Abstract
Background
Faecal incontinence (FI) affects 1–19% of the general population and carries significant physical and psychological morbidity. Treatment strategies vary greatly with respect to morbidity and efficacy and relatively little is known regarding the role of mechanical devices such as anal and vaginal inserts. This is an up-to-date systematic review of the use of these devices in the management of patients with FI.
Methods
A systematic electronic search was performed of the Medline, Pubmed and Embase databases using the key words and/or MeSH ‘anal plug’, ‘anal insert’, ‘vaginal insert’ and ‘faecal incontinence’. Only articles that reported clinical outcomes for these devices for FI in the English language were included. Review articles were excluded to avoid duplication of data.
Results
Thirteen articles fulfilled the eligibility criteria. Two articles reported outcomes for the Eclipse vaginal insert and 11 articles reported on three types of anal inserts; the Coloplast ‘Tulip’ design (6), the Procon/ProTect device (2) and the Renew insert (3). When tolerated, both anal and vaginal inserts significantly improved continence, bowel function and quality of life where reported. Adverse effects included discomfort, leakage and slippage. Long-term compliance and benefit are yet to be determined.
Conclusions
Vaginal and anal inserts may be a useful treatment for FI. Better quality of evidence is needed to define its effectiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Faecal incontinence (FI) is the involuntary passage of solid or liquid stool and may cause major physical and psychological morbidity [1]. Prevalence is between 1 and 19% in the adult population [2] although this may be higher in older adults and nursing home residents [3]. There may be significant under-reporting, given the social embarrassment associated with this condition, making patients reluctant to seek help.
Treatment for FI includes dietary modification, biofeedback and pelvic floor physiotherapy, sacral and tibial nerve stimulation, sphincter reconstruction, injection of bulking agents and stoma. However, conservative measures aside, many of the invasive strategies vary significantly in efficacy, and carry morbidity that may be unacceptable to the patient.
Mechanical insert devices work by providing a physical obstruction to the passage of stool and flatus. Vaginal inserts have a posteriorly directed balloon that provides controlled occlusion of the rectum. Anal inserts provide a direct physical barrier by occluding the lower rectum and anal canal. By doing this, it is hoped that episodes of incontinence can be prevented. They represent a potentially safe and cost-effective treatment for FI. However, little has been reported on the different types of available insert, their effectiveness in comparison to other treatments, long-term efficacy and impact on quality of life (QoL).
The aim of this review was to evaluate and summarise the current evidence in support of their use for FI.
Materials and methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [4]. Two of the authors (PH and GPT) performed an electronic search of the Medline, Pubmed Embase and Cochrane databases using the keywords and/ or MeSH “anal plug”, “anal insert”, “vaginal insert”, “device” and “faecal incontinence”. Further relevant studies were identified by cross referencing from relevant articles and abstracts. There was no restriction on date of publication and the search included all published articles as of March 2020.
Inclusion criteria were studies reporting original outcome data on vaginal or anal insert devices for FI and English language publications only. Articles were excluded if they were reviews or contained duplicate data from another study.
Results
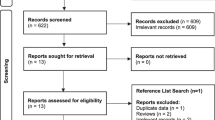
The search produced an initial 285 citations. Two hundred and sixty-nine were excluded from this on the basis that they were not written in English or did not report any outcome data or both. Three of the remaining 16 articles were reviews. Thirteen studies fulfilled the eligibility criteria (Fig. 1). Two reported on vaginal inserts and 11 on anal devices. Data from prospective multicentre trials, single-centre case series and a randomized controlled trial (RCT) were included.
The Coloplast plug single group series
The first study to report on anal continence plugs [5] evaluated 3 variations of the Coloplast polyurethane sponge design, originally designed as a colostomy plug [6]. These plugs were the size of a conventional suppository and expanded on insertion into the anal canal. The two different sized versions of this plug had a ‘tulip’ design with the sponge sitting at the anorectal junction and with gauze string in the anal canal (Fig. 2). The third type was shaped like a wine glass, with the sponge extending down the anal canal from the anorectal junction. Plugs were inserted after a bowel motion and expelled by pulling on the string or during a normal evacuation. Ten patients (8 women and 2 men) were recruited and for each patient, the three types of plug were tested over a 3-week period with 1 per week.
