Abstract
Surgical site infections occur in up to 24 % of patients after surgical excision of sacrococcygeal pilonidal sinus disease with primary wound closure. Local administration of antibiotics by a gentamicin collagen sponge could reduce this infection rate. The objective of this systematic review and meta-analysis was to evaluate the effect of a gentamicin collagen sponge on outcome after surgical excision in patients with sacrococcygeal pilonidal sinus disease. A structured literature search was performed in the PubMed, Embase, The Cochrane Library, and Scopus databases. Studies comparing surgical excision of sacrococcygeal pilonidal sinus disease with versus without a gentamicin collagen sponge were included. Outcome measures were surgical site infection, wound healing, and recurrence. The search strategy yielded six studies with a total of 669 patients. Three randomized controlled trials, comparing excision of pilonidal sinus disease and primary wound closure with versus without gentamicin collagen sponge, were eligible for inclusion in the meta-analysis (319 patients), demonstrating a trend towards reduced surgical site infections after administration of gentamicin collagen sponge [absolute risk reduction 20 %, 95 %-confidence interval (CI) 1–41 %, p = 0.06]. The wound healing (absolute risk reduction 22 %, 95 % CI 32–77 %, p = 0.42) and recurrence rate (absolute risk reduction 8 %, 95 % CI 7–22 %, p = 0.30) were not significantly different between both groups. Administration of a gentamicin collagen sponge after surgical excision of sacrococcygeal pilonidal sinus disease showed no significant influence on wound healing and recurrence rate, but a trend towards a reduced incidence of surgical site infections. Therefore, additional larger well-designed randomized controlled trials are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sacrococcygeal pilonidal sinus disease (SPSD) is an acquired disorder of the skin and subcutaneous tissue. It is most common among young adults, affecting men twice more often than women [1–3]. Patients present with recurrent or persistent discharge, discomfort and/or pain in the natal cleft. The treatment of SPSD involves eradication of the sinus tract by surgical excision, deroofing or phenol application. Radical excision of the sinus, however, is the most commonly applied treatment option. Healing of the overlying skin can be achieved by primary wound closure, in-midline, off-midline, i.e. Karydakis flap reconstruction, or rarely, with Limberg flap reconstruction. In recent decades, off-midline closure has become the preferred method due to a lower recurrence rate [4, 5] and the Karydakis Flap reconstruction is advised for treatment of uncomplicated SPSD [6]. The disadvantage of primary closure, however, is the high rate of surgical site infection, occurring in up to 24 % of patients [1, 3, 4, 7]. Another commonly applied method after radical excision is secondary healing of the wound by open granulation; however, this results in a longer wound healing time [4, 5].
Administration of systemic antibiotics after primary wound closure may be an option to reduce the incidence of surgical site infections. However, several randomized controlled trials (RCTs) have not shown any significant benefit [8–11]. Therefore, local application of a gentamicin collagen sponge in the wound cavity after excision of the SPSD has been introduced to reduce the incidence of surgical site infections. Compared to systemic antibiotics, this local administration leads to prolonged and higher local therapeutic concentrations [12, 13]. Several RCTs have been performed comparing primary wound closure with the administration of a gentamicin collagen sponge after surgical excision of SPSD versus either primary wound closure [14–16] or secondary wound healing [17, 18], both without a gentamicin collagen sponge. The outcome regarding surgical site infection, wound healing, and recurrence rate is quite different in these studies. Therefore, to date, a consensus on the optimal treatment for SPSD with regard to the local administration of antibiotics does not exist.
The objective of this systematic review and meta-analysis was to analyze whether the local intraoperative administration of a gentamicin collagen sponge after excision of SPDS benefits the outcome with regard to surgical site infection, wound healing, and recurrence rate.
Methods
A systematic review and meta-analysis was conducted according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) [19].
Search strategy
A search of the literature was conducted in the databases of PubMed, Embase, and the Cochrane Library on October 30, 2014. Different synonyms for “SPSD”, “gentamicin”, “local antibiotics”, “surgical site infection”, “wound healing,” and “recurrence” were used as search terms (Table 1). For the search in PubMed, additional MeSH terms were used. No search limitations were applied. Additionally, cited references of the included articles were screened using the Scopus database. Finally, reference lists of the included articles were manually searched in order to identify potentially eligible studies.
