Abstract
Background
Matrix metalloproteinases (MMPs) and their endogenous tissue inhibitors (TIMPs) play important roles in the pathophysiology of renal diseases. Imbalanced MMPs/TIMPs are implicated in the vascular alterations of uremic patients on hemodialysis (HD). We have investigated the plasma levels of MMP-2, MMP-9, TIMP-1 and TIMP-2 in uremic patients and the effects of a course of HD on the changes in these factors.
Methods
There were 382 uremic patients on regular HD treatment and 50 healthy controls enrolled in this study. The plasma MMP-2 and MMP-9 levels were detected by gelatin zymography, and TIMP-1 and TIMP-2 concentrations were determined by ELISA assay.
Results
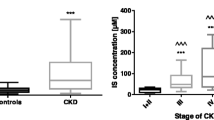
Significantly higher plasma MMP-2 and MMP-9 and decreased TIMP-1 in the uremic patients were detected compared with those in the controls. Therefore, there were markedly higher MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios in the uremic patients. In the course of a single HD session, the plasma MMP-2 level was significantly decreased from pre-HD to post-HD. TIMP-1 concentration was significantly increased from pre-HD to post-HD. Although the HD session did not have a significant effect on the levels of plasma MMP-9 and TIMP-2, both plasma MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios were significantly decreased from pre-HD to post-HD levels.
Conclusion
HD session could decrease MMP-2 and increase TIMP-1 level in the circulation of uremic patients. The physiological significance of reduced MMPs/TIMPs ratio due to a single HD session is required to further validate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endoproteinases that cleave many components of extracellular matrix (ECM) and play key roles in the physiologic processes of embryonic development, morphogenesis and tissue remodeling [1]. MMP activities are inhibited by interactions with their endogenous inhibitors, i.e., tissue inhibitors of MMPs (TIMPs) [2]. The MMPs and TIMPs regulation might be interrelated with the pathogenesis of several diseases [3] because of the abnormal ECM deposition that is caused by imbalanced MMPs and TIMPs. Besides cardiovascular diseases [4, 5], there is now clear and growing evidence indicating that abnormal MMP activities and/or imbalanced MMPs/TIMPs have been implicated in progressive chronic kidney disease (CKD) [6, 7]. Importantly, imbalanced MMPs/TIMPs induce a disequilibrium between ECM synthesis and degradation, i.e., ECM metabolism, and this pathologically and physiologically promotes vascular, glomerular and tubular alterations related with end-stage kidney disease (ESKD) [6, 8]. Imbalanced MMPs/TIMPs in ESKD may also promote cardiovascular disease [4, 5]. It is well documented that increased morbidity and mortality of patients with ESKD is highly associated with predictable cardiovascular complications, especially in ESKD patients on hemodialysis (HD) [9].
There are currently at least 28 MMPs identified. Among known MMPs, gelatinases (MMP-2, 72 kDa; MMP-9, 92 kDa) are the most studied because accumulated data show that both enzymes play important roles in the pathogenesis of cardiovascular diseases and CKD [4, 8]. TIMPs (21–28 kDa) are specific endogenous molecule that bind MMPs and inhibit MMPs activity [2]. TIMP-1, -2, -3 and -4, have been identified in humans and their expression is regulated during development and tissue remodeling [10]. TIMPs can generally inhibit all MMPs; however, the evidence indicates that TIMP-2 and TIMP-1 are the main inhibitors of MMP-2 and MMP-9 activity, respectively [11]. Therefore, the changes in serum or plasma TIMP-1, TIMP-2, MMP-2, and MMP-9 levels associated with several disease pathogenesis have been usually discussed. Several studies have been performed to examine serum or plasma MMP-2, MMP-9, TIMP-1 or TIMP-2 levels in the ESKD patients on HD [12–21]. However, conflicting results have been reported. These differences among studies may be explained by the variances in genetic factors, causal diseases, clinical conditions, and so on. However, it is noticeable that a small number of patients (less than one hundred patients for each study) were enrolled in these studies.
