Abstract
Background
This study presents a laparoscopic surgical protocol for right hemicolectomy and D3 lymphadenectomy (R-D3L) in right colon cancer and reports the oncological outcomes based on a prospective series.
Methods
The study comprises two phases. In the first phase, a dynamic demonstration of the R-D3L surgical protocol is provided through textual explanation, illustrations, and edited surgical videos. The protocol emphasizes technical steps such as dissection of the embryological plane of the right mesocolon, high tie of ileocolic vessels, surgical trunk of Gillot dissection, and high tie of superior right colic vein (SRCV). In the second phase, a prospective observational study was conducted involving patients undergoing R-D3L surgery with this protocol between July 2015 and July 2021. Demographic, perioperative, and postoperative variables are analyzed, along with anatomopathological variables and oncological outcomes.
Results
A total of 33 patients were analyzed. Median operative time was 202 min. Perioperative bleeding occurred in 6%. Postoperative complications were mild (Clavien–Dindo III in 2%). Postoperative ileus was observed in 15%. No anastomotic dehiscence was reported. The median postoperative stay was 7 days. The median number of resected lymph nodes was 26, with 27% having positive nodes and 70% were classified as stage T3 or T4. After a median follow-up of 45 months, local recurrence, distant recurrence, and carcinomatosis rates were 0%. Mortality rate from other causes was 9%.
Conclusion
The surgical protocol shown in the present study could help in the implementation of this technique in those units that consider it appropriate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oncologic right hemicolectomy has a local recurrence rate of 8–14% [1, 2] and a distant recurrence rate of 10–15% [3]. Right complete mesocolic excision (R-CME) in western countries [4] and D3 lymphadenectomy (R-D3L) [5] in the east are aimed at decreasing these rates. The technical differences between R-CME and R-D3L are currently the subject of discussion between different authors.
R-D3L is a technically demanding procedure. The aim of the present study is to propose a laparoscopic surgical protocol for R-D3L and to show the oncological results of this protocol from a prospective series.
Materials and methods
First phase: Dynamic demonstration of the R-D3L surgical protocol by means of explanatory text, illustration, and edited video of each surgical step. The demonstration of the surgical protocol is based on recordings of a real laparoscopic R-D3L surgery performed by a colorectal surgeon (AGG) and a surgeon in training.
In those patients where the possibility of infiltration of the retroperitoneal margin was observed, the retroperitoneal fat area was incorporated en bloc into the surgical specimen.
In those patients in whom the possibility of infiltration of a neighboring anatomical structure was observed, this structure was partially or completely excised en bloc into the surgical specimen.
Written signed informed consent was obtained from all patients during the first and second phase of this study.
Second phase: Prospective observational study of patients undergoing surgery between July 2015 and July 2021 for R-D3L using the protocol shown in the first phase. Surgical interventions were performed in two colorectal surgery units, at the Hospital Universitario y Politécnico la Fe in Valencia and Son Espases University Hospital in Palma de Mallorca.
The anatomopathological study of the surgical specimens was carried out at each center by an anatomopathologist specifically trained for this purpose by means of a predetermined protocol [6, 7] and classified according to the TNM 7th edition [8]. Oncological follow-up was carried out by the oncology units of each center.
Patient selection was based on the recommendations of the Japanese Colorectal Surgery Guidelines [9]. Selected patients had suspected positive nodes preoperatively or intraoperatively and in T2 according to surgical criteria.
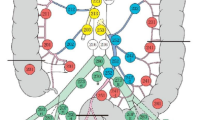
Inclusion criteria: laparoscopic approach, pathological anatomy of the surgical specimen of adenocarcinoma, and complete D3 lymphadenectomy according to the standards described by the same working group (Fig. 1) [6, 7].
Exclusion criteria: preoperative or intraoperative presence of metastases, intraoperative presence of distant metastases or peritoneal carcinomatosis.