Study selection
Studies were screened on title, abstract, and full texts for identifying potentially relevant studies according to predefined inclusion criteria. Studies were included if the patients had SPSD. The intervention consisted of application of a gentamicin collagen sponge versus no gentamicin collagen sponge after surgical excision of the SPSD. The primary outcomes were surgical site infection, wound healing, and/or recurrence. All types of study design were included. Studies describing patients with an abscess and treatment options other than surgical excision were excluded from further analysis.
Quality assessment
The included studies were methodologically assessed, according to the items described in the Cochrane handbook for systematic reviews of interventions, version 5.1.0 [20]. Additionally, the level of evidence was assessed according to the Centre for Evidence Based Medicine at the University of Oxford [21].
Data acquisition
Data of the included studies were acquired by using a standard data extraction form, collecting information on the year of publication, study design, sample size, wound closure technique, size and number of gentamicin collagen sponges, duration of follow-up, surgical site infection rate, wound healing rate, time to wound healing, and recurrence rate.
Statistical analysis
Data were analyzed by using RevMan 5.2 software (Review Manager Version 5.0: The Nordic Cochrane Centre, Copenhagen; The Cochrane Collaboration, 2012). Outcome parameters were summarized per individual study using absolute risks (AR), absolute risk reduction (ARR), and the number needed to treat (NNT) with corresponding 95 % confidence intervals (95 % CI). Statistical heterogeneity of the pooled data was assessed by using the Chi-square test and I 2 statistic. Heterogeneity was considered statistically significant with p < 0.1 and I 2 > 75 %. Forest plots were made for the absolute risk differences (RD) using a random effects model, since significant statistical heterogeneity was present.
Results
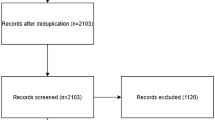
The original search yielded 40 articles. After removal of duplicates, 22 articles remained which were screened on title and abstract according to predefined inclusion criteria. Subsequently, ten articles remained and were screened on full text. Eventually, five RCTs [14–18] and one retrospective case–control study [22] were eligible for inclusion (Fig. 1).
The methodological quality assessment of the five included RCTs is shown in Table 2. Andersson et al. [14] had no negative score on any of the assessed items. The method of randomization was not reported in two studies [16, 17]. Studies performed by Vogel et al. [15] and Rao et al. [18] had a loss to follow-up of more than 10 %. In four trials, blinding of patients, surgeons, and assessors for the intervention and whether analysis was performed according to the intention to treat principle was not reported [15–18]. Doll et al. [22] executed a retrospective case–control study (excision of SPSD with or without gentamicin collagen sponge). The study groups and interventions were adequately described, and outcomes were adequately assessed according to predefined criteria.
The level of evidence according to the Oxford Centre for Evidence Based Medicine for the five RCTs [14–18] was 1b and for the individual cohort study [22] was 2b.
Primary closure with versus without gentamicin collagen sponge
Three RCTs [14–16] and one retrospective case control study [22] were conducted comparing surgical excision followed by primary closure with or without a gentamicin collagen sponge.
Andersson et al. [14] executed a double-blinded multicenter RCT in Sweden comparing primary closure with (77 patients) versus without a gentamicin collagen sponge (82 patients) after surgical excision of SPSD (Table 3). This study showed no significant differences in terms of surgical site infection rates at 2 weeks after surgery (Table 4). The wound healing and recurrence rate at one-year follow-up were also not significantly different between the groups (Tables 5 and 6, respectively).
Vogel et al. [15] performed a RCT in Germany, with 40 patients in each group, comparing application of a gentamicin collagen sponge versus no gentamicin collagen sponge after surgical excision (Table 3). One to four gentamicin collagen sponges were administrated depending on the size of the wound. With regard to surgical site infections, the ARR was 42.5 % (95 % CI 25.0–60.0, p < 0.001) in favor of the gentamicin collagen sponge group with a corresponding number needed to treat (NNT) of 3.0 (95 % CI 1.7–4.0) (Table 4). The absolute risk reduction (ARR) of the rate of non-healed wounds was 50.0 % (95 % CI 31.8–68.2), p < 0.001) in favor of application of a gentamicin collagen sponge (Table 5). No recurrences occurred at follow-up (Table 6).