In the present study, we compared plasma MMP-2, MMP-9, TIMP-1, TIMP-2, MMP-2/TIMP-2 and MMP-9/TIMP-1 levels in 382 uremic patients on HD with those found in healthy volunteers. We also examined the effects of a single HD session on these circulating biochemical factors.
Materials and methods
Patient population and treatment
The present study included 382 uremic patients (aged 65.9 ± 13.8; range 33–96 years; average HD therapy time was 7.6 years) who are on regular HD treatment and 50 healthy subjects (aged 63.5 ± 14.5; range 31–87 years) (Table 1). The combinations of beta-blocker and angiotensin-converting enzyme inhibitor were mandatory for hypertension control in the HD patients (i.e., uremic patients).
The control group consisted of 50 healthy subjects that were carefully selected for any symptoms, including outpatient diagnosis, clinical examinations and laboratory tests, to exclude either cardiovascular or renal disease. The study protocol (IRB20130811R) was reviewed and approved by the Bioethics Committee of Shin Kong Wu Ho-Su Memorial Hospital (Taiwan) and written informed consent was obtained from all participants.
HD procedure
The uremic patients were routinely dialyzed three times per week for 3–4 h with a polysulfone hollow-fiber membrane of Fresenius Medical Care 4008S (Deutschland GmbH, Bad Homburg, Germany), bicarbonate dialysate, and standard heparin anticoagulation. Reverse osmosis was used for water treatment and the dialysate was regularly checked for the presence of endotoxin. Heparinized, fasting venous blood was sampled just before and at the end of the HD session (referred to as pre-HD and post-HD, respectively). The blood samples were centrifuged at 1000g for 10 min and plasma fractions were immediately stored at –80 °C until they were used for biochemical measurements.
Hematological and biochemical assays
Hematological and biochemical parameters, including total protein (TP), albumin (ALB), glucose AC, cholesterol, triglyceride, blood urea nitrogen (BUN), uric acid, creatinine, mean corpuscular volume (MCV), hematocrit (Hct), platelet, hemoglobin (Hb), and the red blood cells count (RBC) as well as white blood cells count (WBC), were determined by routine procedures using an automated analyzer (ADVIA1800; SIEMENS, Munich, Germany).
Zymography for the assay of plasma MMP-2 and MMP-9 levels
The activities of gelatinases (MMP-2 and MMP-9) in plasma were measured by gelatin zymography as our previous report [22]. The plasma was mixed with 2 × zymography sample buffer [0.125 M Tris–HCl, pH 6.8, 20 % (v/v) glycerol, 4 % (w/v) SDS, and 0.005 % bromophenol blue], incubated for 10 min, and then loaded into SDS-PAGE that was performed on 8 % acrylamide gels containing 0.1 % (w/v) gelatin (Sigma–Aldrich, St. Louis, MO, USA). After electrophoresis, the gel was washed twice for 30 min in zymography renaturing buffer (2.5 % Triton X-100) with gentle shake to remove SDS, then incubated for 18 h at 37 °C in reaction buffer (50 mM Tris–HCl, pH 7.4, 200 mM NaCl, and 5 mM CaCl2). The gels were then stained with Coomossie blue for 30 min prior to destain with destain buffer (50 % methanol, 10 % acetic acid, and 40 % ddH2O). Finally, gelatinolytic activities on the gels were presented as transparent bands on the blue background. Gelatinolytic activities were identified as clear zones and calculated densitometric value of the lyses against a dark blue background on zymography gels using ImageJ software (National Institutes of Health, Bethesda, MD, USA), which quantified both the surface and the intensity of the lysis bands after scanning of the gels. The levels of MMP-2 and MMP-9 gelatinolytic activity of certain clear zones were then normalized to the levels in recombined human MMP-2 (#M9070; Sigma–Aldrich, St. Louis, MO, USA) and MMP-9 (#M8945; Sigma–Aldrich) as controls on the same zymography gel, respectively.
