glomerular filtration rate (eGFR). However, very few studies have examined how the speed of kidney function decline before initiating dialysis relates to mortality after dialysis initiation. Here, we report our examination of the relationship between the speed of eGFR decline in the 3 months prior to dialysis initiation and mortality.
Methods
The study included 1292 new dialysis patients who were registered in the Aichi Cohort Study of Prognosis in Patients Newly Initiated into Dialysis. The subjects were placed in 4 groups based on the speed of eGFR decline in the 3 months before initiating dialysis (eGFR at 3 months before initiation—eGFR at initiation) <2: ≥2, <4: ≥4, <6: ≥6 mL/min/1.73 m2. All-cause, cardiovascular, and infection-related mortality rates were compared using Kaplan–Meier curves. A multivariate analysis using the Cox proportional hazard model was used to extract the factors that contributed to all-cause mortality.
Results
The group with faster eGFR decline exhibited significantly more heart failure symptoms when dialysis was initiated. Rapid eGFR decline correlated with prognosis (log-rank test: all-cause mortality p < 0.001, cardiovascular mortality p < 0.001). The speed of eGFR decline was related to elevated all-cause mortality rates [eGFR decline 10 mL/min/1.73 m2, HR (95 % CI) = 1.53 (1.12–2.08)].
Conclusions
This study showed that patients with rapid eGFR decline in the 3 months before initiating dialysis more often presented with heart failure symptoms when dialysis was initiated and had poorer survival prognoses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The timing for initiating dialysis is not definitively decided through a comprehensive assessment of objective markers of kidney function such as estimated glomerular filtration rate (eGFR), presence of uremia symptoms, the degree of anemia as well as the fluid, electrolyte, and acid–base balance [1, 2]. Many past studies have shown that prognosis is good if dialysis is initiated when kidney function is still relatively well-maintained [3–5]. However, in 2010, the initiating dialysis early and late (IDEAL) study found no connection between early initiation and improved prognosis [6], which cast doubt on the merits of early initiation into dialysis. On the other hand, an observational study from Japan showed that prognosis was poor in patients who had high eGFR when dialysis was initiated [7]. Various countries and regions have created guidelines by referencing articles, that addressed the relationship between the timing of dialysis initiation and mortality after initiating dialysis. While all of these guidelines place importance on symptoms and findings that accompany kidney function decline, eGFR is used as the criteria for determining when to initiate dialysis [8–12].
Although these guidelines include eGFR values as criteria for initiating dialysis, none describes the speed of kidney function decline. Patients who are initiated into dialysis early include those who needed to begin dialysis because of over-hydration that is difficult to manage conservatively, electrolyte abnormalities that are difficult to rectify, and other such conditions. In these patients, kidney function rapidly declines immediately before initiating dialysis, so they begin dialysis while in an unstable overall condition, which could have a negative impact on survival prognosis after dialysis initiation. However, very few studies have dealt with the relationship between the speed of kidney function decline and survival prognosis immediately before initiating and after initiating dialysis, respectively. The above points suggest that if patients’ prognoses after initiating dialysis are taken under consideration, the speed of kidney function decline could serve as a marker for deciding when to begin dialysis.
Thus, we investigated the relationship between the speed of kidney function decline in the 3 months before initiating dialysis and survival prognosis after dialysis initiation in subjects who registered with the Aichi Cohort Study of Prognosis in Patients Newly Initiated into Dialysis (AICOPP), which is a multi-center, prospective cohort study.
Materials and methods
Subjects
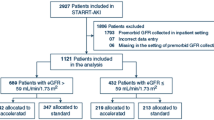
The subjects were patients who were newly initiated into dialysis at the 17 Aichi Cohort Study of Prognosis in Patients Newly Initiated into Dialysis (AICOPP) group centers from October 2011 to September 2013. Patients who were withdrawn from dialysis while hospitalized, died while hospitalized, or did not agree to be registered were excluded. The study screened 1524 patients who were at least 20 years old, had CKD, and provided written consent. We excluded patients whose eGFR values 3 months before initiating dialysis were not measured and whose prognoses were unknown, as determined by a survey conducted at the end of March 2015. We enrolled 1292 subjects into the study (Fig. 1).