The preoperative variables analyzed were gender, age, American Society of Anesthesiologists (ASA) classification, preoperative carcinoembryonic antigen (CEA), tumor location, possible lymphadenopathies on preoperative computed tomography (CT) scan, preoperative contained perforation/abscess in tumor area, scheduled or urgent surgery.
Intraoperative variables were the type of surgery (right hemicolectomy or extended right hemicolectomy), operative time, intraoperative bleeding due to superior mesenteric vein or gastrocolic trunk of Henle injury, and conversion to laparotomy. An extended right hemicolectomy was when central ligation of the middle colic vessels was performed.
Postoperative variables were classified according to Clavien–Dindo [10] and were analyzed independently for reoperation for hemoperitoneum, postoperative ileus, anastomotic dehiscence, surgical site infection, and postoperative length of stay.
Perioperative bleeding was considered when there was the need for reoperation for hemoperitoneum, intraoperative bleeding requiring hemostatic surgical movements, or the need for transfusion of red blood cells during surgery or postoperatively.
Postoperative ileus was defined as the absence of passing of gas or feces for more than 6 days after surgery.
Anastomotic dehiscence was considered when there was the need for percutaneous drainage due to perianastomotic collection, surgical re-intervention with an intraoperative finding of anastomotic dehiscence or need of antibiotic treatment due to radiological report of perianastomotic collection or perianastomotic pneumoperitoneum.
As for the microscopic anatomopathological variables, the degree of differentiation, total lymph nodes resected, patients with positive lymph nodes for infiltration, proximal/distal border infiltration, retroperitoneal infiltration, vascular infiltration, lymphatic infiltration, perineural infiltration, pathological node (N), and pathological tumor depth (T) were recorded. An R0 type resection was considered if the radial or retroperitoneal surgical margins were free of infiltration within more than 2 mm. Finally, it was recorded whether patients received pre- and/or postoperative chemotherapy, presence of tumor recurrence, mortality due to oncological disease progression, and mortality due to other causes. The types of tumor recurrence were classified into clinical and/or pathologically confirmed (divided into adenopathy local recurrence or carcinomatosis) and clinical and/or anatomopathological confirmation of distant recurrence.
The qualitative variables were expressed as sample size and percentage while the quantitative variables were expressed as the median and range.
Results
First phase
Technical steps of laparoscopic R-D3L. Trocars: Hasson periumbilical, first surgeon trocars: 12 mm in left iliac fossa, 5 mm suprapubic, first assistant trocars: 5 mm left subcostal. Occasionally an additional 5 mm trocar is used for the second assistant in the right hypochondrium.
1. Dissection of the embryological plane of the right mesocolon (Video 1): The operation should be started in a Trendelenburg position and left lateral rotation. The authors recommend using the ileocolic vessels as a reference for the medial access and not to divide them until reaching the hepatocolic ligament through Toldt’s fascia (Fig. 2A). Completely release the second duodenal portion and the head of pancreas to the lateral plane of the superior mesenteric vein (SMV) through division of Fredet’s fascia (Fig. 2B). The recommended anatomical reference is the visualization of the right superior colic vein (SRCV). Once this vein has been identified, it is useful to place a gauze in this plane which will be located in stage 4 [11].
2. High tie of ileocolic vessels (Fig. 3A) (Video 2): This should be performed after visualization of the SMV both proximal and distal to the origin of the ileocolic vein.
Photographic description of A high ligation of the ileocolic vessels with intact right mesocolic sail, B dissection of lympho-adipose tissue over superior mesenteric vein or surgical trunk of Gillot, C identification of superior right colic vein draining into the trunk of Henle, and D central ligation of the latter, E central ligation of right branch of the middle colic vessels. SMV superior mesenteric vein, ASPDV anterosuperior pancreatoduodenal vein
3. Surgical trunk of Gillot (STG) dissection (Fig. 3B) (Video 3): The end of the divided ileocolic vessels should be used as a reference. It is essential to access the plane between the SMV and the lympho-adipose/nerve tissue above the SMV. It is sometimes necessary to complete the division of Fredet’s fascia at this point.