Enzyme-linked immunosorbent assay (ELISA) for TIMPs
The concentrations of TIMPs in plasma were determined using human TIMP-1 and TIMP-2 ELISA kits (Abcam, Cambridge, MA, USA) according to the manufacturer’s recommendations. The samples were diluted appropriately to fall within the standard range of the assay. Each recombinant human TIMP-1 and TIMP-2 were used as a standard. The diluted plasma was incubated in ELISA plates, in which wells had been coated with anti-human TIMP-1 and TIMP-2 primary antibodies. Following the addition of biotinylated secondary antibodies, the plates were washed and reacted with horseradish peroxidase (HRP)-conjugated streptavidin. Tetramethylbenzidine (TMB) one-step substrate was used to detect the targeted protein and the product was measured at 450 nm using a micro-plate reader (Thermo Scientific Multiskan EX, Waltham, MA, USA).
Statistical analysis
Statistical analyses were performed using the SPSS statistical software package (version 19.0 for Windows; SPSS, Chicago, IL, USA). All parameters are presented as the mean with the standard deviation (SD). Clinical features were compared between the groups using the unpaired Student’s t test or Mann–Whitney’s test. A value of P < 0.05 was considered statistically significant.
Results
General clinical characteristics of the HD patients
The clinical characteristics of study subjects, including 382 uremic patients (or named as HD patients; the patients on hemodialysis) and 50 healthy controls are listed in Table 1. Generally, glucose AC in the uremic patients were significantly higher than those in the controls (P < 0.001), whereas a lower Hct, platelet, Hb and the number of RBC was detected in the HD patients compared with those in the control (P < 0.001). The levels of total protein, albumin, cholesterol, triglyceride, MCV and the number of WBC in both groups were insignificantly different (Table 1).
Two major uremic toxins, BUN (71.2 ± 17.4 vs. 14.2 ± 5.0 mg/dL, P < 0.001) and creatinine (9.03 ± 2.11 vs. 0.87 ± 0.34 mg/dL, P < 0.001), and CRP (4.92 ± 3.61 vs. 0.51 ± 0.30 mg/L, P < 0.001), a biomarker of physiological inflammation, in the uremic patients were markedly higher than those in the healthy controls (Table 2).
Plasma MMPs and TIMPs in the HD patients
Plasma MMP-2 and MMP-9 levels in the HD patients were significantly higher than those in the healthy controls (567 ± 117 vs. 430 ± 81 ng/mL for MMP-2 and 329 ± 90 vs. 255 ± 23 ng/mL for MMP-9, P < 0.001). The plasma TIMP-1 concentration was significantly lower in the HD patients compared with that in the healthy controls (325 ± 90 vs. 373 ± 60 ng/mL, P < 0.001). There were no significant alterations in the TIMP-2 concentration in the HD patients compared with that in the healthy subjects (116 ± 34 vs. 123 ± 57 ng/mL, P = 0.172) (Table 2). Increasing plasma MMP-2 and MMP-9 levels as well as decreasing TIMP-1 concentration resulted in significantly increasing MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios in the HD patients compared with both in the healthy controls (5.39 ± 2.19 vs. 4.02 ± 2.43 for MMP-2/TIMP-2 and 1.11 ± 0.47 vs. 0.77 ± 0.19 for MMP-9/TIMP-1, P < 0.001) (Table 2).
The correlations between plasma MMPs, TIMPs, MMPs/TIMPs, and clinical and biochemical characteristics in the HD patients were shown in Table 3. The significantly positive correlations were observed between Glucose AC and plasma MMP-2, MMP-2/TIMP-2, MMP-9 as well as MMP-9/TIMP-1 in the HD patients. Hct, MCV and Hb were significantly correlated with MMP-9 or MMP-9/TIMP-1, but not with MMP-2 and MMP-2/TIMP-2. All measured clinical and biochemical characteristics in the HD patients were not correlated with the TIMP-1 and TIMP-2 plasma levels.
Changes during the HD session
The parameters of BUN, creatinine, CRP, MMP-1, MMP-9, TIMP-1 and TIMP-2 in the uremic patients before (pre-HD) and after (post-HD) a single HD session have been determined and compared. The results show significant reductions of BUN and creatinine in post-HD, whereas the serum CRP level in the uremic patients was similar between pre-HD and post-HD (Table 4).