One patient withdrew from the study due to discomfort and in the nine remaining patients, the optimum plug (large tulip) had a median wear-time of 12 h and there were no episodes of incontinence in 82% of the time the plug was in place. The wine glass-shaped plug proved to be the least effective, perhaps due to its stem hampering removal and insertion and only one patient expressed a preference for this plug. The results of this small pilot study prompted further evaluation of this tulip design plug in a separate study of 14 patients [7] by Christiansen et al. Their results were less encouraging. Only 9 (64%) of these patients were fully continent when using the plug. Furthermore, 6 (43%) reported occasional slippage of the plug and 10 (71%) experienced discomfort. This led to 11 patients withdrawing prematurely from the study before the intended end at 4 weeks. The authors reported no correlation between anorectal physiology studies and plug efficacy. However, it was noted that all felt a degree of reassurance when using the plug. This was irrespective of discomfort and they would use the plug on occasions where leakage would be particularly embarrassing. This study highlighted the limitations of the tulip Coloplast plug. It suggested the need for an improved design that would be better tolerated for long-term use. At this point, the authors concluded that the tulip plug offered only a short-term solution for incontinence.
In a similar study assessing the two different sizes of the tulip plug, Norton and Kamm [8] reported that 14 out of 20 patients could not tolerate the plug leading to early withdrawal. Only 4 patients (20%) found the plug comfortable and would use the plug on a regular basis after the study. For those patients who could tolerate the plug, continence was greatly improved and there was no clear preference for the smaller or larger plug. However, it was not possible to predict with the use of anorectal physiology, which patients would benefit from plug use.
Van Winckel et al. [9] evaluated the efficacy and tolerance of the Coloplast anal plug in children with persistent FI secondary to spina bifida or anal atresia. Sixteen patients were recruited (seven with congenital imperforate anus and nine with spina bifida) in a prospective 6-week crossover study to determine which diameter of plug (37 or 45 mm) was more effective. Outcome measures included soiling episodes, stool frequency and consistency and number of diapers used with and without the plug. Marginally better results were reported from the anal atresia group (57% (4/7) achieved full continence using two plugs daily, compared to 22% (2/9) in the spina bifida group). In keeping with previous studies, 25% (4/16) of patients did not complete the study due to loss, pain or discomfort.
Coloplast plug comparative studies
The early articles reported above highlighted a lack of high-quality, comparative or randomized data to support the efficacy of anal plugs in managing FI. Subsequent Coloplast plug studies sought to address this.
Cazemier et al. [10] investigated the long-term benefit of Coloplast plugs and retrograde colonic irrigation (RCI) in 201 patients including adults and children with FI or constipation. Outcome measures were QoL scores and side effects of their treatment. Only 101 (50%) responded to the questionnaire. No differences were reported between responders and non-responders with respect to pathology, age or sex. Of the 14 adults with FI, who were prescribed an anal plug, 8 returned the questionnaire. Five of these stopped using the plug immediately, one stopped after 20 months leaving only 2 patients on long-term use. Similarly, of the 16 children, who were prescribed anal plugs, only 7 responded to the questionnaire. Two stopped using the plug immediately and after 5 years five reported ongoing satisfactory use. The study reports similar data for RCI. Whilst the authors did not perform a direct comparison between anal plugs and RCI in the management of FI, it was the first to evaluate the long-term use of anal plugs. In selected patients, intermittent use of up to 5 years was possible and was of some benefit in children. However, the low response rate and small numbers in both adult and child groups temper significantly these conclusions.
Bond and colleagues [11] were the first to conduct an RCT to compare 1-year use of the Coloplast anal plug with a control group who managed their condition preemptively or protectively. Patients used the plug as a complete alternative or adjunct to existing management. Study patients included both children and adults with either congenital, acquired or neurogenic FI. Outcome measures included QoL questionnaires, interviews, bowel charts and diaries. The primary outcome measure was a non-validated symptom score ranging from 0 to 100, where 0 represented the most severe FI and 100 the least severe. Forty-eight patients (53% of the target recruitment) were recruited (28 children and 20 adults) and randomisation was adjusted to a 2:1 intervention:control to maximize plug data. Thirty-one patients (16 children and 15 adults) were recruited to the intervention arm and 17 (12 children and 5 adults) to the control group.