4. Release of the hepatic flexure and division of the right parietocolic ligament (Video 4): To do this, the authors recommend changing the patient’s position to anti-Trendelenburg and use the left subcostal trocar. The assistant ascends the gastroepiploic arcade while the first surgeon descends the transverse colon and tenses the greater omentum. In this way the gastrocolic ligament is transected (Fig. 4A) and the embryological plane of the lesser sac of the omentum is accessed on its right side and the greater omentum can be incorporated into the surgical specimen. The hepatocolic ligament is then divided (Fig. 4B) and the gauze lodged in stage 1 is located. At this point, the main surgeon may use the left subcostal trocar to complete the dissection of the hepatocolic ligament (Fig. 4B) and its continuation known as the parietocolic ligament to the right iliac fossa.
5. Identification and division of the right gastroepiploic vein (RGEV) (Video 5): This step is the beginning of the dissection of the gastrocolic trunk of Henle (GCTH). The assistant again ascends the gastroepiploic arcade and thus tensing the right gastroepiploic vein (RGEV) (Fig. 4C). The surgeon must dissect the RGEV to its drainage into the GCTH and divide it at that point. Prior to perform stage 6, the authors recommend placing a gauze over the head of the pancreas, between the gastroepiploic arcade and the transverse mesocolon.
6. High tie of the right superior colic vein (Fig. 3C, D) (Video 6): The patient should be placed back in the Trendelenburg position. The surgeon accesses below the right mesocolon and ascends it until the SRCV shows tension. This maneuver may cause the SRCV to tear as it is not accompanied by an artery that increases its fixation. Dissection of the SRCV must be done until it enters the GCTH.
At this point it is ligated and incorporated into the surgical specimen. The gauze lodged in stage 5 gives us security to avoid injuring the right gastroepiploic artery.
7. High tie of the right branch of the middle colic vessels (Fig. 3E) (Video 7): Finally, the surgeon increases the traction of the right mesocolon after the division of the SRCV to cause tension of the middle colic vessels. Once the origin of the colic vessels has been identified, the division of the transverse mesocolon is started from the right side until the origin of the right branch of the middle colic vessels is identified. At that time, they are ligated and divided.
In all patients, the surgical specimen was removed by means of a supraumbilical minilaparotomy and the anastomosis was performed by manual extracorporeal ileocolic anastomosis with double anterior and double posterior sutured plane.
Second phase
Thirty-three patients were analyzed. The median age was 74 years old and 55% were men. Table 1 shows the demographic and perioperative variables.
All surgeries were performed on a scheduled basis, with right hemicolectomy being the most frequent procedure (90.9%). The median operative time was 202 min (150–215). Two patients (6%) had perioperative bleeding, one due to intraoperative bleeding due to injury to the middle colic vessels and one reoperated for right gastroepiploic artery bleeding. No patients were classified as Clavien–Dindo IV or V and two patients as III. Postoperative ileus occurred in 15%. The percentage of reoperation or need for percutaneous drainage due to anastomosis dehiscence was 0%. Surgical site infection occurred in 12%. The median postoperative stay was 7 days (4–20).
Table 2 shows the macroscopic and microscopic anatomopathological variables and oncological findings. The median number of resected nodes was 26 (9–90). Twenty-seven percent of patients had positive lymph nodes for tumor infiltration; 70% were classified as stage T3 or T4. None of the patients received preoperative chemotherapy and 21% received postoperative chemotherapy.
After a median follow-up of 45 months, the percentages of local recurrence, distant recurrence, and carcinomatosis were 0%. The mortality rate was 9%, none of them due to disease progression.