Yetim et al. [16] conducted a RCT in Turkey with 80 patients, comparing local administration of a gentamicin collagen sponge to postoperative oral antibiotic therapy for 7 days after surgical excision with primary midline closure (Table 3). For the outcome surgical site infections, a significant ARR of 15.0 % (95 % CI 0.9–29.0, p = 0.04) was achieved after application of a gentamicin collagen sponge (Table 4). The mean time to wound healing was reported to be shorter after application of a gentamicin collagen sponge (8.9 vs. 15.1 days, p = 0.001) [16]. Additionally, a significant ARR of 15.0 % (95 % CI 4.0–26.1, p = 0.01) in favor of the gentamicin collagen sponge group was demonstrated with regard to recurrence at one-year follow-up (Table 6).
Doll et al. [22] retrospectively examined a population of 187 men with SPSD who underwent excision with primary midline closure with or without a gentamicin collagen sponge (Table 3). Application of a gentamicin collagen sponge yielded a significant ARR of 13.6 % (95 % CI 0.9–26.2, p = 0.03) with regard to surgical site infections (Table 4). All wounds were healed after 12 days. No statistically significant difference in the recurrence rate existed between the groups (Table 6).
Primary closure with gentamicin collagen sponge versus secondary wound healing without gentamicin collagen sponge
Holzer et al. [17] executed a multicenter RCT in Austria that included 103 patients comparing primary closure with a gentamicin collagen sponge versus secondary wound healing without a gentamicin collagen sponge after surgical excision of SPSD (Table 3). In the gentamicin collagen sponge group, 27.5 % (95 % CI 17.0–41.0 %) of the wounds were not healed at 2-week follow-up. The median time to healing in the primary closure with gentamicin collagen sponge group was 17 days (range 7–39 days) versus 68 days (range 10–161 days) in the secondary wound healing group (p < 0.001) [17]. Two patients in the gentamicin collagen sponge group developed a surgical site infection in the first 2 weeks after surgery, which required conversion to open treatment (Table 4). The surgical site infection and wound healing rates for the secondary wound healing group were not reported. After a follow-up period of 26 weeks, one recurrence was seen in the primary closure group versus none in the open treatment group (Table 6).
Rao et al. [18] performed a single-center RCT in Northern Ireland that enrolled 60 patients who underwent surgical excision of SPSD. In the primary closure group (30 patients), one or two gentamicin collagen sponges were implanted in the wound depending on the size of the wound. The surgical site infection rate was not reported. The rate of non-healed wounds at 4-week follow-up was significantly higher in the group of patients, who underwent surgical excision followed by secondary wound healing without a gentamicin collagen sponge (Table 5). Furthermore, the median wound healing time (interquartile range) was also significantly shorter in the gentamicin collagen sponge group [10 (10–26) days vs. 50 (40–90) days; p < 0.001] [18]. At 5-year follow-up, there was no significant difference in terms of recurrence (Table 6).
Pooling of data
The study data from three RCTs [14–16] comparing surgical excision followed by primary closure with versus without a gentamicin collagen sponge were pooled. The risk difference (RD) for surgical site infections was 20 % (95 % CI, range 1–41 %) in favor of the treatment with the gentamicin collagen sponge, although this was not statistically significant (p = 0.06) (Fig. 2A). Pooled data of two RCTs [14, 15] reported no significant difference in rate of non-healed wounds at one-year follow-up after administration of local antibiotics (RD 22 %, 95 % CI, range 32–77 %, p = 0.42) (Fig. 2b). Additionally, heterogeneity was significantly present for both outcome parameters. There was no significant difference regarding recurrence rate between both treatments at one-year follow-up (Fig. 2c).
Discussion
This systematic review summarizes the available literature with regard to the effect of intraoperative local administration of a gentamicin collagen sponge after surgical excision of SPSD. Meta-analysis of three RCTs that investigated surgical excision of SPSD followed by primary closure with versus without a gentamicin collagen sponge demonstrated a trend towards less surgical site infections with the application of a gentamicin collagen sponge. However, the wound healing and recurrence rate were not significantly influenced [14–16]. Additionally, a retrospective cohort study showed a significant reduction in surgical site infection with the administration of a gentamicin collagen sponge, but there was no statistically significant difference in wound healing and recurrence rates [22].