The statistical power of this RCT was limited by inadequate patient numbers; therefore, comparison of the two groups was descriptive. The intervention group showed a greater improvement in child health questionnaire scale scores (physical and psychosocial) compared to the intervention group. However, there was no statistically significant difference in overall symptoms severity score between the intervention and control group in adults and children. This included follow-up scores at 1 year and change in score after treatment for both groups, the latter approached statistical significance (79.32 vs 70.04, p=0.053). The qualitative data reported by this study appear more valuable. Subjective interview data reported the plug to confer better control, more regulated bowel movement and prevention of anal skin rashes and soreness. Reported disadvantages were difficulty with insertion, retention, leakage and discomfort. In contrast to previous studies, plug retention and tolerability was better. Twenty out of 23 reported the plug as comfortable or very comfortable. Despite its limitations, this study was the first to evaluate the use of an anal plug for FI with a randomised comparative methodology and demonstrated some benefit to the majority of patients.
The renew anal insert
Lukacz et al. [12] reported the first evaluation of an alternative plug to the Coloplast device. The Renew anal insert device is a soft silicone plug that is self-inserted with a fingertip applicator. In contrast to the tulip design of the Coloplast version, the Renew plug has two discs. The uppermost disc sits at the anorectal junction to help prevent leakage of stool whilst the lowermost disc remains outside the anus to help prevent displacement of the plug upward. Between these two discs lies the stem of the plug which sits within the anal canal (Fig. 3). The plug is expelled during a voluntary bowel movement or removed by pulling on the bottom disc.
The authors conducted a multicentre prospective study of 73 patients who completed the intended 12 weeks of continuous plug use from 91 originally recruited (20% dropped out). Outcome measures were bowel diary use, Wexner FI score, subjective assessment and complications. The results were encouraging: 77% (56) reported a 50% or more reduction in FI frequency. Median FI frequency reduced from 0.9 per day to 0.2 per day (82% reduction) and the mean Wexner FI score improved by 32.4%. Seventy-eight percent of patients were satisfied or extremely satisfied with the device, with only 3 moderate adverse events recorded (faecal urgency, soreness and bleeding haemorrhoids). Eighty percent of respondents ‘liked’ the inserts. An average of 2.6 inserts was used per day.
Despite the lack of randomisation, QoL measures and a control group, the results of this study for the Renew device compare favourably to outcomes for the Coloplast plug. Segal et al. [13] conducted a further evaluation of the Renew device within a single-centre prospective study. The authors investigated the acceptability, effectiveness and safety of the Renew plug in 15 patients with FI who had undergone restorative proctocolectomy (RPC) with ileal pouch-anal anastomosis (IPAA). Inclusion criteria included passive FI for more than 2 weeks and the absence of pouchitis. Patients were asked to use the Renew insert for 14 days following conventional biofeedback therapy, with at least 1 plug to be used during the day and 1 at night. Outcome measures included a stool diary for 14 days and a standardized incontinence questionnaire (International Consultation on Incontinence Questionnaire Bowels; ICIQ-B) conducted before and at the end of the 2-week study period.
There were no significant differences between pre- and post-intervention scores for the majority of outcomes. Only night seepage demonstrated significant improvement with use of the Renew plug. With respect to acceptability, eight patients who completed the study (one patient was lost to follow-up) were satisfied with the plug, two were ambivalent and four were dissatisfied. Six found the device effective, two were neither satisfied nor dissatisfied, and six found it to be ineffective. Therefore, the plug was deemed acceptable to 8/15 (53%) of patients and demonstrated effectiveness in 6/15 (40%) of patients. Despite the small sample size, the Renew plug was associated with an improvement in bowel habit and daytime seepage though this did not reach significance. The plug proved most effective at reducing nighttime seepage when sphincter tone is likely to be lower.