Discussion
The present work is not intended to respond to the current discussion on the oncological benefit of R-D3L versus D2 lymphadenectomy, but rather to demonstrate a laparoscopic surgical protocol for those surgeons who believe it is appropriate to routinely or selectively perform it on a routine or selective basis. Previous studies have demonstrated the usefulness of applying surgical protocols in colorectal oncological surgery [12].
The indication for D3 lymphadenectomy in right colon cancer is currently under scientific discussion in most forums on colorectal oncological surgery. Some meta-analyses published in the last 2 years conclude that it improves oncological outcomes in stage III and even stage II patients [13]. On the other hand, a recent prospective study questions the benefit of this technique [14].
The R-CME and R-D3L concur in the access to the embryological plane and high tie of the ileocolic vessels and right branch of the middle colic vessels. R-D3L adds dissection of the STG and GCTH [15, 16].
The STG is located over the SMV and not over the superior mesenteric artery (SMA) [15]. In fact, recent publications have emphasized that it is not necessary to include lympho-adipose tissue located over the SMA as there is more presence of nerve tissue and it increases the likelihood of intestinal transit alterations, either as early postoperative ileus or as late postoperative diarrhea episodes [17].
The importance of en bloc excision of the STG with the surgical specimen is that it allows pathologists to classify surgical specimens as complete or absent D3 lymphadenectomy using the term “right mesocolic sail” [6, 7].
To facilitate dissection of the GCTH, it would be sufficient to include the SRCV in the specimen by its high tie [18]. The presence of the SRCV is described in 95% of cases [16].
The central control of the GCTH is not routinely recommended because of an increased risk of direct injury to the SMV and subsequent added difficulty for hemostatic control [19].
The vascular variability of the GCTH site and the potential for SMV injury when resecting the lymphovascular tissue located in this area require a training plan to be mapped prior to its application [20]. The results of the present study show a percentage of intraoperative or postoperative bleeding of 6%.
Some authors prefer to start the retroperitoneal approach inferior to the mesoileum rather than inferior to the ileocolic vessels (caudal-cranial). This has the advantage of keeping the mesocolon intact, but the disadvantage of using the ileocolic vessels as an anatomical reference [21].
The presence of a positive retroperitoneal margin has been associated with an increased likelihood of local recurrence but has not been the subject of the same number of publications as the need for central ligation [22].
Some authors highlight the need to improve the preoperative diagnostic accuracy for possible preoperative infiltration of the retroperitoneal margin (currently 79% for CT scans) and to extend the oncological dissection beyond the mesocolon [23].
In the present study, the smooth surface of the posterior surface of the surgical specimen indicating satisfactory mesocolon quality was considered as an inclusion criteria [24]. In this study the presence of satisfactory mesocolon was 100%. However, one patient had a positive retroperitoneal margin.
In oncological surgery of the right colon, it is recommended to include the right portion of the greater omentum in the surgical specimen [25]. Whether or not the right gastroepiploic vein (RGEV) is divided, identifying it and separating it by a few millimeters from the GCTH facilitates dissection and posterior ligation of the SRCV. This fact is one of the reasons why some authors propose a cranial (cranial-caudal) access instead of medial approach in R-D3L [26].
Recent publications show a 15–20% rate of distant recurrence and 8–10% of local recurrence after oncological right hemicolectomy [1,2,3].
The average time to onset of local recurrence is 1 year and systemic or local tumor recurrence appears within the first 3 years in 80% of cases [27]. The present series shows systemic and local recurrence rates of 0% after almost 4 years of average follow-up. These results are similar to other personal series such as that of Xie et al., with a local recurrence rate of 0% after 36 R-D3L procedures [28].
The authors of the present study are aware that these oncological results may be so optimistic because of the absence of several risk factors for local recurrence and distant recurrence such as the absence of urgently indicated surgeries. However, these results are encouraging, as other risk factors are present, such as 27% of patients who had positive lymph nodes or 70% who were classified as stage T3 or T4 [29, 30].