Speed of kidney function decline
To evaluate the speed of kidney function decline, we measured the speed by which the eGFR declined in 3 months, or the difference between eGFR 3 months before and when initiating dialysis, respectively (eGFR 3 months before dialysis—eGFR at initiation). The eGFR was calculated using the Japanese Society of Nephrology’s estimation formula (eGFR = 194 × serum creatinine − 1.094 × age − 0.287 [×0.739 for women]) [13]. The subjects were classified into 4 groups based on speed of eGFR decline (Group 1, G1: <2 mL/min/1.73 m2, Group 2, G2: ≥2, <4 mL/min/1.73 m2, Group 3, G3: ≥4, <6 mL/min/1.73 m2; Group 4, G4: ≥6mL/min/1.73 m2). Moreover, to verify the validity of the cutoff described above, subjects were also evaluated after speed of eGFR decline being divided into quartiles.
Patient characteristics and data when dialysis was initiated (baseline)
BMI was measured at the first dialysis session. Diabetes were defined as fasting blood glucose ≥126 mg/dL, casual blood glucose ≥200 mg/dL, HbA1c (NGSP) ≥6.5 %, use of insulin, or oral hypoglycemic agents. History of cardiovascular disease (CVD) was defined as a history of heart failure requiring hospitalization, coronary artery disease requiring coronary artery intervention or heart bypass surgery, stroke, aortic disease requiring surgery, or peripheral artery disease requiring hospitalization. The period by nephrology care was established, based on patients’ medical records, as the period from referral to the nephrologist until the initiation of dialysis. Medication use referred to the drugs taken at dialysis initiation. Aortic calcification was defined as the presence of calcification in the aortic arch in a frontal chest radiograph and the presence of mitral valve or aortic valve calcification in a cardiac ultrasonography, at the time of dialysis initiation, respectively. Blood tests were performed on samples taken before the first dialysis session.
Condition at the first dialysis session
Blood pressure was measured before the first dialysis session. Symptoms of heart failure were defined as the presence of subjective symptoms, pulmonary congestion, or pleural fluid on a chest radiogram, and hypoxemia. Gastrointestinal symptoms included subjective symptoms such as nausea, vomiting, and lack of appetite in the absence of clear signs of gastrointestinal diseases. The cardio-thoracic ratio was calculated from a frontal chest radiogram taken immediately before the first dialysis session.
Survey of survival prognosis
Survival prognosis as of March 31, 2015, was determined by surveying medical records. For patients who were transferred to other institutions, information was obtained by mailing out survey forms.
Outcomes
The study outcomes included: (1) Comparisons of all-cause mortality rates in the 4 groups as categorized by speed of eGFR decline; (2) Comparisons of CVD-related and infection-related mortality rates in the above 4 groups. CVD deaths were defined as deaths resulting from heart failure, coronary artery disease, cardiogenic sudden death, stroke, or aortic diseases. (3) Extraction of factors, including speed of eGFR decline that contributed to the all-cause mortality.
Statistical processing
The results were expressed as mean ± standard deviation (median values were used for nephrology care, CRP, and ultrafiltration volume). The easy R (EZR) was used for statistical processing [14]. Comparisons of characteristics, baseline data, and condition at the first dialysis session between the 4 groups of patient were performed using the Mann–Whitney U test for continuous variables and Fisher’s exact test for nominal variables. All-cause, CVD-related, and infection-related mortality rates were compared using the log-rank test for the Kaplan–Meier curves. The post hoc analysis tested differences between the groups using the Bonferroni method. Factors contributing to the different mortality rates were examined using univariate Cox proportional hazard regression analysis, with the speed of eGFR decline as the continuous variable. In addition to the speed of eGFR decline, factors that were significant in the univariate analysis served as explanatory variables for the multivariate Cox proportional hazard analysis using the stepwise method (i.e., age, sex, BMI, dialysis method, history of CVD, Charlson comorbidity index (CCI), systolic blood pressure, diastolic blood pressure, use of RAS inhibitors, use of loop diuretics, use of vitamin D receptor activators (VDRAs), aortic calcification, valve calcification, heart failure symptoms, gastrointestinal symptoms, cardiac-thoracic ratio, hemoglobin, serum albumin, adjusted serum calcium, Log CRP, blood bicarbonate concentration). Factors that were believed to compete with the speed of eGFR decline were excluded (i.e., serum creatinine, BUN/creatinine, eGFR at dialysis initiation, eGFR 3 months before initiation). p values less than 5 % were considered statistically significant.