A larger retrospective audit of 30 patients from the same institution [14] demonstrated improved outcomes with the Renew device. Twenty-four (80%) patients liked the device and 17 (57%) wanted to use the plug in the long-term. Furthermore, there was a significant improvement in incontinence scores (15 vs 10, p < 0.001) with use of the plug at a median follow-up time of 11 weeks (range 8–14 weeks) whilst 67% reported improvement in their symptoms with only 10% experiencing deterioration. Four (13%) patients stopped using the plug due to pain. These results are comparable to those of Lukasz et al. [12]. However, patient numbers were small and data acquisition limited by incorrect use of some bowel diaries that made retrospective completion vulnerable to recall bias.
The Procon and ProTect incontinence device
The Procon incontinence device as described by Giamundo et al. [15] employs a balloon that acts as a mechanical barrier to the passage of stool. The device is inserted in the rectum and constitutes a disposable rubber catheter with an infrared sensor connected to a ‘beeper’. The catheter is secured in place by a 20-ml balloon which presents a physical barrier to stool leakage. Stool entering the rectum sets off the alarm system such that the patient can voluntarily deflate the balloon and remove the catheter to permit evacuation (Fig. 4).
The Procon Incontinence device (reproduced with permission from Giamundo et al. [15]). a Distal tip of catheter with infrared sensor and flatus vent holes. b Inflatable 20-ml balloon. c Flatus venting charcoal filter. d Cuff fill valve. e Monitor connector. f Monitor (‘beeper’)
In this preliminary study of 7 patients (5 women and 2 men), the Procon device was used for 14 consecutive days. Selection criteria included Cleveland Clinic Incontinence Score > 7, absence or complete loss of rectal sensation, FI interfering with lifestyle, no acute proctitis or previous low rectal anastomosis. Outcomes were assessed with QoL scores and daily logs of bowel activity and incontinence episodes which were completed before and at the end of the study.
Five of the seven patients (71.4%) reported complete satisfaction with significant improvements (reduction) in FI scores (5.2 vs 12.7, p=0.0119) and QoL (135.5 vs 95.3, p=0.0026). Two rated the device as being partially satisfactory, limited by difficulty in handling the device. There were no reported major adverse effects or complications. Three of the seven patients (42.8%) reported hypersensitivity of the sensor due to continuous activation of the alarm by the constant presence of faecal matter in the rectum. All three of these patients’ baseline function included watery diarrhoea and/or continuous seepage and mucous discharge. Such factors may inform future selection. Despite this, none of these patients experienced FI with the device in situ.
Encouraging as these findings are, only 7 out of 18 patients completed the 14-day study period. In addition to hypersensitivity (n=3), eight found the device too difficult to operate due to reduced manual dexterity (n=5) or general physical limitations (n=3).
The ProTect device, reported by the same author [16], is a later version of the Procon device that includes new features. These are a protective sleeve to prevent false alarms from mucous, introducing wedge-shaped absorbent disc to provide additional protection against leakage and a different-shaped balloon to seal the anorectal junction. A multicentre trial involving seven institutions was conducted. A prospective cohort study of 17 patients was recruited. Assessment was over a 14-day period to assess the device. Two QoL questionnaires and daily log of bowel activity and incontinent episodes were completed before and during the study. Eleven patients completed the study and six withdrew due to the inability to follow study protocol (n=1), non-compliance and lack of motivation (n=2) and frequent alarm activation due to persistent liquid stool in rectum (n=3). Seven of the 11 patients (64%) who completed the study reported an overall positive experience and personal satisfaction with the device in preventing FI episodes. Mean incontinence scores reduced significantly with a corresponding reduction in the number of FI episodes and frequency of pad usage. Flatus continence did not improve in most. There was significant improvement in all QoL scales including lifestyle, depression and self-perception, coping, behaviour and embarrassment. Global QoL scores were significantly higher when using the device. As with the earlier Procon model, the ProTect device achieved better results in patients with formed or semi formed stool. The device continued to be hampered by its inability to differentiate between small and significant quantity of faeces resulting in ‘false alarming’ that may be unacceptable to some patients. Despite technical improvements, strong psychological and clinical support are required for patients to use the device effectively. Both studies commented were limited by the 14-day study period. Encouragingly, five patients who continued to use the device for 6 months reported similarly improved scores.