In this meta-analysis, the results did not reach a statistical significant difference in terms of surgical site infection, probably due to relatively small sized and therefore underpowered RCTs. Heterogeneity of the included studies was present as well. However, the results showed a trend towards a reduction in the rate of surgical site infections (p = 0.06). These results are supported by a systematic review and meta-analysis performed by Chang et al. [23], consisting of fifteen RCTs, which confirmed that gentamicin collagen sponges significantly reduce the incidence of surgical site infections after different types of surgery [odds ratio (OR) = 0.51; 95 % CI 0.33–0.77; p = 0.01]. Although current evidence is not yet overly convincing, the advantage of applying a gentamicin collagen sponge is that the antibiotics remain localized and do not enter the systemic circulation. Moreover, no adverse events due to the application of a gentamicin collagen sponge were reported in the included trials.
The results of this systematic review showed no significant difference with regard to wound healing with the use of gentamicin collagen sponges, therefore this still remains a problem in a substantial proportion of patients. It should be noted that primary midline closure was applied in the included studies, whereas several meta-analyses have shown that off-midline closure should be the treatment of choice considering the lower rate of surgical site infections, faster healing rates and lower recurrence rates associated with this type of closure [4, 5]. Additionally, wound healing could be impeded when the gentamicin collagen sponge is inserted between both edges of the wound, as this may become a barrier to adequate wound healing. Some included studies reported details regarding the size [16, 17] and number [14, 15, 18] of inserted sponges in the wound cavity. However, they did not report whether the gentamicin sponge was inserted in the wound as a whole, although some images included in the articles demonstrate that the gentamicin collagen sponge was in situ in the wound after surgical excision as a whole [16–18]. In order to promote wound healing, however, the gentamicin collagen sponge can be cut into small pieces before insertion in the wound cavity. Whether cutting the sponge into small pieces will improve wound healing needs further investigation.
There also were two RCTs [17, 18] included in this systematic review that compared primary wound closure with a gentamicin collagen sponge versus secondary wound healing (without a gentamicin collagen sponge) after excision of SPSD. The surgical site infection rate was not adequately reported in both studies, and there was no statistically significant difference in recurrence rate between both groups [17, 18]. Both studies reported that in terms of wound healing, primary closure with a gentamicin collagen sponge was superior to secondary wound healing. However, it is commonly known that primary closure accelerates wound healing. Therefore, the additional effect of a gentamicin collagen sponge on wound healing cannot be determined from these studies.
There are a few limitations to this systematic review, which are mainly due to the quality, heterogeneity, and size of the included studies. First, four RCTs [15–18] did not state details about concealment of allocation, and whether blinding for participants, personnel, and patients was performed. Therefore, these studies are at risk for selection bias, performance bias, and detection bias, respectively. Second, two studies [15, 16] reported a remarkable recurrence rate of 0 %, which leads us to question the validity of these studies as this seems, in our opinion, unlikely in this patient population. Third, most RCTs [15–18] did not record whether their studies were appropriately powered. Fourth, it is remarkable that the relatively smaller RCTs showed statistically significant differences with regard to surgical site infections [15, 16], wound healing rate [15], and recurrences [16] by adding a gentamicin collagen sponge to the surgical treatment, whilst the largest RCT [14] does not support these findings. This may be due to publication bias, where statistically significant results may be more likely to be published than non-significant results regardless of the size, design, and methodology of the study. This could lead to overestimation of the effect of the gentamicin collagen sponge. Finally, to the best of our knowledge, the cost-effectiveness of the application of a gentamicin collagen sponge in patients with SPSD has not yet been evaluated. The cost-effectiveness of local application of a gentamicin collagen sponge has been confirmed in the prevention of sternal wound infections following cardiac surgery [24]. However, future research has to be performed to determine whether the application of gentamicin implants in patients with SPSD will also be cost-effective.
Conclusions
This systematic review and meta-analysis has demonstrated that the administration of a gentamicin collagen sponge after surgical excision of SPSD does not accelerate wound healing or reduce the recurrence rate, but there is a trend towards less surgical site infections. Therefore, larger well-designed RCTs are needed in order to demonstrate a more reliable and accurate effect of the application of a gentamicin collagen sponge on the outcome after surgical excision of SPSD.