Ethical considerations
This study was conducted by following the Japanese Ministry of Health, Labor, and Welfare’s “ethical guidelines for clinical research” (created July 30, 2003; full revision December 28, 2004; full revision July 31, 2008) and the Helsinki Declaration (revised 2013), and was approved by the clinical research ethics committees at each AICOPP group facility (the approval number: 20110823-3). The subjects received oral and written explanations of the purpose of the study, and provided their consent in writing. The study was registered with the University Hospital Medical Information Network (UMIN) on January 18, 2012 (ID: 000007096).
Results
Comparison of patient characteristics and baseline data
Table 1 shows the patient characteristics and baseline data in the 4 groups. Groups with faster eGFR decline had smaller proportions of women, larger proportions of patients with a history of CVD, increased CCI, shorter periods of nephrology care, lower usage rates of RAS inhibitors, ESA, and vitamin D receptor activators; lower hemoglobin concentration, lower serum albumin, higher serum phosphorus, and higher CRP.
eGFR change in the 3 months before dialysis initiation
Figure 2 shows eGFR values for the 4 groups at 3 months before and when dialysis was initiated, as well as the change in eGFR. The eGFR at 3 months before dialysis in G1, G2, G3, and G4 were 6.57 ± 1.92, 8.04 ± 1.71, 10.11 ± 1.57, and 17.71 ± 8.52 mL/min/1.73 m2, respectively. The eGFR at the time of dialysis initiation in G1, G2, G3, and G4 were 5.62 ± 2.07, 5.17 ± 1.63, 5.21 ± 1.48, and 6.21 ± 3.57 mL/min/1.73 m2, respectively. Differences between the 4 groups were significant. The range and median values for speed of eGFR decline during the 3 months before dialysis initiation and differences in these values when groups were divided into quartiles are displayed in Table 2.
Comparison of the eGFR 3 months before dialysis and when dialysis was initiated, as well as the change in eGFR over the 3 months between the 4 study groups. Change in eGFR over the 3 months eGFR = 3 months before dialysi − eGFR at dialysis initiation. (G1: <2 mL/min/1.73 m2; G2: ≥2, <4 mL/min/1.73 m2; G3: ≥4, <6 mL/min/1.73 m2; G4: ≥6 mL/min/1.73 m2)
Comparison of condition at first dialysis session
Table 3 shows the conditions at the first dialysis session in the 4 groups. In groups with faster eGFR decline, systolic blood pressure was lower, the proportion of patients with heart failure symptoms was higher, cardio-thoracic ratio was higher, and ultra-filtration volume at the first dialysis session was larger.
Comparison of all-cause mortality
Figure 3 shows Kaplan–Meier curves for the cumulative survival rates of the 4 groups. There were 216 deaths during the follow-up period (G1, 79 cases; G2, 68 cases; G3, 29 cases; G4, 40 cases). Significant differences were observed between the 4 groups’ cumulative survival rates (p < 0.001). In the post hoc analysis, the all-cause mortality rate for G4 was significantly higher than those of other groups (G1, G2, G3 vs G4: p < 0.001). Figure 4 shows Kaplan–Meier curves for the cumulative survival rates of the 4 groups classified according to quartile. Significant differences were similarly observed between the 4 groups’ cumulative survival rates (p = 0.023).
Comparison of CVD-related mortality, infection-related mortality
Figure 5 shows Kaplan–Meier curves for the cumulative survival rates of the 4 groups for CVD- and infection-related mortality rates. There were 70 CVD- and 37 infection-related deaths during the follow-up period. Significant differences between the 4 groups were observed for CVD-related mortality (p < 0.001) but not for infection-related mortality (p = 0.053). The breakdown of CVD deaths was as follows: heart failure: 23 subjects, acute coronary syndrome: 13 subjects, cardiogenic sudden death: 11 subjects, cerebral hemorrhage: 12 subjects, cerebral infarction: 6 subjects, ruptured aortic aneurysm: 3 subjects, acute aortic dissection: 1 subject, pulmonary embolism: 1 subject.
