Abstract
Background
The aim of this study was to evaluate the feasibility of robotic total/subtotal colectomy procedures with the Xi robot and to compare its short-term outcomes with those of conventional laparoscopy.
Methods
Between October 2010 and September 2018, consecutive patients with colonic neoplasia, inflammatory bowel disease, familial adenomatous polyposis or colonic inertia who underwent elective robotic or laparoscopic total/subtotal abdominal colectomy at two specialized centers in Turkey were included. Data on perioperative characteristics and 30-day outcomes were compared between the two approaches.
Results
There were a total of 82 patients: 26 and 56 patients in the robotic and laparoscopic group, respectively (54 men and 28 women, mean age 54.7 ± 17.4 years). The groups were comparable regarding preoperative characteristics. All the robotic procedures were completed with a single positioning of the robot. Estimated blood loss (median, 150 vs 200 ml), conversions (0% vs 14.3%), and complications (0% vs 7.1%) were similar but operative time was significantly longer in the robotic group (median, 350 vs 230 min, p < 0.001). No difference was detected in the length of hospital stay (7.9 ± 5.7 vs 9.5 ± 6.0 days, p = 0.08), anastomotic leak (3.8% vs 8.3%), ileus (15.4% vs 19.6%), septic complications, reoperations (7.7% vs 12.5%), and readmissions (19.2% vs 12.5%). The number of harvested lymph nodes in the subgroup of cancer patients was significantly higher in the robotic group (median, 66 vs 50, p = 0.01).
Conclusions
In total/subtotal colectomy procedures, the robotic approach with the da Vinci Xi platform is feasible, safe, and associated with short-term outcomes similar to laparoscopy but longer operative times and a higher number of retrieved lymph nodes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In colorectal surgery, the use of robotic platforms for the last two decades has revolutionized the minimally invasive approach, with its inherent technical advantages such as three-dimensional vision, wrist-like motion, tremor filtering with stable instrumentations, and improved ergonomics [1, 2].
Although the implementation of robotic segmental colectomies such as right-, left-sided colonic resections and proctectomies has increased worldwide [3,4,5], the number of extended colonic resections such as total or subtotal colectomies performed with the robotic platforms remains low [6]. The reason for this is the fact that extended colonic resections involve a multiquadrant access, requiring repositioning of the patient-side surgical cart in order to access multiple areas of the abdomen [6, 7], increasing operative time and workload [7]. To further advance robotic technology, the manufacturer launched the fourth-generation robotic system, da Vinci Xi robot, with the aim of minimizing the aforementioned limitations of robotic systems for multiquadrant surgery. This robotic platform integrates a range of the latest technologies to provide better access to the abdomen without the need to reposition the robot [6,7,8].
Although the applicability of the Xi platform has been evaluated in a variety of colorectal procedures, data are still very limited regarding the use of this platform in extended colonic resections involving manipulation in all four quadrants of the abdomen. In the present study, we examined the feasibility of robotic total/subtotal colectomy procedures performed with the Xi robot and compared its short-term outcomes with those of conventional laparoscopy.
Patients and methods
This study was approved by the Institutional Review Board. Eighty-two consecutive patients with the diagnosis of either synchronous colonic cancer, obstructive cancer, multiple dysplasia, inflammatory bowel disease, familial adenomatous polyposis or colonic inertia who had robotic or laparoscopic total or subtotal colectomy at one of two specialized centers in Turkey between October 2010 and September 2018. Operations were performed by one of five surgeons, each highly experienced in minimally invasive colorectal surgery (DB, EB, TK, IH, and BB). Total colectomy was defined as a resection of the entire colon and subtotal colectomy as a resection of the entire colon without the sigmoid colon. Patients with an American Society of Anesthesiologists (ASA) score > 3 and those who had emergency colectomy were excluded. We divided the patients into robotic and laparoscopic groups and results in the two groups were compared.
Data were collected in a prospective fashion and analyzed retrospectively. Clinical characteristics included demographics, body mass index (BMI), ASA score, preoperative comorbidities, disease types, and previous abdominal surgery. Operative variables included surgical procedure (total or subtotal colectomy), anastomotic technique (stapled or handsewn), stoma status, operative time, estimated blood loss, additional procedures, complications, and conversions. Postoperative 30-day outcomes included time to first flatus, first bowel movement and resume soft diet, length of hospital stay, anastomotic leak, surgical site infections (SSIs), ileus, hemorrhage, blood transfusion, reoperation, readmission, mortality, and histopathologic data in patients with colon cancer.