Vaginal devices
Richter et al. [17] were the first to report on a vaginal bowel-control device. The Eclipse system consists of a vaginal insert attached to a pump. The insert is a silicone coated stainless steel base with a posteriorly located balloon. The balloon inflates to occlude the distal rectum and deflates to permit evacuation via a detachable and discreet pump (Fig. 5).
Eclipse system, reproduced with permission from Richter et al. [17]. a Uninflated device to permit evacuation. b Inflated device to prevent stool leakage
In this multicentre study of six centres, women with a minimum of four FI episodes over 2 weeks were recruited. The primary endpoint was treatment success, defined as a ≥50% reduction in the number of FI episodes over 1 month. Secondary endpoints included symptom improvement as assessed by validated questionnaires. Participants were invited to extend their study period by another 2 months. Out of an initial 110 recruits, 61 (55.5%) had a successful fitting, and started treatment. At 1 month, 78.7% (48) of the intention to treat population reported treatment success. Significant improvement was also observed for Incontinence Quality of Life and Modified Manchester scores at 1 month with 98.2% of participants stating they would recommend the device to a friend. Forty-four participants undertook the 2-month extended study period and at 3 months, 86.4% (38/44) reported further significant improvement in all symptom questionnaire scores. There were no major adverse events but 21% (13) of patients reported side effects of pelvic discomfort (n=6), urinary urgency or frequency (n=2), vaginal symptoms (n=3) and pelvic pain (n=2). Whilst encouraging, a significant proportion (49/110,44.5%) failed the fitting stage. This precluded them from further study participation.
A secondary analysis of the same trial [18] took a more detailed look at bowel function, aside from FI, which may impact on QoL. They reported the effect of the Eclipse system on bowel motion frequency, faecal urgency, stool consistency and completeness of evacuation in 56 patients as assessed by pre-treatment and post-treatment bowel diaries. After 1 month of use, there was a significant reduction observed in the proportion of all bowel movements reported as liquid, associated with urgency and incomplete evacuation. Subgroup analysis of continent bowel movements demonstrated a reduction in urgency-related events at 1 month (21% vs 37%, p=0.0016) but no significant changes in liquid motions (15% baseline vs 12% 1 month, p=0.48) or sensation of incomplete evacuation (31% baseline vs 22% 1 month, p=0.08). In addition, whilst 11% (6/56) of participants reported urgency with 100% of their bowel movements at baseline, this subset of patients experienced urgency with 42% of bowel movements after treatment with the device. This is important because whilst the authors have demonstrated improved parameters of overall bowel function with this device, the Eclipse system may be of particular benefit in treating stool urgency. A suggested mechanism for this is the physical occlusion of the anal canal during device inflation which increases anorectal angulation.
As with the earlier study, there were no serious adverse device-related events and these decreased with time: 72% during the fitting period, 20% at 1 month and 10% at 3 months. Given the relative novelty of the device, this is not entirely unexpected with both clinicians and patients on a learning curve. Whilst the available data are only for short-term outcome assessment, 91% of patients viewed the device as a long-term treatment. Longer term outcomes are awaited from this trial to assess the durability of these preliminary findings.
Discussion
From our review of the literature (Table 1), it is clear that data relating to the use of anal and vaginal insert devices for the management of FI are limited by small numbers, short-term outcome assessment and a lack of randomised and comparative data. Only one study [11] randomised patients, whilst others were reliant on comparisons between baseline and post-intervention measures. In addition, outcome measures often included non-validated qualitative or subjective assessments of patients’ experiences making them susceptible to recall bias and limiting statistical analysis.