The plasma MMP-2 level was significantly decreased from pre-HD 567 ± 117 ng/mL to post-HD 524 ± 89 ng/mL (P < 0.001). The plasma MMP-9 level was also decreased due to HD treatment, however, the difference did not reach significance (329 ± 90 vs. 317 ± 81, P = 0.067). Plasma TIMP-1 concentration was significantly increased from pre-HD 325 ± 90 ng/mL to post-HD 358 ± 102 ng/mL (P < 0.001). However, TIMP-2 concentration in pre-HD (116 ± 34 ng/mL) was similar to that in post-HD (119 ± 40 ng/mL) (Table 4).
Decreasing MMP-2 and increasing TIMP-1 result in significantly reduced plasma MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios in the uremic patients after an HD session comparing both ratios in the uremic patients before HD session. The plasma MMP-2/TIMP-2 and MMP-9/TIMP-1 ratio was significantly reduced from pre-HD 5.39 ± 2.19 to post-HD: 4.98 ± 2.03 (P < 0.01) and from pre-HD 1.11 ± 0.47 to post-HD: 0.97 ± 0.41 (P < 0.01), respectively (Table 4).
Dehydration induced by HD treatment may affect the concentration of circulating TIMPs, therefore, TIMP-1 and TIMP-2 plasma levels normalized by serum total protein in the samples of pre-HD and post-HD have been calculated and compared in Table 4. Indeed, the difference of TIMP-1 plasma level between pre-HD and post-HD was little significance after including the factor of HD dehydration. However, a significant difference (P < 0.05) of plasma TIMP-1 is still could be obtained.
Discussion
We reported increased MMP-2 levels as well as decreased TIMP-1 concentrations in the circulation of uremic patients compared with those in the healthy controls; therefore, there were higher ratios of circulating MMP-2/TIMP-2 and MMP-9/TIMP-1 in the uremic patients on HD. In the course of an HD session, decreased MMP-2 and increased TIMP-1 in the circulation were observed. These results point to the reductions of circulating MMP-2/TIMP-2 and MMP-9/TIMP-1 ratio after a single HD session.
MMP-2 and MMP-9 play important roles in the pathogenesis of progressive CKD. A lot of studies had been executed to examine MMP-2 and MMP-9 activities in patients with different stages of CKD (Table 5). The results of most of these studies showed increased MMP-2 levels in ESKD patients compared with healthy controls [13, 16, 17, 19] and proposed that increasing MMP-2 maybe associated with kidney damage. In this study, we revealed a statistically significant increase in the plasma MMP-2 level in uremic patients. The result is consistent with the most previous work. The experimental evidence showed an early upregulation of MMP-2 expression in the areas of elastin degradation and smooth muscle cells phenotype change in CKD course, which is associated with increased circulating MMP-2 level [4, 23]. These alterations clearly promote vascular medial layer calcification and the increases in MMP-2 level correlated positively with vascular stiffness and phosphate concentrations in CKD patients [8]. In line with our results showing increased MMP-2 level in uremic patients, the elevated circulating MMP-2 levels have also been described as an indicator of cardiovascular disease in HD patients [13, 24].
Compared to MMP-2, very conflicting results on MMP-9 level in progressive CKD and ESKD patients have been reported (Table 5). Our result of increased circulating MMP-9 level in the uremic patients was in agreement with the results obtained by previously reports [12, 16–18]; however, contrasting results have been presented by Rysz et al. [20] who have reported a decreased MMP-9 level in ESKD or HD patents. Although a few papers reported decreased or unaltered circulating MMP-9 levels in ESKD patients [12, 20], several studies including ours have indicated significantly elevated MMP-9 associated with progressive CKD, ESKD or patients on HD (Table 5). However, it is well known that when measuring circulating MMP levels, care should be taken to avoid artificial results caused by sample preparation and there are also remarkable differences between plasma and serum samples [25].