Factors contributing to all-cause mortality (univariate analysis)
Table 4 shows the results of the univariate analyses that were performed to extract factors influencing all-cause mortality. The factors that were extracted included old age, male gender, low BMI, CVD history, high CCI, low blood pressure, no use of RAS inhibitors, use of loop diuretics, no use of VDRAs, aortic and valve calcification, heart failure and gastrointestinal symptoms at the first dialysis session, anemia, low serum albumin, low serum creatinine, high BUN/creatinine, high eGFR 3 months before dialysis, high eGFR at dialysis initiation, large change in eGFR in the 3 months before dialysis, high adjusted serum calcium, and high CRP level.
Factors contributing to all-cause mortality (multivariate analysis)
Figure 6 shows the results of the multivariate analysis performed to extract factors contributing to all-cause mortality. Survival prognosis after initiating dialysis was poorer at faster speeds of eGFR decline (speed of eGFR decline faster than 10 mL/min/1.72 m2; HR = 1.527). Other factors contributing to poor survival prognosis included old age, male gender, high CCI, low systolic blood pressure, aortic calcification, and elevated adjusted serum calcium.
Discussion
This study showed the correlation between the speed of eGFR decline in the 3 months before initiating dialysis and mortality after dialysis initiation. Several studies have examined the relationship between survival prognosis after initiating dialysis and eGFR values at dialysis initiation [3–7]. However, since very few studies have addressed the relationship between the speed of kidney function decline immediately before dialysis and mortality after dialysis initiation, we believe the results of this study have major clinical significance.
The speed of kidney function decline was previously reported to correlate with factors such as sex, blood pressure, primary disease, and urinary protein [15–18]. However, the observation periods in many of these studies were at least 1 year or more during the pre-dialysis period, indicating that very few studies had evaluated short and limited periods similar to our study (3 months pre-dialysis). The cutoff values for rate of eGFR decline used in this study were 2, 4, and 6 mL/min/1.73 m2. Although a comparison against the total mortality rate in quartiles was also conducted to verify the validity of this cutoff value, the results obtained were similar. Nevertheless, when subjects were divided into quartiles, the lower limit cutoff value for Quartile 4 was 4 mL/min/1.73 m2 or lower, and detecting the difference was believed to be difficult due to the inclusion of cases involving comparatively gradual declines in eGFR speed. Moreover, we believe that using cutoff values of 2, 4, and 6 mL/min/1.73 m2 will be clearer when applying the results of this study clinically.
In regular medical care, heart failure symptoms are considered an important factor for deciding whether to initiate dialysis at all. Fabbian et al. found that 73 % of patients who had intractable congestive heart failure when dialysis was initiated needed emergency dialysis [19]. Crews et al. found that patients who were initiated into dialysis early (eGFR = 10 mL/min/1.73 m2 or higher), had higher incidence of acute kidney injury (AKI), congestive heart failure, and hospitalization for other reasons [20]. With regards to this point, our study showed that patients with faster eGFR decline in the 3 months before initiating dialysis often had a history of CVD, frequently had heart failure symptoms when dialysis was initiated, had high cardio-thoracic ratios, and had greater ultra-filtration volume at the first dialysis session. We believe this is because the state of over-hydration that accompanies reduced kidney function often results in a precipitous decline in kidney function in patients with reduced cardiac function.