References
Søndenaa K, Andersen E, Nesvik I, Søreide JA (1995) Patient characteristics and symptoms in chronic pilonidal sinus disease. Int J Colorectal Dis 10:39–42
Akinci OF, Bozer M, Uzunköy A, Düzgün SA, Coşkun A (1999) Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg 165:339–342
Khanna A, Rombeau JL (2011) Pilonidal disease. Clin Colon Rectal Surg 24:46–53
Al-Khamis A, McCallum I, King PM, Bruce J (2010) Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev (1): CD006213
Enriquez-Navascues JM, Emparanza JI, Alkorta M, Placer C (2014) Meta-analysis of randomized controlled trials comparing techniques with primary closure for chronic pilonidal sinus. Tech Coloproctol 18:863–872
Ates M, Dirican A, Sarac M, Aslam A, Colak C (2011) Short and long-term results of the Karydakis flap versus the Limberg flap for treating pilonidal sinus disease: a prospective randomized study. Am J Surg 202:568–573
Hull TL, Wu J (2002) Pilonidal disease. Surg Clin North Am 82:1169–1185
Søndenaa K, Diab R, Nesvik I et al (2002) Influence of failure of primary wound healing on subsequent recurrence of pilonidal sinus. Combined prospective study and randomised controlled trial. Eur J Surg 168:614–618
Kronborg O, Christensen K, Zimmermann-Nielsen C (1985) Chronic pilonidal disease: a randomized trial with a complete 3-year follow-up. Br J Surg 72:303–304
Chaudhuri A, Bekdash BA, Taylor AL (2006) Single-dose metronidazole vs 5-day multi-drug antibiotic regimen in excision of pilonidal sinuses with primary closure: a prospective, randomized, double-blinded pilot study. Int J Colorectal Dis 21:688–692
Lundhus E, Gjøde P, Gottrup F, Holm CN, Terpling S (1989) Bactericidal antimicrobial cover in primary suture of perianal or pilonidal abscess. A prospective, randomized, double-blind clinical trial. Acta Chir Scand 155:351–354
Ruszczak Z, Friess W (2003) Collagen as a carrier for on-site delivery of antibacterial drugs. Adv Drug Deliv Rev 55:1679–1698
Musella M, Guido A, Musella S (2001) Collagen tampons as aminoglycoside carriers to reduce postoperative infection rate in prosthetic repair of groin hernias. Eur J Surg 167:130–132
Andersson RE, Lukas G, Skullman S, Hugander A (2010) Local administration of antibiotics by gentamicin-collagen sponge does not improve wound healing or reduce recurrence rate after pilonidal excision with primary suture: a prospective randomized controlled trial. World J Surg 34:3042–3048
Vogel P, Lenz J (1992) Treatment of pilonidal sinus with excision and primary suture using a local, resorbable antibiotic carrier. Results of a prospective randomized study. Chirurg 63:748–753
Yetim I, Ozkan OV, Dervişoglu A, Erzurumlu K, Canbolant E (2010) Effect of gentamicin-absorbed collagen in wound healing in pilonidal sinus surgery: a prospective randomized study. J Int Med Res 38:1029–1033
Holzer B, Grüssner U, Brückner B, EMD study group et al (2003) Efficacy and tolerance of a new gentamicin collagen fleece (Septocoll) after surgical treatment of a pilonidal sinus. Colorectal Dis 5:222–227
Rao MM, Zawislak W, Kennedy R, Gilliland R (2010) A prospective randomised study comparing two treatment modalities for chronic pilonidal sinus with a 5-year follow-up. Int J Colorectal Dis 25:395–400
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341
Higgins J, Green S (2011) Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The cochrane collaboration. http://www.cochrane-handbook.org. Accessed 22 Feb 2014
Centre for evidence based-medicine at University of Oxford (2011) The Oxford 2011 Levels of evidence. http://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf. Accessed 22 Feb 2014
Doll D, Evers T, Matevossian E, Hoffmann S, Krapohl B, Bartsch D (2011) Does gentamycin affect long term recurrence rate in pilonidal sinus surgery? Eur Surg 43:236–243
Chang WK, Srinivasa S, MacCormick AD, Hill AG (2013) Gentamicin-collagen implants to reduce surgical site infection: systematic review and meta-analysis of randomized trials. Ann Surg 258:59–65
Friberg O, Dahlin LG, Levin LA et al (2002) Cost effectiveness of local collagen-gentamicin as prophylaxis for sternal wound infections in different risk groups. Scand Cardiovasc J 40:117–125
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Nguyen, A.L., Pronk, A., Furnée, E.J.B. et al. Local administration of gentamicin collagen sponge in surgical excision of sacrococcygeal pilonidal sinus disease: a systematic review and meta-analysis of the literature. Tech Coloproctol 20, 91–100 (2016). https://doi.org/10.1007/s10151-015-1381-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-015-1381-7