Despite this, there is evidence to suggest both anal and vaginal insert devices may be of benefit to the majority of patients with FI. The major drawback for both, however, appears to be long-term acceptability with significant drop out rates reported for the Coloplast plug, Eclipse vaginal system and Procon device. In these studies, patients could not complete the intended study period or did not have an initial successful fitting of the device (which precluded study participation). A recent Cochrane review [19] of anal plugs used to manage FI that did not include the Renew, Procon or ProTect devices reported similarly high drop out rates (12.5–68%). Reasons for this included discomfort or pain, plug loss and non-compliance with study protocol.
Encouragingly from our review, drop out rates were lower with the more recently developed Renew anal plugs and ProTect device. In particular, the Renew plug compared favourably to the more established Coloplast device with respect to leakage, irritation, urgency and overall acceptability. The Renew device appears to be better tolerated because it is considerably smaller and is composed of soft silicone which is inserted with an applicator. Only the smallest (n=15) of the three Renew studies failed to show a statistically significant improvement in bowel control and QoL with the plug though there was a trend towards improvement [13].
The Procon and ProTect anal devices incorporated a balloon as a mechanical barrier that could be voluntarily inflated and deflated depending upon faeces within the rectum. The theoretical advantage to this is to empower the user with more regulated evacuation due to the presence of a photosensor that detects faeces within the rectum. However, hypersensitivity of the alarm system in patients with loose stool or mucous leakage appears to hinder its usefulness, even with the more recent ProTect device. Furthermore, it is hindered by the need for strong patient motivation and a degree of manual dexterity that may be challenging for some patients. Whilst the outcome data are too heterogenous to enable formal comparisons, it would appear that the anal pump devices do not confer a significant advantage over anal plugs, particularly the Renew device. Further comparative studies would be helpful.
The use of the vagina to influence bowel function is a relatively new concept in managing FI. The Eclipse system is a dynamic vaginal insert that like the ProTect device uses an inflatable balloon to occlude the rectum under voluntary control. The reported 90% patient satisfaction must be tempered by the 45% of patients who could not undergo a successful fitting of the device. Long-term outcomes and comparison to other devices are needed.
It is presumed that both anal and vaginal insert devices are beneficial predominantly for those patients with passive incontinence and all but two of the studies focused on this patient group. Sharma et al. [18] demonstrated a reduction in urgency-related events with the Eclipse system whilst Lukasz et al. [12] included patients with urgency. Future studies enabling formal subgroup analysis would help inform selection policy for the different types of devices. Accordingly, only three of the studies [8, 13, 15] included anal resting pressures and whilst one confirmed low resting pressures in study participants [13], the other two failed to report any correlation between anorectal sensitivity and patient comfort [8, 15]. It is feasible that an anal pump device would be of particular use within a nursing care setting where the alarm can forewarn carers of an imminent bowel motion and, therefore, prevent faecal soiling and potential pressure ulcers.
Given the significant overall heterogeneity of study participants that is apparent from Table 1, it remains difficult to predict which device would be most effective in any given patient. Inclusion criteria for all studies overlapped considerably with common denominators being FI to liquid and solid stool, idiopathic FI, ambulant self-caring patients and the absence of any ongoing low rectal or anal inflammation. Comparable patient characteristics have been reported for more invasive and morbid procedures such as sacral nerve stimulation [20] and bulking agents [21]. Exclusion criteria were more numerous with the pump devices and in particular, the Eclipse system, suggesting a smaller group of patients that would benefit from this device.
Cost and availability are additional important considerations. The Coloplast and Renew anal plugs cost £3.30 and £2.60 per insert respectively and are available on prescription on the National Health Service (NHS) in England. The ProTect and Eclipse devices are currently not available on the NHS and their cost remains to be confirmed. However, both of these pump devices are intended for longer use (1 month for ProTect) making them potentially more cost-effective in the long term.
Given the breadth and complexity of FI management, there is a need to better define the use of anal and vaginal insert devices within the treatment pathway. A recent survey found that more than half of UK continence advisors feel inadequately trained [22]. This is an up-to-date systematic review of all nonsurgical insert devices used to treat FI. Given their low morbidity and improving acceptability and effectiveness, we believe that the use of these devices falls between conservative measures such as diet, pads, medication, biofeedback and more invasive techniques such as bulking agents and nerve stimulation. Equally, they may be used as an adjunct to existing treatment. Future larger randomized comparative studies to other treatment modalities using validated QoL and continence questionnaires are needed to further assess the long-term efficacy of these devices. This in turn would significantly improve existing FI management strategies and algorithms.