Overall operative time was defined as the time from the first skin incision to the end of skin closure. Conversion was defined as the completion of any part of the robotic procedure with a standard laparoscopic or open technique, and any part of the laparoscopic procedure with an open technique, excluding the delivery of the specimen and placement of the stapler anvil for stapled ileocolic or ileorectal anastomosis. Anastomotic leak was defined as clinically apparent leak sign (such as the emission of gas, pus, or feces from the drain) or extravasation of an endoluminally administered water-soluble contrast medium according to the postoperative computed tomography scan. The diagnosis of SSIs was made based on the definitions stated in the guidelines reported by the CDC’s NNIS system [9]. Complications were graded according to the Clavien–Dindo classification [10].
Robotic total/subtotal colectomy
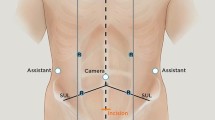
The operation was performed with the medial-to-lateral dissection technique using the da Vinci Xi robotic system (Intuitive Surgical Inc., Sunnyvale, CA, USA). A total of seven trocars (five robotic and two 5-mm assistant trocars) were placed as follows: an 8-mm robotic trocar in the supraumbilical area, a 12-mm robotic trocar in the right iliac fossa, an 8-mm robotic trocar in the right lower quadrant, two 8-mm robotic trocars in the left upper quadrant, one assistant trocar in the right-upper and the other one in the left lower quadrant (Fig. 1). The operation was completed in two stages: the first stage involved dissection of the right colon up to the level of the mid-transverse colon and, for this, the four 8-mm robotic trocars (right-lower-quadrant, supraumbilical and two left-upper-quadrant trocars) and the left-lower-quadrant assistant trocar were used. The second stage was for the dissection of the remaining transverse colon and left colon. In this stage, the right-iliac, right-lower-quadrant, supraumbilical and one left-upper-quadrant robotic trocars and the right-upper-quadrant assistant trocar were used. The 12-mm trocar served for stapler insertion to perform colon transection and bowel anastomosis in the second stage of the operation.
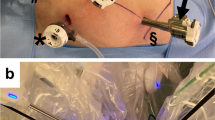
In the first stage, the patient was placed in a 15° Trendelenburg position with a 30° left-side tilt. The patient-side robotic cart was docked from the right side of the patient (Fig. 2). Dissection was initiated by scoring the peritoneum over the ileocolic vascular pedicle. The ileocolic vein and artery were isolated individually, clipped with Hem-o-lok clips (Weck Closure Systems, Research Triangle Park, NC,USA) near its origin and divided. Dissection was carried out superiorly along the superior mesenteric vein axis, dissecting out the entire mesocolic tissue in the embryological plane until the second portion of the duodenum. The right colic vessels, if present, were also clipped and divided near their origins in the same fashion. Mesenteric dissection was continued up to the root of the middle colic vessels, which were then divided between the clips. With caudal traction on the transverse colon, the bursa omentalis was entered and the gastrocolic ligament was divided from left to right. The hepatic flexure and lateral attachments of the ascending colon were mobilized craniocaudally. Demounting of the robotic arms completed the first stage of the operation.
Patient-side surgical cart is positioned on the right side of the patient and this position is maintained throughout the operation. After completing dissection of the right colon up to the mid-transverse colon, the boom of the robotic system was rotated 180° from the right side of the patient to the left to perform dissection of the remaining transverse colon and left colon
In the second stage, without moving the patient-side cart, the boom of the robotic system was rotated 180° from the right side of the patient to left. The operating table was tilted 30° to the right. The peritoneum was incised at the level of sacral promontory and the aorta-mesenteric window was entered. The inferior mesenteric artery (IMA) was clipped and divided near its origin in total colectomy procedures. In subtotal colectomy procedures, the IMA was divided at a point just distal to its superior rectal artery branch. The inferior mesenteric vein was divided at the inferior border of the pancreas. The mesocolon was separated from the pancreas, the omental bursa was re-entered and dissection was continued to the level of the splenic flexure. After completing medial dissection, the omentum was completely separated from the transverse colon and the left colon was freed from its lateral attachments. A robotic EndoWrist stapler was used to divide the rectosigmoid junction or the sigmoid colon depending on the total or subtotal colectomy procedure.