Decreased TIMP-1 in the uremic patients was detected in this study. TIMP-1 is the major member of four TIMPs identified in humans [10]. Pleiotropic functions and clinical implications of TIMP-1, including cancer, cardiovascular disease and CKD, have been reported [17, 26]. Musial and Zwolinska [27] have showed that the increase in serum TIMP-1 concentrations in children with CKD appears only in the late stages of renal failure and proposed that increased TIMP-1 is an anti-fibrotic response to ECM accumulation.
Significantly increased plasma MMP-2 and MMP-9 levels and decreased TIMP-1 concentrations in the uremic patients were detected in our study. Therefore, there were markedly higher MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios in the HD patients. There are evidence for imbalanced MMPs/TIMPs ratios and increased ECM deposition in the development of CKD [6, 7, 28]. Marson et al. [7] wrote a detailed review evaluating the role of imbalanced MMPs/TIMPs in the pathogenesis of cardiovascular alterations found in ESKD patients and validate the contributions of using MMP inhibitors in the treatment for ESKD. So far, the change in MMPs/TIMPs balance in ESKD specifically associated with impaired renal function is not well understood. Further studies are necessary to conclude a causal association between the regulation of MMPs/TIMPs and progression of CKD. A particular imbalance between MMP-2 and its endogenous inhibitor, TIMP-2, has been implicated in the vascular alterations of ESKD [8]. The vascular alterations in ESKD may be related to imbalanced MMP-2/TIMP-2 in serum or plasma [4, 8, 23]. These propositions were supported the findings in our study, i.e., higher MMP-2 levels in the uremic patients with original cause of hypertension.
In the present study, we demonstrated higher concentration of blood glucose AC in the uremic patient indicated diabetic uremia (Table 3). There were around 30 % of the HD patients enrolled in this study with original disease caused by diabetes mellitus (DM). Additionally, our results indicated significantly positive correlations between glucose AC and plasma MMP-2 as well as MMP-9, that also resulted the significantly positive correlations between glucose AC and MMP-2/TIMP-2 as well as MMP-9/TIMP-1 ratios in the HD patients. DM is associated with an increased risk of vascular disease [29, 30] and impaired regulation of ECM remodeling by MMPs may contribute to the development of vascular complications in diabetic nephropathy (DN) [10, 31]. Several MMPs are increased in the plasma and urine of patients with diabetes [32]. Increased levels of both MMP-2 and MMP-9 have been demonstrated in the serum from patients with type 1 diabetes [33]. In a recent large scale clinical study, the data showed that circulating MMP-2 level starts to rise early before the onset of microalbuminuria induced by diabetes and increased almost twofold in the patient with a microalbuminuria duration of more than 5 years [34]. According to the results, the authors proposed MMP-2 as potential marker of early nephropathy in type 1 diabetes.
In 2002, Chou et al. [35] first reported the MMP-2, MMP-9, TIMP-1 and TIMP-2 in HD patients to look into the effect of HD process on the MMPs and TIMPs changes. In the study, the authors showed decreased circulating MMP-2 and MMP-9, increased TIMP-2 and unaltered TIMP-1 in the patients post an HD session; however, no data addressing the MMPs/TIMPs ratio was reported, and only 40 HD patients were enrolled. Rysz et al. [20] have also simultaneously assessed the changes of plasma MMP-2, MMP-9, TIMP-1 and TIMP-2 in patients on HD. This study showed significantly decreased plasma MMP-2 and TIMP-1 as well as slightly increased MMP-9 and MMP-9/TIMP-1 after an HD session. However, there were only 18 patients on regular HD treatment enrolled in the study. In the present study, we observed that the plasma levels of MMP-2, MMP-9, TIMP-1 and TIMP-2 were differentially affected by HD session in 382 uremic patients and the results show a significant decrease in MMP-2 and increase in TIMP-1 after a single HD session.