Conclusions
Anal and vaginal insert devices may be a useful treatment for faecal incontinence. Better quality of evidence is needed to define their effectiveness.
References
Damon H, Guye O et al (2006) Prevalence of anal incontinence in adults and impact on quality-of-life. Gastroenterol Clin Biol 30:37–43
Sharma A, Tuan L, Marshall RJ et al (2016) Systematic review of the prevalence of faecal incontinence. Br J Surg 103:1589–1597
Nelson R, Furner S, Jesudason V (1998) Faecal incontinence in Wisconsin nursing homes: prevalence and associations. Dis Colon Rectum 41:1226–1229
Shamseer L, Moher D et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349:g7647
Mortensen N, Humphreys MS (1991) The anal continence plug: a disposable device for patients with anorectal incontinence. The Lancet 338(8762):295–297
Burcharth F, Ballan A et al (1986) The colostomy plug: a new disposable device for a continent colostomy. The Lancet 2(8515):1062–1063
Christiansen J, Roed-Petersen K (1993) Clinical assessment of the anal continence plug. Dis Colon Rectum 36(8):740–742
Norton C, Kamm MA (2001) Anal plug for faecal incontinence. Colorectal Dis 3:323–327
Van Winckel M, Van Biervliet S, Van Laecke E, Hoebeke P (2006) Is an anal plug useful in the treatment of fecal incontinence in children with spina bifida or anal atresia. J Urol 176:342–344
Cazemier M, Felt-Bersma RJF, Mulder CJJ (2007) Anal plugs and retrograde colonic irrigation are helpful in fecal incontinence or constipation. World J Gastroenterol 14(22):3101–3105
Bond C, Youngson G et al (2007) Anal plugs for the management of fecal incontinence in children and adults. A Randomized Control Trial. J Clin Gastroenterol 41(1):45–53
Lukacz E, Segall M, Wexner S (2015) Evaluation of an anal insert device for the conservative management of fecal incontinence. Dis Colon Rectum 58:892–898
Segal JP, Leo CA et al (2018) Acceptability, effectiveness and safety of a renew anal insert in patients who have undergone restorative proctocolectomy with ileal pouch-anal anastomosis. Colorectal Dis 21:73–78
Leo CA, Thomas GP et al (2019) The Renew anal insert for passive faecal incontinence: a retrospective audit of our use of a novel device. Colorectal Dis 21:684–688
Giamundo P, Welber A et al (2002) The Procon incontinence device: a new nonsurgical approach to preventing episodes of fecal incontinence. Am J Gastroenetrol 97(9):2328–2332
Giamundo P, Altomare DF et al (2007) The ProTect device in the treatment of severe fecal incontinence: preliminary results of a multicentre trial. Tech Coloproctol 11(4):310–314
Richter H, Matthews C et al (2015) A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynaecol 125(3):540–547
Varma M, Matthews C et al (2016) Impact of a Novel Vaginal Bowel Control System on Bowel Function. Dis Colon Rectum 59:127–131
Deutekom M, Dobben AC (2015) Plugs for containing faecal incontinence. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005086.pub2
Maeda et al (2014) Outcome of sacral nerve stimulation for fecal incontinence at 5 years. Ann Surg 259(6):1126–1131
Luo et al (2010) Systematic review on the efficacy and safety of injectable bulking agents for passive faecal incontinence. Colorectal Dis 12:296–303
Leo CA, Maeda Y et al (2017) Current practice of continence advisors in managing faecal incontinence in the United Kingdom: results of an online survey. Colorectal Dis 19(9):O339–O344
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
None.
Ethics approval
Not applicable.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
How, P., Trivedi, P.M., Bearn, P.E. et al. Insert devices for faecal incontinence. Tech Coloproctol 25, 255–265 (2021). https://doi.org/10.1007/s10151-020-02317-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-020-02317-3