Then, the robotic arms were demounted and a transverse suprapubic incision was made to extract the colectomy specimen. The terminal ileum was divided and an anvil of a 29- or 31-mm circular stapler was secured in the ileum and the ileum was returned into the abdomen. The suprapubic incision was closed, pneumoperitoneum was re-established and the robotic arms were remounted. An intracorporeal side-to-end ileorectal anastomosis was carried out using a circular stapler placed transanally. In subtotal colectomy procedures, a side-to-side ileosigmoid anastomosis was performed intracorporeally using robotic staplers. A diverting loop ileostomy was created based on surgeons’ preferences.
Laparoscopic total/subtotal colectomy
We used one 15-mm, one 10-mm and three 5-mm trocars, as illustrated in Fig. 3. The laparoscopic procedure followed similar operative steps of the robotic approach, including the patient positioning, dissection technique, specimen extraction and intracorporeal bowel anastomosis. An ultrasonic device or a vessel sealer was used for dissection. Following completion of the first stage of the operation with mobilization of the right colon up to the mid-transverse colon, the surgeon and the assistant moved from the left side of the patient to the right to complete the second stage involving dissection of the left colon, as described above. The side-to-end ileorectal anastomosis was performed using a circular stapler introduced transanally and side-to-side ileosigmoidostomy was performed using either an extracorporeal handsewn technique or a laparoscopic linear stapler introduced through the 15-mm trocar.
Statistical analysis
Statistical analysis was performed using the SPSS 22.0 software package (IBM Corp. in Armonk, NY, USA). Qualitative variables were analyzed using frequencies and percentages and quantitative variables were analyzed using medians and ranges or means and standard deviations. For categorical variables, group comparisons were conducted by Chi square test or Fisher’s exact test. Normal distribution was assessed by the Kolmogorov–Smirnov test. Continuous data that followed approximately normal distribution were compared by the Student’s t test while continuous data that departed from normal approximation were compared by the Mann–Whitney U test. A p value < 0.05 was considered significant.
Results
A total of 82 patients (54 men and 28 women) were included in this study. The mean age of the patients was 54.7 ± 17.4 years and the mean BMI was 25.0 ± 4.6 kg/m2. Forty-eight patients had colon cancer, 17 had ulcerative colitis, five had familial adenomatous polyposis, four had Crohn’s disease, four had multiple foci of dysplasia, and four had colonic inertia. Forty-six patients underwent total colectomy and 36 patients underwent subtotal colectomy.
The robotic and laparoscopic group included 26 and 56 patients, respectively. The two groups were comparable in terms of age, gender, BMI, ASA status, diagnoses, comorbidities, plasma albumin level, medication use and previous abdominal surgery (p > 0.05) (Table 1).
Regarding the intraoperative outcomes (Table 2), no significant differences were found between the groups in surgical procedure (total or subtotal colectomy), anastomotic technique, diverting ileostomy status (7.7% vs 7.1%, p = 0.63), intraoperative blood loss, additional surgery (19.2% vs 14.3%), intraoperative complications, and conversions. Bowel anastomosis was performed in all the patients in the robotic group and in 48 patients in the laparoscopic group. The median intraoperative blood loss was 150 ml and 200 ml in the robotic and laparoscopic group, respectively (p = 0.44). The median operative time was longer in the robotic group (350 vs 230 min, p < 0.001). Regarding intraoperative complications, there were none in the robotic group and four in the laparoscopic group (0 vs 7.1%, p = 0.3). These complications were bleeding due to mesenteric vascular injury in two patients, managed laparoscopically, and iatrogenic colonic perforation in two patients, which required conversion. No conversion occurred in the robotic group whereas eight laparoscopic procedures were converted to open approach (0 vs 14.3%, p = 0.051). The reasons for conversion were adhesions in three patients, colonic dilatation in two, iatrogenic colonic perforation in two, and retroperitoneal tumor invasion in one patient.