Several studies have analyzed the effects of an HD session on the circulating levels of MMP-2 and MMP-9. However, conflicting results have been reported (Table 6). In line with our findings, most studies have shown that HD treatment reduced or had no effect on MMP-2 and MMP-9 levels [14, 19, 35, 36]. Variable results have also been reported for the effects of HD on TIMP-1 and TIMP-2 levels (Table 6) [17, 20, 35]. Rysz et al. [20] showed significantly decreasing plasma TIMP-1 and no significant change on TIMP-2 level after an HD session. Comparing our data with this previous report, significantly decreased MMP-2 after an HD session is consistent; however, the increases in MMP-9 and MMP-9/TIMP-1 are controversial results [36]. These differences between our data and the previous reports may be explained by differences in causal diseases, age, genetic factors, type of dialysis membrane, clinical conditions and pharmacological interventions. Another important issue is the serum used in the study by Rysz et al. [20] and plasma used in this study. It is not clear why TIMP-1 (a 21-kDa molecule) concentration was increased and the level of MMP-2 (72 kDa) was decreased during a single HD session. The larger size of MMP-2 should have precluded its filtration, thus suggesting that a mechanism other than ultrafiltration is certainly involved. Interestingly, although HD activates inflammatory responses, which promote MMPs release [19], no study has shown increased MMP-2 levels after an HD session.
Neutrophil and monocyte activation in CKD patients under HD have been reported [37]. It is expected that increasing neutrophil MMP-9 could be detected in the circulation of HD patients under HD. However, our results do not match this expectation (Table 4). It is very complications of the effects on plasma MMP-9 activity during HD. Grzela et al. [38] have indicated that heparin treatment could decrease plasma MMP-9 activity, but not MMP-2, in the patients with abdominal aortic aneurysm. Additionally, MMP-9 is also produced and secreted by several cell types, including vascular endothelial cells. HD removing uremic toxins can decrease MMP-9 expression of vascular endothelial cells [39]. Overall the discussion above, insignificant difference of plasma MMP-9 activity between pre-HD and post-HD detected could be reasonably explained.
It has been shown that HD treatment increased circulating TIMP-1 level and the results of the current study support this report [36]. However, the contradicting result, i.e., decreased TIMP-1 due to HD process, also have been reported [17, 20]. In the present study, decreased MMP-2, increased TIMP-1 and insignificantly altered MMP-9 as well as TIMP-2 levels in the circulation after a single HD session were detected. A few papers have reported the effects of the HD process on circulating MMPs/TIMPs ratios. Rysz et al. [20] showed that MMP-2/TIMP-2 and MMP-9/TIMP-1 were not affected by HD process and Marson et al. [36] indicated that MMP-9 genetic variants could modify the effects of HD on circulating MMP-9 level and an increased MMP-9/TIMP-1 ratio detected in the circulation after HD treatment. Our study is the first to report significant reductions in both MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios after a HD session.
In spite of the small changes of MMPs/TIMPs come from a single session of HD, uremic patients need to receive HD session more than three times per week and should live a long time on HD treatment. Therefore, we proposed that a little reduced MMPs/TIMPs ratio during each HD session could meet some of significantly biological consequences for long-term HD treatment. Increased circulating MMPs production and/or MMPs/TIMPs ratio seem to correlate more with increased thrombotic incidence and atherosclerosis observed in the patients with CKD [4, 5]. Zafeiropoulou et al. [39] have demonstrated that removal of circulating uremic toxins by HD treatment could reduce the MMPs (MMP-2 and MMP-9) and enhance TIMPs (TIMP-1 and TIMP-2) production by vascular endothelial cells in vitro as well as their enzymatic activity. This proposition is confirmed by Rysz et al. [20], they indicated that route HD-related washout of uremic toxins would partially explain the decrease of MMP-2 in the patients. Therefore, regular HD related with the decreases of MMPs activity and/or MMPs/TIMPs ratio might be reduce the risk of cardiovascular incidents observed in the patients with CKD.
Conclusion
The alteration in MMPs, TIMPs and MMPs/TIMPs ratio in HD patients might be explicitly related to impaired renal function and the pathogenesis of renal diseases. The decrease in plasma MMP-2, increase in plasma TIMP-1 levels and imbalanced MMPs/TIMPs ratio might play a key role in the progress of CKD and the effects of a single HD session. The preliminary data present herein could form the basis of further study.