Although kidney function was previously thought to decline in a linear manner over time [21–23], there have been recent reports of patients who exhibited a mix of periods of stability and progressive decline [24, 25]. O’Hare et al. examined the state of kidney function decline and prognosis after initiating dialysis in 8 time periods during the 2 years before initiating dialysis [26]. The group with rapid kidney function decline had shorter periods of care under nephrologists, higher AKI incidence, and poorer prognosis at 1 year after dialysis initiation. However, these groups were divided based on eGFR levels at 2 years before dialysis initiation. In the group with rapid kidney function decline, mean eGFR at 3 months before dialysis was 30 mL/min/1.73 m2. The higher proportion of patients with AKI compared with our study may have been due to these differences in the subject populations. However, one study examined eGFR 3 months before and when dialysis was initiated, which is similar to the period that we examined in our study [27]. Among the patients with eGFR above 30 mL/min/1.73 m2 before initiating dialysis, 8 out of 105 subjects (7.6 %) and 9 out of 71 subjects (12.7 %) at the 2 centers, respectively, died. Among the 17 total deaths, 10, 3, 2, and 2 were from acute tubular necrosis, cholesterol embolisms, drugs, and other causes, respectively. They thus concluded that the presence of heart disease speeds up declines in kidney function. Their results also indicate that patients who exhibit rapid eGFR decline immediately before initiating dialysis most likely have CKD and AKI. 21 cases in the present study exhibited an eGFR of 30 mL/min/1.73 m2 or higher. While there is a possibility that AKI cases may be included among these 21 cases, these were added to the evaluation performed in this study as a detailed description of an underlying disease was not provided for all 1292 cases.
This study has the following limitations. First, we did not use uniform criteria for initiating dialysis. Rather, it was left to the discretion of the attending physician. The timing for initiating dialysis changes over time depending on the available evidence, which is based on various guidelines that guide clinical management. Nevertheless, given that the nephrologists decided on the timing for initiating dialysis in all the subjects in this study and the 2-year registration period that started in October 2011, the decisions on when to initiate dialysis were made in the same historical period and major differences between institutions and attending physicians are unlikely. Second, while the groups were determined based on the speed of GFR decline in the 3 months before initiating dialysis, we did not examine cases with rapid kidney function decline in more detail. Although we discussed the trajectory of GFR decline above, GFR may decline in at less than 3 months, particularly in the presence of acute heart failure. Third, although we were able to demonstrate a correlation between speed of eGFR decline and mortality, because this was an observational study, our results will have no impact on determining appropriate timing for initiation of dialysis.
To conclude, our results confirmed that patients who exhibit rapid GFR decline in the 3 months before initiating dialysis often present with heart failure symptoms when dialysis is initiated and have poor prognoses after initiating dialysis. In the future, the presence of rapid kidney function decline could be referenced when attempting to identify these cases early and determine the appropriate time for initiating dialysis.
References
Slinin Y, Ishani A. What drives early dialysis initiation and how do we optimize timing of RRT? Clin J Am Soc Nephrol. 2014;7:1671–3.
Lin ZH, Zuo L. When to initiate renal replacement therapy: The trend of dialysis initiation. World J Nephrol. 2015;4:521–7.
Bonomini V, Feletti C, Scolari MP, et al. Benefits of early initiation of dialysis. Kidney Int. 1985;17(Suppl):S57–9.
Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–53.
Hakim RM, Lazarus JM. Initiation of dialysis. J Am Soc Nephrol. 1995;6:1319–28.
Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–19.
Yamagata K, Nakai S, Iseki K, et al. Late dialysis start did not affect long-term outcome in Japanese dialysis patients: long-term prognosis from Japanese Society for Dialysis Therapy Registry. Ther Apher Dial. 2012;16:111–20.
Hemodialysis Adequacy Work G. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2–90.
Levin A, Hemmelgarn B, Culleton B, et al. Guidelines for the management of chronic kidney disease. CMAJ. 2008;179:1154–62.
European Best Practice Guidelines Expert Group on Hemodialysis, European Renal Association. Section I. Measurement of renal function, when to refer and when to start dialysis. Nephrol Dial Transpl. 2002;17(Suppl 7):7–15.
Kelly J, Stanley M, Harris D. The CARI guidelines. Acceptance into dialysis guidelines. Nephrology (Carlton). 2005;10(Suppl 4):S46–60.
Watanabe Y, Yamagata K, Nishi S, et al. Japanese Society for dialysis therapy clinical guideline for “Hemodialysis Initiation for Maintenance Hemodialysis”. Ther Apher Dial. 2015;19(Suppl1):93–107.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statics. Bone Marrow Transplant. 2013;48:452–8.
Hanratty R, Chonchol M, Havranek EP, et al. Relationship between blood pressure and incident chronic kidney disease in hypertensive patients. Clin J Am Soc Nephrol. 2011;6:2605–11.