The postoperative 30-day outcomes are presented in Table 3. The two groups were similar in terms of mean time to first flatus (2.3 vs 2.3 days) and first bowel movement (3.0 vs 3.2 days), except for the time to oral intake, which was longer in the robotic group (3.5 vs 2.6 days, p < 0.01). No difference was found in the mean length of hospital stay (7.9 vs 9.5 days). The rate of anastomotic leak (3.8% vs 8.3%), overall SSIs (19.2% vs 16.1%), sepsis (3.8% vs 3.6%), ileus (15.4% vs 19.6%), hemorrhage (3.8% vs 5.4%), cardiac complications (3.8% vs 3.6%), pulmonary complications (7.7% vs 7.1%), and urinary tract infection (11.5% vs 5.4%) was similar. Reoperation was required in 2 (7.7%) patients in the robotic group (one patient with anastomotic leak and one patient with organ/space SSI) and seven patients (12.5%) in the laparoscopic group (four patients with anastomotic leak, two patients with extended ileus, one patient with postoperative hemorrhage) (p = 0.71). The readmission rate was similar between the groups (19.2% vs 12.5%, p = 0.51). No mortality occurred in either group. According to Clavien–Dindo classification scale, no differences were observed in the distribution of postoperative complications between the two groups (p = 0.9).
A subgroup analysis of histopathological results in patients with cancer revealed that the two groups had similar oncologic features in terms of pT class, pTNM stage, tumor size, length of specimen, and surgical margin involvement, but the robotic group had a significantly higher number of harvested lymph nodes (median; 66 vs 50, p = 0.01) (Table 4).
Discussion
In this study, we evaluated the feasibility of robotic total and subtotal colectomy procedures and compared the perioperative short-term outcomes with those of conventional laparoscopy in both benign and malignant conditions. Our results show that robotic surgery with the da Vinci Xi platform has perioperative outcomes similar to laparoscopy, but a higher number of retrieved lymph nodes in cancer patients, and longer operative times.
Robotic technology is an evolving field with the introduction of new robotic systems in colorectal surgery. Compared with the prior robotic platforms, the da Vinci Xi robot has important distinctive features, including easier docking, a wider range of motion with its thinner arms, patient clearance design and an ability to attach the endoscope to any arm [8]. More importantly, the rotating boom-mounted system feature of this robot facilitates easier access in multiquadrant surgery without the need for multiple patient-side cart positioning [6,7,8]. All the robotic procedures in this study were completed with a single positioning of the patient-side cart and without the need for a laparoscopic assistance.
Although the feasibility of the Xi platform has been evaluated in a variety of colorectal procedures, this platform is not widely used for extended colectomy procedures. To our knowledge, there is only one study specifically evaluating the role of the Xi platform in total colectomy procedure. In this study including 23 patients [6], Jimenez-Rodriguez et al. report their experience with 15 robotic total colectomy procedures and compared the short-term outcomes with those of eight laparoscopic procedures. Results from this relatively small series show that the robotic approach is associated with a shorter length of stay (4 vs 6 days, p = 0.047), and similar operative time (243 vs 263 min), conversion rate (0 vs 6%), and complication rate (25% vs 0). The authors conclude that the da Vinci Xi robotic platform may overcome some of the disadvantages of older-generation platforms and is associated with similar operative time for this specific complex operation.
Our study is the second of its kind with more powered data to compare robotic approach with laparoscopy in multiquadrant colonic resections. A notable difference between our technique and that described by Jimenez-Rodriguez et al. [6] is that we position the patient-side surgical cart on the right side of the patient instead of positioning the cart between the patient’s legs. We prefer this cart position to avoid working space limitation for the assistant surgeon during the stapled bowel anastomosis stage of the operation. All the robotic procedures in this study were successfully completed in a fully robotic fashion with a single positioning of the patient-side cart via its rotating boom-mounted system.
Analysis of our groups revealed that the two groups had equivalent preoperative characteristics, suggesting that the groups were well comparable. As seen from the results, there were no significant differences between the two approaches in terms of intraoperative outcomes and overall short-term complications except that the robotic approach was associated with longer operative times. In our robotic group, the median operative time was 350 min, which is higher than the 243 min reported by Jimenez-Rodriguez et al. [6]. It should be taken into account that additional surgery was performed in nearly 20% of our robotic procedures and all the anastomoses were performed with the robot as opposed to the laparoscopic assistance used by these authors. These two factors might collectively have contributed to increased operative times in our robotic series.
With respect to conversion rates and length of hospital stay, there was a trend toward better results in the robotic group. Our conversion rate was lower in the robotic group (0 vs 14%, p = 0.051) perhaps owing to easier multiquadrant manipulation provided by better dexterity with stable wristed instrumentations. Regarding length of stay, there was a 1.5-day difference favoring the robotic group (7.9 ± 5.7 vs 9.5 ± 6.0 days, p = 0.08). We believe that, although not statistically significant, these results are clinically important.