References
Szarvas T, vom Dorp F, Ergün S, Rübben H. Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat Rev Urol. 2011;8:241–54.
Clutterbuck AL, Asplin KE, Harris P, Allaway D, Mobasheri A. Targeting matrix metalloproteinases in inflammatory conditions. Curr Drug Targets. 2009;10:1245–54.
Amălinei C, Căruntu ID, Giuşcă SE, Bălan RA. Matrix metalloproteinases involvement in pathologic conditions. Rom J Morphol Embryol. 2010;51:215–28.
Chen NX, O’Neill KD, Chen X, Kiattisunthorn K, Gattone VH, Moe SM. Activation of arterial matrix metalloproteinases leads to vascular calcification in chronic kidney disease. Am J Nephrol. 2011;34:211–9.
Spinale FG, Villarreal F. Targeting matrix metalloproteinases in heart disease: lessons from endogenous inhibitors. Biochem Pharmacol. 2014;90:7–15.
Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol. 2007;292:F905–11.
Marson BP, Poli de Figueiredo CE, Tanus-Santos JE. Imbalanced matrix metalloproteinases in cardiovascular complications of end-stage kidney disease: a potential pharmacological target. Basic Clin Pharmacol Toxicol. 2012;110:409–15.
Chung AW, Yang HH, Kim JM, Sigrist MK, Chum E, Gourlay WA, et al. Upregulation of matrix metalloproteinase-2 in the arterial vasculature contributes to stiffening and vasomotor dysfunction in patients with chronic kidney disease. Circulation. 2009;120:792–801.
Paloian NJ, Giachelli CM. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol. 2014;307:F891–900.
Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–62.
Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73.
Chang HR, Yang SF, Li ML, Lin CC, Hsieh YS, Lian JD. Relationships between circulating matrix metalloproteinase-2 and -9 and renal function in patients with chronic kidney disease. Clin Chim Acta. 2006;366:243–8.
Pawlak K, Pawlak D, Mysliwiec M. Long-term erythropoietin therapy does not affect metalloproteinases and their inhibitor levels, oxidative stress and inflammation in hemodialyzed patients. Am J Nephrol. 2007;27:221–5.
Polañska B, Makulska I, Augustyniak D, Niemczuk M, Zwoliñska D, Jankowski A. Serum levels of MMP-9 in children and young adults with chronic kidney disease treated conservatively and undergoing hemodialysis. Central Eur J Immunol. 2007;32:66–71.
Rysz J, Banach M, Stolarek RA, Pasnik J, Cialkowska-Rysz A, Koktysz R, et al. Serum matrix metalloproteinases MMP-2 and MMP-9 and metalloproteinase tissue inhibitors TIMP-1 and TIMP-2 in diabetic nephropathy. J Nephrol. 2007;20:444–52.
Friese RS, Rao F, Khandrika S, Thomas B, Ziegler MG, Schmid-Schonbein GW, et al. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens. 2009;31:521–33.
Musiał K, Zwolińska D. Matrix metalloproteinases (MMP-2,9) and their tissue inhibitors (TIMP-1,2) as novel markers of stress response and atherogenesis in children with chronic kidney disease (CKD) on conservative treatment. Cell Stress Chaperones. 2011;16:97–103.
Musiał K, Zwolińska D. Matrix metalloproteinases and soluble Fas/FasL system as novel regulators of apoptosis in children and young adults on chronic dialysis. Apoptosis. 2011;16:653–9.
Pawlak K, Mysliwiec M, Pawlak D. Peripheral blood level alterations of MMP-2 and MMP-9 in patients with chronic kidney disease on conservative treatment and on hemodialysis. Clin Biochem. 2011;44:838–43.
Rysz J, Banach M, Stolarek RA, Mikhailidis DP, Cialkowska-Rysz A, Pokoca L, et al. Serum metalloproteinases MMP-2, MMP-9 and metalloproteinase tissue inhibitors TIMP-1 and TIMP-2 in patients on hemodialysis. Int Urol Nephrol. 2011;43:491–8.