In conclusion, the surgical protocol shown in the present study could help in the implementation of this technique in those units that consider it appropriate.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Green BL, Marshall HC, Collinson F et al (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100(1):75–82
Park JH, Kim MJ, Park SC et al (2015) Difference in time to locoregional recurrence between patients with right-sided and left-sided colon cancers. Dis Colon Rectum 58:831–837
Malakorn S, Ouchi A, Hu CY et al (2021) Sidedness, recurrence, and survival after curative resection of localized colon cancer. Clin Colorectal Cancer 20(1):e53–e60
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis 11:354–365
Kanemitsu Y, Komori K, Kimura K, Kato T (2013) D3 lymph node dissection in righthemicolectomy with a no-touch isolation technique in patients with colon cancer. Dis Colon Rectum 56(7):815–824
Garcia-Granero A, Gianluca P, Giner F et al (2020) A proposal for novel standards of histopathology reporting for D3 lymphadenectomy in right colon cancer: the mesocolic sail and superior right colic vein landmarks. Dis Colon Rectum 63:450–460
Alfonso-Garcia M, García-Granero A, Pellino G, Giner F, Valverde-Navarro A, Primo-Romaguera V (2023) How to know if a D3 lymphadenectomy has been actually performed in a right hemicolectomy: focus on the pathology report. Dis Colon Rectum 66:e51–e52
Quirke P, Morris E (2007) Reporting colorectal cancer. Histopathology 50:103–112
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25:1–42. https://doi.org/10.1007/s10147-019-01485-z
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Garcia-Granero A, Pellino G, Frasson M, Fletcher-Sanfeliu D, Bonilla F, Sánchez-Guillén L, Domenech Dolz A, Primo Romaguera V, Sabater Ortí L, Martinez-Soriano F, Garcia-Granero E, Valverde-Navarro AA (2019) The fusion fascia of Fredet: an important embryological landmark for complete mesocolic excision and D3-lymphadenectomy in right colon cancer. Surg Endosc 33:3842–3850
Bertelsen CA, Bols B, Ingeholm P, Jansen JE, Neuenschwander AU, Vilandt J (2011) Can the quality of colonic surgery be improved by standardization of surgical technique with complete mesocolic excision? Color Dis 13:1123–1129
Balciscueta Z, Balciscueta I, Uribe N et al (2021) D3-lymphadenectomy enhances oncological clearance in patients with right colon cancer. Results of a meta-analysis. Eur J Surg Oncol 47:1541–1551
Benz SR, Feder IS, Vollmer S et al (2022) Complete mesocolic excision for right colonic cancer: prospective multicentre study. Br J Surg 110:98–105
Sica GS, Vinci D, Siragusa L et al (2023) Definition and reporting of lymphadenectomy and complete mesocolic excision for radical right colectomy: a systematic review. Surg Endosc 37:846–861
Mike M, Kano N (2013) Reappraisal of the vascular anatomy of the colon and consequences for the definition of surgical resection. Dig Surg 30:383–392
Lin Z, Yang C, Wang Y, Yan M, Zheng H (2022) Comparison of prolonged postoperative ileus between laparoscopic right and left colectomy under enhanced recovery after surgery: a propensity score matching analysis. World J Surg Oncol 20:68
Ignjatovic D, Spasojevic M, Stimec B (2010) Can the gastrocolic trunk of Henle serve as an anatomical landmark in laparoscopic right colectomy? A postmortem anatomical study. Am J Surg 199:249–254
Kim NK, Kim YW, Han YD et al (2016) Complete mesocolic excision and central vascular ligation for colon cancer: principle, anatomy, surgical technique, and outcomes. Surg Oncol 25:252–262
Emmanuel A, Haji A (2016) Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: is it worth that extra effort? A review of the literature. Int J Colorectal Dis 31(4):797–804
Yi X, Liao W, Zhu B et al (2023) “Caudal to cranial” versus “medial to lateral” approach in laparoscopic right hemicolectomy with complete mesocolic excision for the treatment of stage II and III colon cancer: perioperative outcomes and 5-year prognosis. Updates Surg 75:1149–1160