For any available robotic system to become an accepted alternative, it must also result in better or at least comparable oncologic outcomes. Considering the cancer cases in this study, we found comparable results between the robotic and laparoscopic approach with regard to oncologic safety except that a significantly higher number of lymph nodes were retrieved in the robotic procedures (median, 66 vs 50 nodes). The number of retrieved lymph nodes is known to be an important prognostic factor for survival in patients undergoing surgery for colon cancer [11, 12].
Notwithstanding these advantages, the robotic approach has been criticized for its increased cost. In a study including 26,721 patients with data from the nationwide inpatient sample (NIS) database [13], Moghadamyeghaneh et al. compared the outcomes of open, laparoscopic, and robotic total colectomy and the authors found that the robotic approach was associated with higher total hospital charges, but a lower conversion rate, and similar length of hospital stay, morbidity and mortality rates compared to the laparoscopic approach. Interestingly, there was no significant difference in hospital charges between robotic surgery and open surgery. This may be due to the shorter length of stay associated with the robotic approach compared to open surgery (8 vs 11 days).
We note that this study has several limitations. First, it is a retrospective study despite the use of prospectively collected data. Second, there might be selection bias although the two groups were closely comparable in terms of preoperative characteristics. This study included the cases of five surgeons at two centers and, therefore, there could be individual surgeon’s bias in selecting patients for the laparoscopic or robotic approach. Finally, our interpretation of the results is limited by the short follow-up period and lack of cost analysis.
Conclusions
In total/subtotal colectomy procedures, the robotic approach with the da Vinci Xi platform is feasible, safe, and associated with short-term outcomes similar to laparoscopy but longer operative times and a higher number of retrieved lymph nodes. The multiquadrant access possible with the da Vinci Xi with its rotating boom-mounted system allows the surgeon to make optimal use of the advantages of robotic surgery in all stages of such extended colonic resections.
References
Zimmern A, Prasad L, Desouza A, Marecik S, Park J, Abcarian H (2010) Robotic colon and rectal surgery: a series of 131 cases. World J Surg 34:1954–1958
D’Annibale A, Morpurgo E, Fiscon V et al (2004) Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum 47:2162–2168
Kim JC, Lee JL, Yoon YS, Kim CW, Park IJ, Lim SB (2018) Robotic left colectomy with complete mesocolectomy for splenic flexure and descending colon cancer, compared with a laparoscopic procedure. Int J Med Robot 14:e1918
Spinoglio G, Bianchi PP, Marano A et al (2018) Robotic versus laparoscopic right colectomy with complete mesocolic excision for the treatment of colon cancer: perioperative outcomes and 5-year survival in a consecutive series of 202 patients. Ann Surg Oncol 25:3580–3586
Baik SH, Kwon HY, Kim JS et al (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16:1480–1487
Jimenez-Rodriguez RM, Quezada-Diaz F, Tchack M et al (2019) Use of the Xi robotic platform for total abdominal colectomy: a step forward in minimally invasive colorectal surgery. Surg Endosc 33:966–971
Protyniak B, Jorden J, Farmer R (2018) Multiquadrant robotic colorectal surgery: the da Vinci Xi vs Si comparison. J Robot Surg 12:67–74
Ozben V, Cengiz TB, Atasoy D et al (2016) Is da Vinci Xi Better than da Vinci Si in robotic rectal cancer surgery? Comparison of the 2 generations of da Vinci systems. Surg Laparosc Endosc Percutan Tech 26:417–423
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13:606–608
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Swanson RS, Compton CC, Stewart AK, Bland KI (2003) The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 10:65–71
Kotake K, Honjo S, Sugihara K et al (2012) Number of lymph nodes retrieved is an important determinant of survival of patients with stage II and stage III colorectal cancer. Jpn J Clin Oncol 42:29–35
Moghadamyeghaneh Z, Hanna MH, Carmichael JC, Pigazzi A, Stamos MJ, Mills S (2016) Comparison of open, laparoscopic, and robotic approaches for total abdominal colectomy. Surg Endosc 30:2792–2798
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
All study participants signed a written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozben, V., de Muijnck, C., Karabork, M. et al. The da Vinci Xi system for robotic total/subtotal colectomy vs. conventional laparoscopy: short-term outcomes. Tech Coloproctol 23, 861–868 (2019). https://doi.org/10.1007/s10151-019-02066-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-019-02066-y