Marson BP, Lacchini R, Belo V, Dickel S, da Costa BP, Poli de Figueiredo CE, et al. Matrix metalloproteinase (MMP)-2 genetic variants modify the circulating MMP-2 levels in end-stage kidneydisease. Am J Nephrol. 2012;35:209–15.
Cheng KS, Liao YC, Chen MY, Kuan TC, Hong YH, Ko L, et al. Circulating matrix metalloproteinase-2 and -9 enzyme activities in the children with ventricular septal defect. Int J Biol Sci. 2013;9:557–63.
Pai A, Leaf EM, El-Abbadi M, Giachelli CM. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178:764–73.
Pawlak K, Tankiewicz J, Mysliwiec M, Pawlak D. Systemic levels of MMP2/TIMP2 and cardiovascular risk in CAPD patients. Nephron Clin Pract. 2010;115:c251–8.
Gerlach RF, Demacq C, Jung K, Tanus-Santos JE. Rapid separation of serum does not avoid artificially higher matrix metalloproteinase (MMP)-9 levels in serum versus plasma. Clin Biochem. 2007;40:119–23.
Ries CH. Cytokine functions of TIMP-1. Cell Mol Life Sci. 2014;71:659–72.
Musiał K, Zwolińska D. Novel indicators of fibrosis-related complications in children with chronic kidney disease. Clin Chim Acta. 2014;430:15–9.
Boor P, Sebekova K, Ostendorf T, Floege J. Treatment targets in renal fibrosis. Nephrol Dial Transplant. 2007;22:3391–407.
Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46:760–5.
Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care. 2006;29:798–804.
Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD. Matrix metalloproteinases and diabetic vascular complications. Angiology. 2005;56:173–89.
Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302:F1351–61.
Gharagozlian S, Svennevig K, Bangstad HJ, Winberg JO, Kolset SO. Matrix metalloproteinases in subjects with type 1 diabetes. BMC Clin Pathol. 2009;9:7.
Fouad M, Boraie M. Matrix metalloproteinase-2 as potential marker of early nephropathy in type 1 diabetes. Am J Intern Med. 2015;3:1–5.
Chou FP, Chu SC, Cheng MC, Yang SF, Cheung WN, Chiou HL, et al. Effect of hemodialysis on the plasma level of type IV collagenases and their inhibitors. Clin Biochem. 2002;35:383–8.
Marson BP, Lacchini R, Belo V, Mattos SG, Da Costa BP, Poli-de-Figueiredo CE, et al. Functional matrix metalloproteinase (MMP)-9 genetic variants modify the effects of hemodialysis on circulating MMP-9 levels. Clin Chim Acta. 2012;414:46–51.
Pereira R, Costa E, Gonçalves M, Miranda V, do Sameiro Faria M, Quintanilha A, et al. Neutrophil and monocyte activation in chronic kidney disease patients under hemodialysis and its relationship with resistance to recombinant human erythropoietin and to the hemodialysis procedure. Hemodial Int. 2010;14: 295–301.
Grzela T, Brawura-Biskupski-Samaha R, Jelenska MM, Szmidt J. Low molecular weight heparin treatment decreases MMP-9 plasma activity in patients with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2008;35:159–61.
Zafeiropoulou K, Bita T, Polykratis A, Karabina S, Vlachojannis J, Katsoris P. Hemodialysis removes uremic toxins that alter the biological actions of endothelial cells. PLoS One. 2012;7:e30975.
Acknowledgments
This work was supported by the grants of MOST 104-2313-B-009 -001-MY3 from the Ministry of Science and Technology and HCH 104-017 from National Taiwan University Hospital Hsin-Chu Branch, Taiwan. This work is also supported by the “Aiming for the Top University Program” of the National Chiao Tung University and the Ministry of Education, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors have no competing interests.
Additional information
L.-C. Lu and C.-W. Yang contributed equally to the manuscript.
About this article
Cite this article
Lu, LC., Yang, CW., Hsieh, WY. et al. Decreases in plasma MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios in uremic patients during hemodialysis. Clin Exp Nephrol 20, 934–942 (2016). https://doi.org/10.1007/s10157-015-1221-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1221-0