McMullan CJ, Lambers Heerspink HJ, Parving HH, et al. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: a post hoc analysis from the RENAAL study and the Irbesartan Diabetic Nephropathy Trial. Am J Kidney Dis. 2014;64:714–22.
Imai E, Ito S, Haneda M, Harada A et al. Effects of blood pressure on renal and cardiovascular outcomes in Asian patients with type 2 diabetes and overt nephropathy: a post hoc analysis (ORIENT-blood pressure). Nephrol Dial Transpl. 2015. [Epub ahead of print].
Yang W, Xie D, Anderson AH, et al. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2014;63:236–43.
Fabbian F, Cantelli S, Molino C, et al. Dialysis initiation and survival in patients with refractory congestive heart failure. Int J Artif. 2009;32(8):492–5.
Mitch W, Walser M, Buffington G, et al. A simple method of estimating progression of chronic renal failure. Lancet. 1976;2:1326–8.
Levey A, Perrone R, Madias N. Serum creatinine and renal function. Ann Rev Med. 1988;39:465–90.
Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the modification of diet in renal disease study. Kidney Int. 1997;51:1908–19.
Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012;59:504–12.
Zhong Y, Muñoz A, Schwartz GJ, Warady BA, Furth SL, Abraham AG. Nonlinear trajectory of GFR in children before RRT. J Am Soc Nephrol. 2014;25:913–7.
Crews DC, Scialla JJ, Liu J, Guo H, Bandeen-Roche K, Ephraim PL, Jaar BG, Sozio SM, Miskulin DC, Tangri N, Shafi T, Meyer KB, Wu AW, Powe NR, Boulware LE, for the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) Patient Outcomes in End Stage Renal Disease Study Investigators. Predialysis health, dialysis timing, and outcomes among older United States adults. J Am Soc Nephrol. 2014;25(2):370–9.
O’Hare AM, Batten A, Burrows NR, Pavkov ME, Taylor L, Gupta I, Todd-Stenberg, Maynard, Rodriguez R, Murtagh FEM, Larson EB, Williams DE. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis. 2012;59(4):513–22.
Lee P, Johansen K, Hsu C. End-stage renal disease preceded by rapid declines in kidney function: a case series. BMC Nephrol. 2011;12:5.
Acknowledgments
We acknowledge the support provided by the following investigators and members of the Aichi Cohort study of Prognosis in Patients Newly Initiated into Dialysis (AICOPP), who participated in this study: Yasuhiro Otsuka, Asami Takeda (Japanese Red Cross Nagoya Daiichi Hospital), Hirofumi Tamai (Anjo Kosei Hospital), Tomohiko Naruse (Kasugai Municipal Hospital), Kei Kurata (Tosei General Hospital), Hideto Oishi (Komaki City Hospital), Isao Aoyama (Japanese Community Healthcare Organization Chukyo Hospital), Hiroshi Ogawa (Shinseikai Daiichi Hospital), Hiroko Kushimoto(Chita City Hospital), Hideaki Shimizu (Chubu-Rosai Hospital), Junichiro Yamamoto(Tsushima City Hospital), Hisashi Kurata (Toyota Kosei Hospital), Taishi Yamakawa (Toyohashi Municipal Hospital), TakaakiYaomura (Nagoya Medical Center), Hirotake Kasuga(Nagoya Kyouritsu Hospital), Shizunori Ichida (Japanese Red Cross Nagoya Daiichi Hospital), Shoichi Maruyama (Nagoya University Graduate School of Medicine), Seiichi Matsuo (Nagoya University Graduate School of Medicine), Noritoshi Kato (Nagoya University Graduate Schoolof Medicine), Shigehisa Koide (Fujita Health University Hospital), and Yukio Yuzawa (Fujita Health University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The Aichi Kidney Foundation funded this study. The authors declare no competing interests.
Additional information
D. Inaguma was an investigator and member of Aichi Cohort Study of Prognosis in Patients Newly Initiated into Dialysis (AICOPP).
About this article
Cite this article
Inaguma, D., Murata, M., Tanaka, A. et al. Relationship between mortality and speed of eGFR decline in the 3 months prior to dialysis initiation. Clin Exp Nephrol 21, 159–168 (2017). https://doi.org/10.1007/s10157-016-1262-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1262-z