Scott N, Jamali A, Verbeke C, Ambrose NS, Botterill ID, Jayne DG (2008) Retroperitoneal margin involvement by adenocarcinoma of the caecum and ascending colon: what does it mean? Colorectal Dis 10:289–293
Seo N, Lim JS, Chung T, Lee JM, Min BS, Kim M (2022) Preoperative computed tomography assessment of circumferential resection margin in retroperitonealized colon cancer predicts disease-free survival. Eur Radiol. https://doi.org/10.1007/s00330-022-09222-3
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P (2010) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 28:272–278
Bertelsen CA, Bols B, Ingeholm P et al (2014) Lymph node metastases in the gastrocolic ligament in patients with colon cancer. Dis Colon Rectum 57:839–845
Koyama M, Miyagawa Y, Kitazawa M et al (2022) Laparoscopic right hemicolectomy with a cranial-first approach for right-sided colon cancer. Tech Coloproctol 26:919–920
Argilés G, Tabernero J, Labianca R et al (2020) Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:1291–1305
Xie D, Yu C, Gao C, Osaiweran H, Hu J, Gong J (2017) An optimal approach for laparoscopic D3 lymphadenectomy plus complete mesocolic excision (D3 + CME) for right-sided colon cancer. Ann Surg Oncol 24:1312–1313
Kinugasa Y, Shiomi A, Yamaguchi T et al (2017) The distribution of lymph node metastases and their size in colon cancer. Langenbecks Arch Surg 402:1213–1221
Pellino G, Frasson M, Roselló S et al (2019) Prognostic impact of pT stage and peritoneal invasion in locally advanced colon cancer. Baguena G Dis Colon Rectum 62:684–693
Funding
The authors declare that no funding or grants were received during the production of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Álvaro Garcia-Granero, Dr. Alejandro Gil-Catalán, Dr. Sebastián Jerí-McFarlane, Dr. Jorge Sancho-Muriel, Dr. Gianluca Pellino, Dr. Margarita Gamundí-Cuesta, Dr. Eduardo Garcia-Granero and Dr. Francisco Xavier Gonzalez-Argenté have no conflicts of interest or financial ties to disclose.
Informed consent
All participants in this study were provided with a written informed consent document, which clearly outlined the purpose, procedures, risks, benefits, and confidentiality measures related to the study. Each participant was given ample opportunity to read the document thoroughly and ask any questions they had. All patients signed the informed consent.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of Balearic Islands Ethics Committee (CE-IB) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Video 1: Description of real surgery step 1. Medial-lateral dissection of the embryological planes; Toldt’s fascia and Fredet’s fascia (MP4 120082 KB)
Supplementary file2 Video 2: Description of real surgery step 2. High tie of the ileocolic vessels (MP4 59618 KB)
Supplementary file3 Video 3: Description of real surgery step 3. Dissection of lympho-adipose tissue over superior mesenteric vein or surgical trunk of Gillot (MP4 14952 KB)
Supplementary file4 Video 4: Description of real surgery step 4. Dissection of the colonic hepatic flexure and division of the right parieto-colic ligament (MP4 96284 KB)
Supplementary file5 Video 5: Description of real surgery step 5. Dissection and division of the right gastroepiploic vein (MP4 25682 KB)
Supplementary file6 Video 6: Description of real surgery step 6. High tie of the right superior colic vein (MP4 60177 KB)
Supplementary file7 Video 7: Description of real surgery step 7. Central ligation of the right branch of the middle vessels (MP4 26295 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia-Granero, Á., Gil-Catalán, A., Jerí-McFarlane, S. et al. Proposal for standardization of laparoscopic D3 lymphadenectomy for right colon cancer. Tech Coloproctol 28, 111 (2024). https://doi.org/10.1007/s10151-024-02974-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10151-024-02974-8